Abstract
Objective: We evaluated the effects of modafinil, a wake-promoting agent, on patient functioning, health-related quality of life, and nighttime and daytime sleep in patients with excessive sleepiness associated with shift work sleep disorder (SWSD).
Method: A 12-week, randomized, double-blind, placebo-controlled study was performed at 31 centers in the United States between February 2001 and March 2002. Adults (N = 278) with excessive sleepiness associated with chronic SWSD (International Classification of Sleep Disorders criteria) were randomly assigned to receive modafinil 200 or 300 mg or placebo, 30 to 60 minutes before each night shift. Effects on patient functioning and quality of life were assessed using the Functional Outcomes of Sleep Questionnaire (FOSQ) and the 36-item Short Form Health Survey (SF-36), respectively. Daily patient diaries were used as a sleep log.
Results: Modafinil 300 mg significantly improved mean FOSQ total score relative to placebo (2.3-point increase from baseline vs. 1.6 for placebo; p < .05). Both doses of modafinil significantly improved mean SF-36 mental component scores relative to placebo (mean changes from baseline of 3.2, 3.7, and 0.7 points in the modafinil 300-mg, modafinil 200-mg, and placebo groups, respectively; p < .05 for each comparison vs. placebo). Modafinil did not adversely affect sleep when sleep was desired or caffeine use. Modafinil was well tolerated. Headache (21.5%) and nausea (12.4%) were the most common adverse events in modafinil-treated patients. Differences between modafinil and placebo for vital sign measurements, physical examination findings, or electrocardiography results were not clinically meaningful.
Conclusions: Modafinil significantly improves functioning and quality of life in patients with SWSD. Modafinil is an effective treatment for excessive sleepiness associated with SWSD.
Shift work sleep disorder (SWSD) is a circadian rhythm sleep disorder that results from the inability of some shift workers to adapt to the major misalignment between the work-rest schedule imposed by their occupation and the internal rhythms that are normally responsible for the physiologic maintenance of sleep and wakefulness. SWSD is characterized by symptoms of excessive sleepiness or insomnia,1 symptoms that can negatively impact daily living. Excessive sleepiness has been shown to impair performance2 and quality of life, regardless of its underlying cause.3,4
More than 15 million Americans work alternative shifts on a permanent or rotating basis, accounting for almost 17% of the workforce.5 Previous research has shown that night and/or rotating shift work has negative physical, personal, and social consequences, including increased incidence of cardiovascular and gastrointestinal complaints6; increased fatigue,7–10 confusion,8,10 tension/anxiety,8 anger/ hostility,8 mood depression,7,8,11 and absenteeism12; decreased vigor8–10; and reduced satisfaction with personal and family pursuits.6 A study of 2570 adult workers in the United States by Drake et al.13 reported a significantly higher prevalence of excessive sleepiness among night and rotating workers compared with day workers and estimated that up to 32% of night and rotating shift workers have symptoms that meet criteria for SWSD. This study reported significantly higher risks for ulcers, depression, sleepiness-related accidents, and missed family and social activities among workers with SWSD compared with workers without SWSD.13 Findings from a study of Air Force air traffic controllers also showed significantly greater anxiety, depression, and quality-of-life impairment in those with SWSD compared with shift workers without the disorder.14
The wake-promoting agent modafinil has been shown to improve wakefulness in patients with excessive sleepiness associated with SWSD.15 Modafinil 200 mg per day improved wakefulness as measured with objective and subjective assessments, overall clinical condition, and ability to sustain attention, in patients with SWSD in a 12-week, randomized, placebo-controlled trial of 209 patients in which sleepiness and performance were assessed in a simulated night shift in a sleep laboratory.15 Data from patient diaries also showed significant reductions in the level of sleepiness at work and on the commute home and in near misses or accidents on the commute home.
The objective of this study was to assess the effect of modafinil on patient functioning and quality of life and the tolerability of modafinil in patients with excessive sleepiness associated with SWSD.
