Abstract
Objective
Patterns of successive saccades and fixations (scan paths) that are made while viewing images are often spatially restricted in schizophrenia, but the relation with cannabis-induced psychosis has not been examined. We used higher-order statistical methods to examine spatiotemporal characteristics of scan paths to determine whether viewing behaviour was distinguishable on a continuum.
Methods
Patients with early acute first-episode paranoid schizophrenia (SCH; n = 11), cannabis-induced psychosis (CIP; n = 6) and unaffected control subjects (n = 22) undertook a task requiring free viewing of facial, fractal and landscape images for 5 seconds while their eye movements were recorded. Frequencies and distributions of saccades and fixations were calculated in relation to image regions examined during each trial.
Results
Findings were independent of image category, indicating generalized scanning deficits. Compared with control subjects, patients with SCH and CIP made fewer saccades and fewer fixations of longer duration. In turn, the spatial distribution of fixations in CIP patients was more clustered than in SCH and control subjects. The diversity of features fixated in subjects with CIP was also lower than in SCH patients and control subjects.
Conclusion
A continuous approach to characterizing scan path changes in different phenotypes suggests that CIP shares some of the abnormalities of SCH but can be distinguished with measures that are sensitive to cognitive strategies active or inhibited during visual exploration.
Medical subject headings: schizophrenia, cannabis, principal component analysis
Abstract
Objectif
Les tracés de saccades et fixations successives (axes de balayage) effectués lorsqu'on visualise des images sont souvent limités dans l'espace dans la schizophrénie, mais on n'a pas étudié le lien avec la psychose provoquée par le cannabis. Nous avons utilisé des méthodes statistiques d'ordre élevé pour examiner les caractéristiques spatiotemporelles des axes de balayage afin de déterminer s'il est possible de distinguer un comportement de visualisation dans un continuum.
Méthodes
Des patients atteints d'une schizophrénie paranoïaque constituant un premier épisode aigu précoce (SCH; n = 11), des sujets atteints d'une psychose provoquée par le cannabis (PPC; n = 6) et des sujets témoins non affectés (n = 22) ont entrepris une tâche les obligeant à regarder librement des images faciales, fractales et panoramiques pendant cinq secondes pendant que l'on enregistrait le mouvement de leurs yeux. On a calculé les fréquences et les distributions des saccades et des fixations par rapport aux régions de l'image regardées au cours de chaque essai.
Résultats
Les résultats n'avaient aucun lien avec les catégories d'images, ce qui indique des déficits généralisés du balayage. Comparativement aux sujets témoins, les patients atteints de SCH et de PPC ont fait moins de saccades et moins de fixations de plus longue durée. En retour, la distribution spatiale des fixations chez les patients atteints de PPC était plus regroupée que chez les sujets atteints de SCH et les sujets témoins. La diversité des caractéristiques fixées chez les sujets atteints de PPC était aussi moins grande que chez les patients atteints de SCH et chez les sujets témoins.
Conclusion
Une approche continue de la caractérisation des changements des axes de balayage chez différents phénotypes indique que la PPC présente quelques-unes des anomalies de la SCH, mais qu'il est possible de les distinguer au moyen de méthodes de mesure sensibles aux stratégies cognitives actives ou inhibées au cours de l'exploration visuelle.
Introduction
Schizophrenia (SCH) is a common form of major mental illness and normally presents early in adult life. Early signs of SCH include different subtle neurological signs (e.g., eye movement dysfunctions), and there is international consensus that genetic and environmental factors interact to determine overall risk.1 Some of the changes present in SCH, such as enlarged ventricles, probably occur prenatally and are neurodevelopmental in origin.2 However, postnatal stressors also operate to increase overall risk of illness. One of the most important stressors is cannabis abuse; cannabis is described as an important component causal factor3 in this early phase of illness. Its relation to psychotic symptoms and the occurrence of undiagnosed psychotic symptoms in the general population provoked several researchers to rethink categorical classifications and to propose a dimensional approach to diagnosis.4 To test such a continuum hypothesis, it is important to investigate whether patients in the early phase of a schizophrenic disorder and of a cannabis-induced psychosis (CIP) can be differentiated with potential trait markers that are commonly associated with SCH (e.g., eye movement dysfunctions). Recent research has focused on risk factors to improve early detection and intervention5 and to better understand the underlying pathophysiology.