METHOD
This 12-week, randomized, double-blind, placebo controlled, parallel-group trial was conducted at 31 U.S. centers between February 2001 and March 2002. It was carried out in accordance with the Helsinki Declaration of 1975 and the Good Clinical Practice: Consolidated Guideline approved by the International Conference on Harmonisation.16 The study protocol was approved by local or national institutional review boards, and patients provided written informed consent before study participation.
Patients were men and women (outpatients), ranging in age from 18 to 60 years, with a current diagnosis of chronic SWSD as defined by International Classification of Sleep Disorders criteria.1 Patients were diagnosed by qualified investigators in sleep medicine and were eligible for participation in the study if they were regular shift workers (worked at least 5 nights per month, 6–12 hours per shift, with at least 6 hours worked between 2200 and 0800 hours), worked at least 3 shifts on consecutive nights, and had complaints of excessive sleepiness. For this study, excessive sleepiness was defined as a clinically significant frequency of sleep episodes or struggling to stay awake during the night shifts. Patients were at least moderately ill with respect to sleepiness at work, including the commute to and from work, as assessed by the Clinical Global Impressions-Severity of Illness subscale (CGI-S; score ≥ 4).17 At baseline, clinical assessment of all patients was conducted to ensure that eligibility criteria were met. Female patients could not be pregnant or lactating, and female patients of childbearing potential were required to use a medically accepted method of birth control throughout and for 30 days after study participation. Patients were not permitted any prior or concomitant medications within 30 days before and up to the end of the double-blind portion of the study. Patients were excluded from study participation if they reported any of the following: diagnosis of a current sleep disorder other than SWSD; excessive caffeine consumption (> 800 mg/day or > 8 cups of coffee/day); use of any prescription drugs disallowed by the protocol (i.e., melatonin, tricyclic antidepressants, lithium, St. John's wort, stimulants, anti-psychotics, benzodiazepines, zolpidem, monoamine oxidase inhibitors, anticoagulants, anticonvulsants, barbiturates, sedating antihistamines, and any other medications that made patients feel sleepy) or clinically significant use of nonprescription drugs; alcohol, narcotic, or drug abuse; clinically significant uncontrolled medical or psychiatric condition as determined by the investigator; or a history of a seizure disorder.
Patients were randomly assigned (1:1:1) to modafinil 200 mg, modafinil 300 mg, or placebo taken orally once daily, 30 to 60 minutes before the start of regularly scheduled night shifts for 12 weeks.
Assessments
Patient functioning
Patient functioning was evaluated using the Functional Outcomes of Sleep Questionnaire (FOSQ), a self-administered, 30-item, validated instrument in a specific sleep disorder (i.e., obstructive sleep apnea) that measures the effect of excessive sleepiness on 5 domains of everyday living and quality of life (vigilance, activity level, general productivity, social outcome, and intimacy).18 The FOSQ was administered at baseline and at weeks 4 and 12. The range of scores for each domain is 1 to 4; higher scores indicate greater function. FOSQ total scores were calculated by totaling the 5 sub-scale scores and, therefore, could range from 5 to 20.
Health-related quality of life
Health-related quality of life was assessed using the self-administered 36-item Short Form Health Survey (SF-36).19 The SF-36 is composed of 8 multi-item subscales that measure the following general health concepts: vitality, role physical, physical functioning, bodily pain, general health, social functioning, mental health, and role emotional. The physical component and the mental component scores are derived from scores of the 8 subscales. Total scores (all 8 subscales) range from 0 to 100, with higher scores denoting better health. The SF-36 was administered at baseline and at weeks 4 and 12.
Nighttime sleep, daytime sleep, and caffeine consumption
Patients completed daily diaries to record information on daytime sleep parameters (total time in bed, sleep duration, number of awakenings, and time awake after sleep onset) for each day after a night shift worked. Sleep efficiency was calculated as the percentage of sleep duration relative to the total time in bed. Patient diaries were also used to record caffeine consumption on a night shift worked and on the day after a night shift. Diaries were collected from patients at baseline and at weeks 4, 8, and 12. Use of the diary was introduced in a protocol amendment after the study started. Data from the 98 patients who provided analyzable diary data are summarized in the results section.