Eye movement dysfunctions have been consistently reported in SCH and other neuropsychiatric disorders.6 There is now increasing evidence for direct links between eye movement deficits and genetic markers from specific chromosomal regions7 and morphological changes8 or both in SCH. These abnormalities may be accompanied by deficits in sensory gating and cognitive processing, as reflected in abnormal event-related potentials.9,10 In addition, these findings are closely intertwined with abnormal performance on tests of executive and attentional function. The relation between the clinical symptoms and severity in SCH and these endophenotypic or trait markers is obviously complex.
Although oculomotor abnormalities have emerged as a candidate marker of the SCH spectrum,11 error-prone smooth pursuit and saccadic control have also been observed in autism,12 Parkinson's disease,13 dyslexia,14 affective disorders and obsessive–compulsive disorders.15 Such findings make it unlikely that the measurement of a single attribute of oculomotor function will have diagnostic specificity. Thus, task and measurement type have a role to play in the characterization and delineation of mental diseases.16
Reports of abnormal eye movement patterns during visual exploration in SCH17,18 suggest there may actually be a more global deficit involved in processing perceptual information. Superficially, it may appear that there are similarities with scanning in other psychiatric illness and organic dementia. However, inspection behaviour in SCH appears to have some unique properties. Using facial stimuli, the spatial extent of successive saccades and fixations (scan paths) in observers with SCH appear significantly restricted and atypical (as if inhibited or linearly distributed), compared with more extensive scanning in healthy observers.19 Inferences have therefore been made concerning illness-specific avoidance of threatening or social cues20 or deficits in attention, working memory and executive function.21 Consequently, scan path assessment has become an attractive constituent of the psychophysiological test repertoire. Experimentally, the protocol is cheap and easy to administer, and interesting results have promoted abnormalities in visual inspection as a putative trait marker of vulnerability to SCH.18,22 The greatest body of data in support of this has come from studies with pictures of facial expressions.17,22–25 However, restricted scan paths are also manifest in responses to natural scenes and schematic patterns lacking social potency.21,26,27 For this reason, the notion of a generalized, global abnormality has emerged. This is important because restricted scanning is typically associated with negative symptoms of SCH28 and may be secondary to the presenting symptoms of anhedonia and flattened affect.21 Conversely, patients with positive symptoms sometimes engage in less inhibited and more extensive visual scanning,28 although eye movements are minimized when they are presented with facial stimuli.17,22,24
To date, most reports on abnormal visual scan paths in SCH concentrated on the analysis of basic parameters such as the number of saccades, fixation duration or scan path length, which all critically depend on the exact stimulus shown27,29 and specific instructions (e.g., to remember certain details of pictures) given to the subjects.30 Whereas such analyses have repeatedly shown abnormalities between groups of patients and control subjects, the group identity of individual subjects cannot be reliably estimated.27 This is not surprising if we accept the continuum hypothesis in psychosis and uncertainties about the validity of currently used diagnostic boundaries in the functional psychoses.31–33 Currently, we do not know whether these techniques are appropriate for stratifying patients into different categories. Scan paths are a complex measure containing both spatial and temporal attributes. A more sensitive method has to be devised to take information generated by scan path testing into account and to address their specificity with the clinical phenotypes under investigation. Only at this point will the power of these potentially highly informative measures emerge.
Here we describe a series of analytical refinements that may be more powerful tools in stratifying patients with functional psychoses. We then apply these to 2 groups of patients for whom there is considerable controversy as to whether they should be looked at as separate or related illnesses. On the surface, CIP seems a separate entity from SCH de novo, but several epidemiological studies suggest that most CIPs occur in people genetically predisposed to SCH.34 Similarities in measures of scan path evolution in CIP and SCH would suggest an overlap in their psychomotor behaviour; however, systematic phenotypic variation would advocate a dimensional interpretation of psychosis. In addition to facial images, natural scenes and fractal patterns were used to verify whether any abnormal scanning of faces extended to nonsocial materials in those patients.
Methods
Participants
Before study entry, we obtained written informed consent from all patients and control subjects in accordance with the Ethical Committee of the University Hospitals of Geneva (HUG) and the Declaration of Helsinki. Patients were recruited from a specialized early psychosis unit of HUG and were rated with the Structured Clinical Interview35 (SCID-patient version) to obtain diagnoses according to the Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV).36 Psychopathology was assessed with the Scale for Assessment of Positive Symptoms (SAPS) and the Scale for Assessment of Negative Symptoms (SANS)37,38 in parallel with demographic data and clinical history. We selected 11 patients with early acute first-episode schizophrenic disorder (DSM-IV: 295.3 paranoid type, 9 men). Six men with CIP (DSM-IV: 292.11) and 22 control subjects (12 men) were also recruited. First-episode SCH patients were treated with second-generation antipsychotic drugs (risperidone, olanzapine, amisulpride and clozapine). Dosages were at near-maximal effective dose level39 (i.e., between 2–3 mg/d for risperidone, 10–20 mg/d for olanzapine and 400 mg/d amisulpride). Three SCH patients received clozapine at dosages between 100–200 mg/day. Three SCH patients had a combination of clozapine (25–150 mg/d) with risperidone (0.5–4 mg/d) or amisulpride (300 mg/d). Three CIP patients received olanzapine (10–20 mg/d), 1 received amisulpride (400 mg/d) and 2 received a combination of clozapine (25–150 mg/d) with either risperidone (2 mg/d) or amisulpride (800 mg/d). At least 3 of the SCH patients took cannabis before hospitalization. All patients had received the same medication for 2 weeks before testing, and urinary toxicology screening was negative for cannabis and other illegal drugs at the time of testing. Patients were in the recovery phase of psychosis (4 wk after commencement of acute phase) and were matched for age (total sample 22.5 [standard deviation {SD} 3.4] yr) and education. Healthy volunteers were screened with the DSM-III-R:40 SCID-nonpatient41 and were matched for education but not for sex (22.8, SD 3.9 yr) to increase the variability of eye movement measures in the unaffected group. This heterogeneous group enhanced interindividual variation and was intended to decrease the likelihood of significant false negative differences between patients and control subjects.27 Exclusion criteria for all groups were organic brain disease and subnormal intelligence. We did not include healthy volunteers with a history of psychiatric or neurological illness or with psychiatric illness in first-degree relatives. Control subjects were also free of any current medication and had never been prescribed psychotropic medication.
Stimuli
We used 20 exemplars each from 3 stimulus categories. Facial images (9 horizontal × 13 vertical° visual angle) of male and female posers showing neutral expressions were presented in grey scale with an elliptical mask to exclude external features. Landscape and fractal patterns (25° × 16°) were shown in colour. Images were presented centrally, surrounded by a black border background to encourage eye movements to remain within the stimulus-relevant portion of the display.
Procedure
Stimuli were presented in pseudorandom order in counterbalanced blocks of faces, landscapes and fractal patterns. Participants were instructed to inspect the images in any manner they chose. Calibrated (3 × 3 grid) 2-dimensional eye movements were recorded at 250 Hz, using a head-mounted infrared EyeLink I (SR Research Ltd., Osgoode Ont, 2001), at a resolution of < 0.3°). Trial viewing time was 5 seconds, interspersed with central refixations lasting ≥ 2 seconds to compensate for intrinsic direct current drift. Saccades were computed as changes in eye position exceeding 30° per second velocity and 8000° per second2 acceleration. Fixations were defined by a minimum threshold of 200 ms to ensure compatibility with previous scan path research. Eye blinks were automatically detected and excluded in EyeLink I software. We acquired the data binocularly and conducted analyses with the dominant eye, as determined by the hole-in-the-card test.