Tolerability
Adverse events were monitored throughout the study. Additional assessments included routine clinical laboratory tests (serum chemistry and hematol-ogy), vital signs (blood pressure and heart rate), electrocardiograms (ECGs), physical examinations, and concomitant medication usage.
Analysis
All data were processed and summarized using Statistical Analysis Systems (SAS) software version 8.2 on the Windows 95 platform (SAS Institute, Cary, N.C.). The SAS type III sum of squares for the statistical inference was used for all analyses of covariance. All statistical tests were 2-tailed at the .05 level of significance.
Treatment comparisons of demographic and baseline characteristics were performed using a 2-way analysis of variance for continuous variables or the Cochran-Mantel-Haenszel test for categorical variables. Patient functioning and quality-of-life analyses included data from all randomly assigned patients who received at least 1 dose of study drug or placebo and had at least 1 postbaseline FOSQ or SF-36 measurement. Patients who withdrew from the study early and received at least 1 dose of study medication were required to return to the clinic for post-baseline assessment. The mean change from baseline to final visit in FOSQ total and domain scores was analyzed using an analysis of covariance with treatment and center as factors and baseline value as a covariate. If there was evidence of treatment by covariate interaction (p value for the interaction < 10) in any of the analyses, the covariate was dropped from the analysis of covariance and a 2-way analysis of variance with treatment and center as factors was used instead; however, no interaction between treatment and baseline was found, thus the plan of using analysis of covariance was followed. The mean change from baseline to final visit in SF-36 physical and mental component scores and domain scores was evaluated using an analysis of covariance in the same manner as described for the FOSQ. Diary-extracted data (sleep efficiency, number of awakenings, time awake after sleep onset, and caffeine consumption) were summarized. All patients who received at least 1 dose of study drug or placebo were included in the safety analysis.
RESULTS
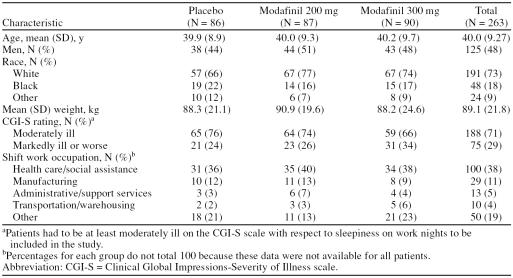
Patient Disposition and Characteristics
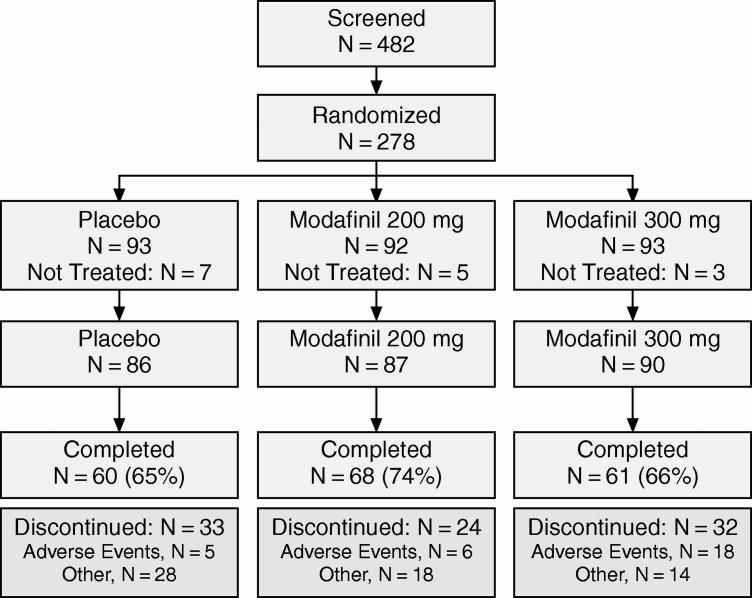
A total of 482 individuals were screened for study participation and 278 patients were randomly assigned to study treatment. The disposition of patients is summarized in Figure 1. Demographic and baseline characteristics for the patients who received at least 1 dose of modafinil or placebo are shown in Table 1. Patient characteristics, including age, sex, race, body weight, and baseline CGI-S ratings, were similar for the 3 groups (p > .05). The majority of patients were permanent night-shift workers (77%), and many were health care/social assistance workers (38%), such as nurses, pharmacists, and health care technologists/technicians. The next most common occupations were in manufacturing (11% overall) and administrative and support services (5%). Patients received study drug on approximately 40 days during the study in each group.
Figure 1.
Patient Disposition in a Randomized, Double-Blind, Placebo-Controlled Trial of Modafinil for Excessive Sleepiness Associated With Chronic Shift Work Sleep Disorder
Table 1.
Demographic and Baseline Characteristics of Patients With Chronic Shift Work Sleep Disorder
Mean FOSQ total scores at baseline were similar in the placebo, modafinil 200-mg, and modafinil 300-mg groups (range: 14.3–14.7 points). Similarly, SF-36 mental component scores ranged from a mean of 45.3 to 47.4 points across the 3 groups and SF-36 physical component scores ranged from a mean of 49.5 to 51.1.
Patient Functioning
Modafinil 300 mg significantly improved patient functioning relative to placebo at the final visit, as shown by the increase from baseline in mean FOSQ total score (2.3 vs. 1.6 points, p < .05). Patients in the modafinil 200-mg group also demonstrated a numerically greater improvement in mean FOSQ total score relative to placebo (2.0 vs. 1.6); however, this difference did not achieve statistical significance (p > .05).
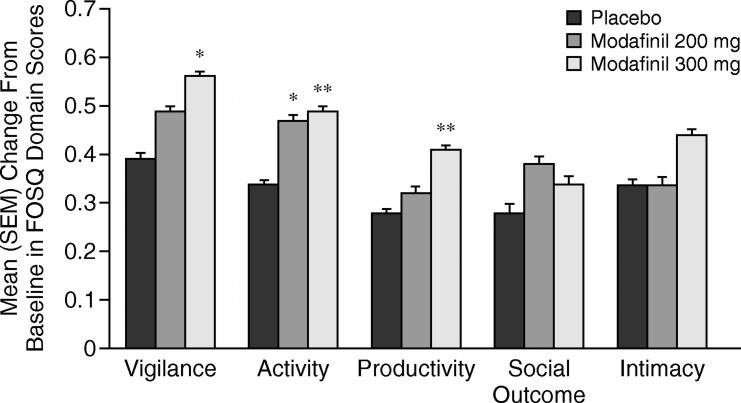
Modafinil 300 mg significantly improved FOSQ domain scores compared with placebo for vigilance (change in baseline of 0.6 for modafinil vs. 0.4 for placebo, p < .05), activity (0.5 vs. 0.3, p < .01), and productivity (0.4 vs. 0.3, p < .01) at final visit (Figure 2). At the final visit, modafinil 200 mg demonstrated significant improvement from baseline compared with placebo in the FOSQ domain score for activity (0.5 vs. 0.3, p < .05). Improvements in social outcome and intimacy domain scores were also observed; however, differences versus placebo did not achieve statistical significance (p > .05).
Figure 2.