Analytical refinements
Fixation clustering
A single measure of the spatial distribution or clustering of fixations can be derived from eye gaze records. Voronoi tessellations are computed for each trial, using fixation position to spatially segment fixations with respect to the image. Cell areas were divided by the mean cell size for that trial, and these normalized values were then accumulated from all trials to form a frequency distribution of normalized cell sizes. The gamma probability function was chosen to fit the derived cumulative distribution, so that skew was quantified by a single free parameter alpha. A small value of alpha corresponds to a highly skewed distribution denoting fixations that are clustered into localized regions of interest (i.e., regions of high visual salience, indicating these saccades are primarily driven by external factors). In terms of visual attention, one might compare this with a visual feature search. Higher alpha denotes less skewed, more dispersed scanning,42 probably driven by more internal factors. This procedure can be separately applied to each observer group and stimulus category.
Fixated image features
Restricted scanning results in the acquisition of a less diverse assortment of image features than those accumulated with widely distributed sampling. Therefore, some of the sources of variation in scanning style can be represented in terms of features acquired (or avoided) during free viewing. Principal component factor analysis (PCA) can be used to describe these sources, first within and then between groups. We used a formal procedure to quantify differences between participant groups.43 Fovea-sized grey-scale subimages (5º) were extracted from stimuli at every fixation location for all trials, and data vectors were formed by concatenating columns of pixel intensities. Because participants will make a variable number of fixations on any given trial, PCA was first used to extract eigenvectors capturing the variability of the input features for each stimulus category and each participant separately. To identify variation in each participant group, PCA was reapplied to their respective participants' eigenvectors. Group-level eigenvectors capture the general pattern of viewing style in a group. We then calculated the magnitude of between-group differences. Within each group, successive principal components were geometrically orthogonal (90°). However, direct pairwise comparison of raw angles between groups' eigenvectors was not meaningful,43 so direct comparison of patients with control subjects is not appropriate. Instead, we calculated the closest subspace common to observers' eigenspaces and quantified differences with respect to those “average” orthogonal vectors. Mathematical proof is given elsewhere.43
Results
Patterns in group level variability: classical scan path analysis
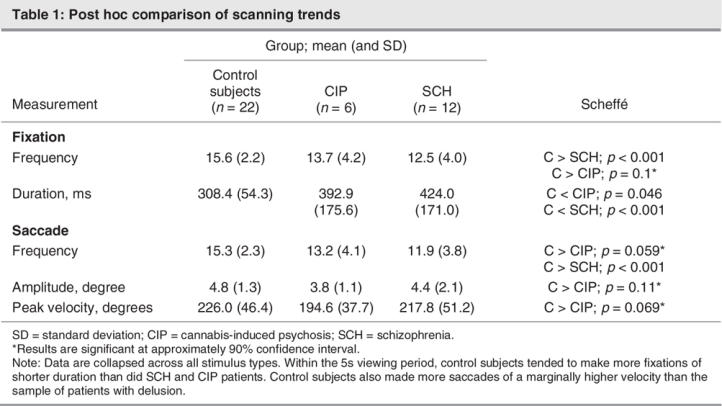
Vision is an active process in which eye movements shift the view several times each second to selected parts of the scene to examine salient features (e.g., eyes or mouth of a face). Saccades bring selected objects or features onto the fovea where, during these fixations, information can be analyzed in detail. Table 1 summarizes findings from frequency analyses using multivariate analyses of variance (ANOVAs) that merely confirmed dichotomous group differences of the type reported in the literature.17,18 Patients' fixations lasted longer and were fewer in number in the viewing period, and control subjects tended to make faster saccades than did patients.