Mean (SEM) Change From Baseline to Final Visit in Functional Outcomes of Sleep Questionnaire (FOSQ) Domain Scores by Treatment Group
Health-Related Quality of Life
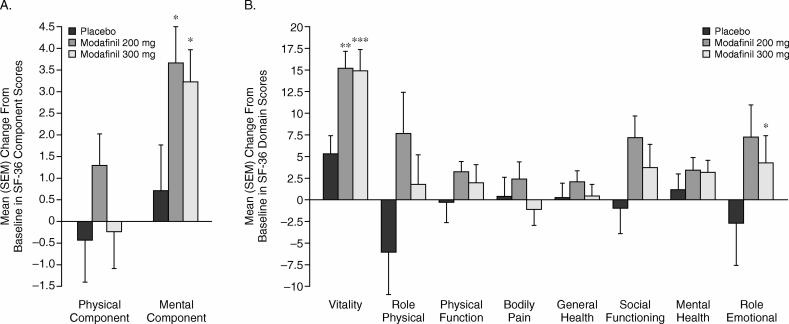
Relative to placebo, both doses of modafinil significantly improved the SF-36 mental component score from baseline to the final visit (mean changes from baseline of 3.2, 3.7, and 0.7 points in the modafinil 300-mg, modafinil 200-mg, and placebo groups, respectively; p < .05 for each comparison vs. placebo; Figure 3A). Modafinil 300 mg also significantly increased the domain score for vitality (14.8 vs. 5.3; p < .0001) and role emotional (4.3 vs. −2.9; p < .05); modafinil 200 mg significantly increased the domain score for vitality (15.0 vs. 5.3; p < .001; Figure 3B).
Figure 3.
Mean (SEM) Change From Baseline to Final Visit in (A) 36-Item Short Form Health Survey (SF-36) Physical and Mental Component Summary Scores and (B) SF-36 Domain Scores by Treatment Group
Nighttime Sleep, Daytime Sleep, and Caffeine Use
Sleep parameters and caffeine use were similar across treatment groups throughout the study period. There were no clinically meaningful changes within the treatment groups for any of the sleep parameters measured. Furthermore, patients reported no clinically meaningful changes from the pretreatment period in their caffeine use, either during or after night shifts.
Tolerability
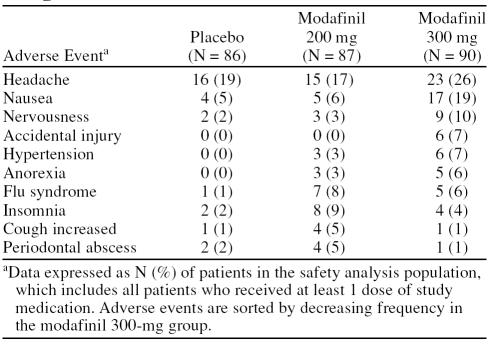
The most common adverse events in modafinil-treated patients were headache (21.5%), nausea (12.4%), and nervousness (6.8%) (Table 2). Adverse events were mild or moderate in nature in 98% of cases. The incidence of some adverse events, such as headache, nausea, nervousness, diarrhea, hypertension, or dizziness, appeared to demonstrate a dose-related trend; with higher rates of occurrence in the modafinil 300-mg group compared with the placebo or modafinil 200-mg groups. One patient treated with modafinil 300 mg had a serious adverse event involving abnormal liver function test values considered possibly related to treatment. The patient was withdrawn from the study, and liver enzymes returned to normal upon discontinuation of modafinil. A total of 28 patients withdrew because of adverse events, with a higher number in the modafinil 300-mg group compared with the placebo and modafinil 200-mg groups (placebo, N = 4; modafinil 200 mg, N = 5; modafinil 300 mg, N = 19). The most common adverse events leading to withdrawal in the modafinil group were headache (N = 4 for modafinil 300 mg, N = 1 for modafinil 200 mg, N = 1 for placebo), insomnia (N = 2 for modafinil 300 mg, N = 1 for modafinil 200 mg, N = 1 for placebo), anxiety (N = 2 for modafinil 300 mg, N = 1 for modafinil 200 mg, N = 0 for placebo), tachycardia (N = 3 for modafinil 300 mg, N = 0 for modafinil 200 mg, N = 0 for placebo), and palpitations (N = 2 for modafinil 300 mg, N = 1 for modafinil 200 mg, N = 0 for placebo).
Table 2.
Most Common Adverse Events Occurring in at Least 5% of Patients in Any Modafinil Treatment Group and Twice as High as Placebo
No clinically meaningful differences in mean clinical laboratory test values, vital sign measurements, physical examination findings, or electrocardiography results were demonstrated between the groups.
DISCUSSION
Patients with SWSD have been shown to be at greater risk for medical, psychological, and social consequences. Impairments in health-related quality of life and functional status not limited to sleepiness intrude on activities outside typical night-shift work hours. Almost half of the patients in this study were from the health care profession, most of whom were nurses, with pharmacists, health care technologists/technicians, and paramedics also included in this group. Another 15% of patients were from the manufacturing and transportation industries. Heightened risk for serious medical conditions seen in patients with SWSD represents an important issue for public health and safety.
The benefits seen with modafinil suggest that improvement of wakefulness during night-shift work in patients with SWSD has wider implications, improving overall well-being, even with intermittent intervention. Specifically, administration of modafinil (30 to 60 minutes before regularly scheduled night shifts) improved aspects of functional status and quality of life as assessed by specific (FOSQ) and general health (SF-36) outcome measures. At final visit, significant improvement in FOSQ total scores was observed with modafinil 300 mg relative to placebo. Both doses of modafinil showed improvement in domain scores for activity level, and the 300-mg dose significantly increased domain scores for vigilance and general productivity relative to placebo. Interestingly, the domains most affected by modafinil treatment are those most affected by excessive sleepiness.18 Examples from the FOSQ include being able to enjoy a theater performance, movie, or concert or to engage in meetings or group discussions, hobbies, or tasks around the house; as well as items such as being active in the morning and evening hours, doing things for family and friends, keeping pace with others your own age, concentrating, remembering, and taking care of financial affairs and paperwork. Modafinil-associated improvements in FOSQ scores in patients with excessive sleepiness are likely the result of improved wakefulness and alertness, which would be expected to impact behaviors requiring sustained attention such as those assessed via the FOSQ vigilance subscale.
Similarly, significant improvements with both doses of modafinil were observed in mean SF-36 mental component scores (> 3 vs. 1 point) as well as the SF-36 domain scores for vitality (15 vs. 5 points) relative to placebo. The 300-mg dose was also associated with a significant improvement in the domain score for role emotional (4 vs. −3 points) relative to placebo. Such improvements suggest that patients with SWSD who use modafinil before night shifts may have more energy and fewer problems with work or other daily activities as a consequence of their modafinil treatment. The improved functional status and quality of life seen with modafinil in this study is consistent with previous research in patients with excessive sleepiness associated with obstructive sleep apnea/hypopnea syndrome20,21 or narcolepsy.22,23
Modafinil has demonstrated the ability to improve alertness, vigilance, and executive function during simulated night shifts24 and improve alertness, overall clinical condition, and ability to sustain attention in patients with chronic SWSD.15 These effects have been attributed to improved wakefulness, including the reduction of the instability of wakefulness caused by microsleeps, and occur in the absence of effects on circadian adaptation. The impact of such effects on work performance and accidents and errors during the night shift requires further research; however, modafinil has been reported to reduce the level of sleepiness and incidence of accidents and near misses during the commute home in patients with SWSD.15