Table 1
Comparison of fixation distributions
A spatial model of fixations was derived with fixation distributions for each group of participants (SCH, CIP, control subjects). Fixation locations were studied for each stimulus category and for each group, using the gamma model. Irrespective of stimulus category, patients with psychosis taking medication exhibited greater fixation clustering and more restricted scanning than did patients with paranoia and control subjects (Table 2). Relative to control subjects, CIP fixation clustering was more pronounced for landscapes and fractals than for faces. Additionally, fixations in response to faces were highly clustered for all participants and were partly determined by the smaller, centralized stimulus, which has consequences for interpreting published data.
Table 2
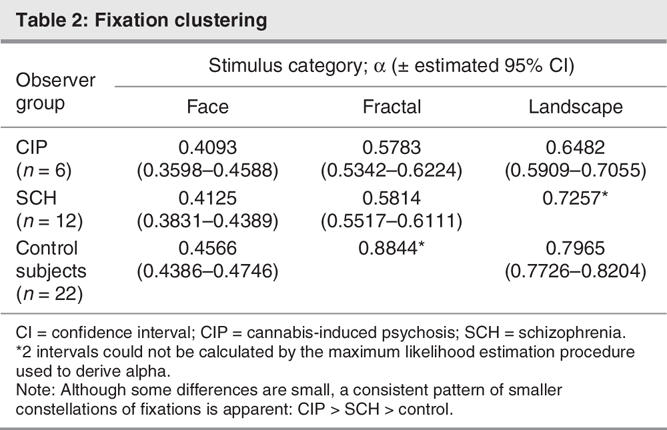
Image features acquired during fixations
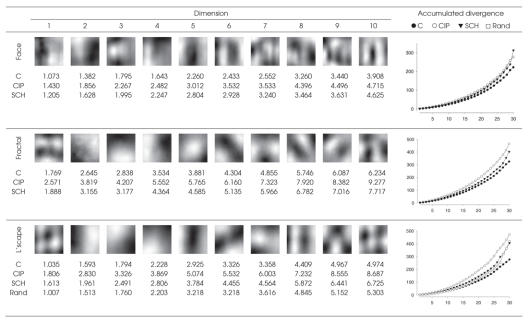
Figure 1 shows the common subspaces (reconstructed as grey-scale images) used to compare our participant samples in each stimulus category. Subjective interpretation of correspondences with actual image features should be avoided at this level of higher-order statistical abstraction. Monte-Carlo simulation of random viewing behaviour of landscape images demonstrated that our observers' scan paths were assembled according to their respective cognitive or behavioural strategies and that statistical divergence from the subspace was not due to chance. Results were not significantly altered when fixation samples were weighted by fixation duration. In line with the gamma model, analysis of visual features acquired during visual exploration revealed that illness type was distributed continuously. Independent of image category, CIP patients tended to “lock on to” particular image features, more so than SCH patients, and did not deviate thereafter. Again, this effect seemed more pronounced for landscapes and fractals than for faces. Unaffected individuals actively acquired a more diverse sample of image features than would be fixated during random viewing.
Fig. 1: Fixated feature subspaces. Dimensions of the subspace closest to all 3 participant groups' principal component spaces are shown for each stimulus category (minimum 86% of total variance). C = control subject, CIP = cannabis-induced psychosis, SCH = first-episode schizophrenic disorder of paranoid type, Rand = random fixation simulation for landscape (L'scape) scenes. For simplicity, only the first 10 of 30 dimensions are shown, reconstructed as eigenimages. The minimum angular deviation (in degrees) between each direction and group is given for each stimulus category and increases with higher dimensions, indicating greater divergence associated with diversity of features fixated. Graph inserts titrate subspace dimension (abscissas) against accumulated angular divergence (ordinates). A clear pattern emerged irrespective of the stimulus category: CIP > SCH > control. Landscapes were chosen as a control because they are commonly experienced, contain complex natural features and lack overt social relevance. Increasing separation (greater angular deviation) of random viewing from the landscape subspace with respect to control subjects indicates staring and unsystematic sampling of image content.
Discussion
To test ideas of a dimensional approach for diagnosis in psychosis,4 we investigated whether patients in an early phase of a schizophrenic disorder and of CIP would be different in their eye movement psychophysiology when free-viewing images. Earlier work has suggested that certain attributes of eye movements made during visual exploration are “abnormal” in patients in the SCH spectrum (e.g., few fixations of longer duration during a fixed viewing period). However, scan paths are also atypical in several other psychiatric illnesses44 and may vary with cannabis abuse or heavy smoking, as seen in visual search,45 pursuit performance46 and saccadic control.47 We used higher-order statistics to capture the sources of variation in viewing behaviour between our observers on a continuum, examining both the distribution of fixations and image features selected or avoided during fixations. These analyses revealed idiosyncratic patterns of viewing strategies among people with CIP or SCH and unaffected individuals. CIP patients engaged in visual scanning in a more limited range than did SCH patients and healthy observers; this was independent of the type of stimulus material used. Because positive symptoms in CIP are generally short-lived,48 compared with SCH, they are unlikely to have been an important factor in our findings.
Cannabis use has been found to be related to a schizotypal personality dimension of proneness to psychosis in healthy individuals.49 Heavy users who go on to develop psychotic symptoms distinct from the intoxication state (including visual hallucinations, expansive mood and ideation, clouded sensoria50 and depersonalization overlapping with SCH51) may be vulnerable to long-term psychotic illness.34 This suggests that CIP symptoms are an important risk indicator. Although the concept of “cannabis psychosis” has been disputed,52 our patient groups clearly inspected images in different ways. Peripubertal-onset long-term cannabis users show less effective, more conservative visual search strategies and frequent reinspection of stimulus features,45 suggesting memory impairments and impoverished internally driven visual information processing. Longer response times and increased saccadic latencies for visually guided eye movements are also produced by the administration of cannabis in healthy individuals.53
The endogenous cannabinoid-1 (CB1) receptor, which is reactive to cannabis use, depresses electrical neural activity, particularly in the receptor-dense hippocampal and substantia nigra pars reticulata regions of the human brain.54 The cognitive consequences of altered signalling in these areas include impaired spatiotemporal processing and memory consolidation and intrusions,55,56 degraded integration of attention and low-level spatial information for movement and saccadic control.57 The increased saccade latency and fixation duration we observed in CIP and SCH patients may be owing to retarded attentional shifts and saccadic programming (“getting stuck”) and information processing dysfunction, all of which are normally regulatory functions of the frontal lobes. In SCH, there may be an increased density of CB1 in the dorsolateral prefrontal cortex independent of recent cannabis ingestion.58 Elevated dopamine release triggered by cannabinoid metabolism in the frontal and mid-brain produces psychotropic effects. Whether psychotic states resembling SCH can persist after cessation of prolonged cannabis abuse requires further endorsement.48,59–62
High levels of the endocannabinoid anandamide in paranoid-type SCH may represent the cannabinoid system's response to dopamine imbalance. Elevated anandaminergic activity in SCH may adaptively enhance inhibition of dopaminergic motor activity,63 thus a desensitized canna-binoid system caused by heavy cannabis use might pre-cipitate psychosis through modulation of dopaminergic transmission. Clearly, our CIP group exhibited viewing behaviour in the direction of, and perhaps beyond, SCH scan paths. Given the small numbers of patients reported here, we would not expect large differences between CIP and SCH groups, since CIP is found in people genetically predisposed to SCH.61,64
Antipsychotic medication was similar for people with SCH and with CIP. Blink rates tended to be slightly elevated in psychosis, but patients treated with risperidone showed no extrapyramidal symptoms (e.g., dystonia or Parkinsonism). Although clozapine and olanzapine have been associated with improvements in social functioning and affective symptoms, this was not evidenced by more normal patterns of eye movements when inspecting faces or other images. At low and moderate doses (50–400 mg/d), amisulpride is effective against negative symptoms of SCH, and in a control study, 300 mg daily consumed by healthy volunteers did not affect eye movements.65