In this study, modafinil did not interfere with patients' capacity to sleep as desired during the daytime (nonwork hours), as recorded in the daily patient diaries. Previously published data in patients with SWSD demonstrated no significant differences between modafinil and placebo in the mean change from baseline to final visit for sleep latency, sleep duration, sleep efficiency, and sleep architecture as measured by daytime polysomnography.15 Additionally, both doses were well tolerated, with modafinil 200 mg better tolerated than the higher dose. The difference in tolerability profile seen in the current study may be due to the lack of titration in the SWSD population. This observation is consistent with findings from the randomized, multicenter studies of modafinil in patients with narcolepsy, which showed an improved tolerability profile when the dose was titrated25 compared with when no titration was used.26 Modafinil 300 mg showed more consistent effects on patient functioning and health-related quality of life, suggesting that dose titration may be required in some patients to achieve clinical effectiveness. The adverse event profile seen in this study is consistent with findings of modafinil studies in other sleep disorders.21,23,27
The findings of the current study must be interpreted in light of the following considerations. Neither the FOSQ nor the SF-36 has been specifically validated for SWSD. However, both have been previously shown to be sensitive to evaluations of excessive sleepiness in other sleep disorders.18,19 Quantitative improvements have been correlated with improved physician and patient assessments. Furthermore, the assessments evaluated symptom status during the work week, not just while the patient was at work (when impairment is most severe). Consequently, these tools may underestimate the true impact of modafinil treatment. To our knowledge, the FOSQ has not been validated as a measure of change and therefore may not have been the most sensitive tool to detect treatment-induced changes, which could potentially explain the small degree of change observed in the present study. However, from our perspective, it was the best tool available at the time the study was conducted. Data from daily dairies may have reduced internal validity because of reliance on patient recall. However, such a limitation would be expected to be equal in both the modafinil and placebo groups, thus minimizing the potential for bias. Nonetheless, future studies in this area may choose to use actigraphy in addition to daily dairies to further minimize potential for recall bias. Another limitation is that 12 weeks might be too short a time frame to fully realize potential improvement in functional status and quality of life from a treatment. Further studies over longer periods are needed to fully define the impact of modafinil on functional status and quality of life in patients with excessive sleepiness associated with SWSD.
Modafinil treatment administered before the start of a night shift significantly improved aspects of functional status and health-related quality of life, without adversely affecting intended sleep, in patients with excessive sleepiness associated with chronic SWSD. Modafinil was well tolerated and was not associated with clinically meaningful changes in safety parameters compared with placebo. Our results support the use of modafinil as treatment for excessive sleepiness associated with SWSD.
Drug names: lithium (Eskalith, Lithobid, and others), modafinil (Provigil), zolpidem (Ambien).
Footnotes
Sponsored by Cephalon, Inc., Frazer, Pa.
Portions of the data have been presented at the 17th annual meeting of the Associated Professional Sleep Societies, June 3–8, 2003, Chicago, Ill.
Data were analyzed by the Biometrics Department at Cephalon. Development of the manuscript was supported by Cephalon, with significant input and revisions to the satisfaction of all authors.
Dr. Erman is a consultant for Cephalon, Mallinckrodt, Neurocrine Biosciences, sanofi-aventis, and Takeda; has received grant/research support from Aventis, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Organon, Pfizer, Pharmacia, ResMed, Sanofi, Schwarz, and Takeda; is a member of the advisory board for Cephalon, Neurocrine Biosciences, sanofi-aventis, and Takeda; is a member of the speakers' bureau for Mallinckrodt, sanofi-aventis, and Takeda; and is a stock shareholder for Cephalon, Forest Laboratories, Merck, Neurocrine Biosciences, Pfizer, sanofi-aventis, Sepracor, and Somaxon. Dr. Rosenberg has received honoraria and grant/research support from Cephalon.
REFERENCES
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 2nd edition: Diagnostic and Coding Manual. Westchester, Ill: American Academy of Sleep Medicine; 2005 [Google Scholar]
- Rajaratnam SM, Arendt J.. Health in a 24-h society. Lancet. 2001;358:999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- Reimer MA, Flemons WW.. Quality of life in sleep disorders. Sleep Med Rev. 2003;7:335–349. doi: 10.1053/smrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- Sforza E, Janssens JP, and Rochat T. et al. Determinants of altered quality of life in patients with sleep-related breathing disorders. Eur Respir J. 2003 21:682–687. [DOI] [PubMed] [Google Scholar]
- Beers T.. Flexible schedules and shift work: replacing the ‘9-to-5’ workday? Monthly Labor Rev. 2000;123:33–40. [Google Scholar]
- Jaffe MP, Smolensky MH, Wun CC.. Sleep quality and physical and social well-being in North American petrochemical shift workers. South Med J. 1996;89:305–312. doi: 10.1097/00007611-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Cavallo A, Jaskiewicz J, Ris MD.. Impact of night-float rotation on sleep, mood, and alertness: the resident's perception. Chronobiol Int. 2002;19:893–902. doi: 10.1081/cbi-120014106. [DOI] [PubMed] [Google Scholar]
- Munakata M, Ichi S, and Nunokawa T. et al. Influence of night shift work on psychologic state and cardiovascular and neuroendocrine responses in healthy nurses. Hypertens Res. 2001 24:25–31. [DOI] [PubMed] [Google Scholar]
- Bohle P, Tilley AJ.. Predicting mood change on night shift. Ergonomics. 1993;36:125–133. doi: 10.1080/00140139308967863. [DOI] [PubMed] [Google Scholar]
- Luna TD, French J, Mitcha JL.. A study of USAF air traffic controller shiftwork: sleep, fatigue, activity, and mood analyses. Aviat Space Environ Med. 1997;68:18–23. [PubMed] [Google Scholar]
- Kaneko SY, Maeda T, and Sasaki A. et al. Effect of shift work on mental state of factory workers. Fukushima J Med Sci. 2004 50:1–9. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Miura K, and Ishizaki M. et al. Sickness absence and shift work among Japanese factory workers. J Hum Ergol (Tokyo). 2001 30:393–398. [PubMed] [Google Scholar]
- Drake CL, Roehrs T, and Richardson G. et al. Shift work sleep disorder:prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004 27:1453–1462. [DOI] [PubMed] [Google Scholar]
- Puca FM, Perrucci S, and Prudenzano MP. et al. Quality of life in shift work syndrome. Funct Neurol. 1996 11:261–268. [PubMed] [Google Scholar]
- Czeisler C, Walsh J, and Roth T. et al. Modafinil for excessive sleepiness associated with shift work sleep disorder. New Engl J Med. 2005 353:476–486. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Guidance for Industry. E6 Good Clinical Practice: Consolidated Guidance. Rockville, Md: Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research; 1996 [Google Scholar]
- Guy W. Clinical Global Impressions. In: ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education and Welfare publication (ADM) 76-338. Rockville, Md: National Institute of Mental Health; 1976 218–222. [Google Scholar]
- Weaver TE, Laizner AM, and Evans LK. et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997 20:835–843. [PubMed] [Google Scholar]
- Ware JE Jr. SF-36 health survey update. [SF-36.org Web site] Available at: www.sf-36.org/tools/sf36.shtml. Accessed July 20, 2005. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Weaver TE.. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4:393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]
- Schwartz JR, Hirshkowitz M, and Erman MK. et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea: a 12-week, open-label study. Chest. 2003 124:2192–2199. [DOI] [PubMed] [Google Scholar]
- Becker PM, Schwartz JR, and Feldman NT. et al. Effect of modafinil on fatigue, mood, and health-related quality of life in patients with narcolepsy. Psychopharmacology (Berl). 2004 171:133–139. [DOI] [PubMed] [Google Scholar]
- Mitler MM, Harsh J, and Hirshkowitz M. et al. Long-term efficacy and safety of modafinil (PROVIGIL) for the treatment of excessive daytime sleepiness associated with narcolepsy. Sleep Med. 2000 1:231–243. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Randazzo AC, and Stone KL. et al. Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep. 2004 27:434–439. [DOI] [PubMed] [Google Scholar]
- US Modafinil in Narcolepsy Multicenter Study Group. Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy. Neurology. 2000;54:1166–1175. doi: 10.1212/wnl.54.5.1166. [DOI] [PubMed] [Google Scholar]
- US Modafinil in Narcolepsy Multicenter Study Group. Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. Ann Neurol. 1998;43:88–97. doi: 10.1002/ana.410430115. [DOI] [PubMed] [Google Scholar]
- Pack AI, Black JE, and Schwartz JR. et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med. 2001 164:1675–1681. [DOI] [PubMed] [Google Scholar]