Saccade amplitude was within the normal range of 2°–15° in each group. For classical fixation analysis, patients made significantly fewer fixations than control subjects; fixation duration was also longer in patients. Measures of saccade amplitude, peak velocity and fixation distributions for people with CIP were both lower and more different from control subjects than for subjects with SCH; it appears that saccade execution is somehow affected in CIP. The tendency of people with CIP and with SCH to minimize the frequency and amplitude of saccades may reflect attempts to suppress smearing of the percept between successive fixations,66 a pronounced and inflexible cognitive style, or attempts to minimize sensorial stimulation. Our findings also indicate that there may be a conceptual relation between the distribution of fixations (gamma model) and the perceptual content of preferred image features that attract those fixations (hierarchical PCA model). In both the CIP and SCH groups, restricted scanning was not limited to facial images but extended to and was more evident for natural (landscapes) and synthetic scenes (fractals); this supports the idea of subtly different but generalized cognitive impairments in gaze and information processing.67 Therefore, it makes sense to ascertain the extent to which networks implicated in abnormal oculomotor and higher cognitive functions overlap with the neural substrates of social cognition.68
A significant problem confronting understanding of the scan path phenotype of SCH concerns its measurement and analysis. Violations of the homogeneity of variance assumption in between–group ANOVAs could arise from several sources, including overreliance on small facial stimuli in the absence of other socially relevant and everyday scenes and presence of natural boundaries and associated variability in the SCH spectrum of illnesses, as defined by scan path variables (compare sensitivity and specificity). Further, without large-scale sampling, frequentist analyses are also vulnerable to arbitrary selection of priors (e.g., α = 0.05) for significance testing that will affect power analyses. Deriving a suitable alternative basis for comparing patients and control subjects along a single continuum is not trivial. This type of dimensionality reduction problem is common in mathematical psychology when dealing with large numbers of parameters, and it can be applied in psychophysiological psychiatry.
Oculomotor dysfunction has long been recognized as a common problem in SCH.11,15 In future experiments, it seems reasonable to test for associations between smooth pursuit and saccadic control69 and fixation stability70,71 in relation to atypical scan paths. From a physiological perspective, deficits in the former domains can produce atypical patterns in the latter without appealing to explanations due to cognitive or perceptual dissonance. Research reports containing both oculomotor and scan path data from the same patients are therefore required to distinguish abnormal eye movements due to attentional or information processing deficits. This would improve our understanding of the effects of cannabis intake on scan path formation. Given that a cognitive element seems to have played a role in fixation behaviour in this study (as reflected in the consistent group difference findings from the PCA), a similar comparison with patients with bipolar and other disorders is appropriate.
The scan path dimensions illustrated in this paper demonstrated a neurocognitive deficit in visual exploration with increasing defect from control subjects to SCH to CIP. Significantly, both gamma and PCA models placed participants on univariate continua. Classical scan path analysis was only able to provide a general description of oculomotor engagement with the stimuli, whereas spatial analysis revealed that these results were due to fixations distributed along a clustered axis through extensive scanning (low through high). PCA suggested that image fixations were selected with particular features of the stimuli or that inferior motor execution of eye movements may be more strongly associated with CIP than with SCH. The degree of absence of normal exploratory fixations suggests that a more accurate estimation of scan path mutability is possible. Thus an understanding of both dimensional and prototypical trait-like behaviour can assist enormously in characterizing the quantitative trait locus of the elected phenotype(s) at heightened risk for schizophrenia and related illnesses.
Acknowledgments
We thank Stéphanie Duhoux for assistance with recruitment and data collection and Philippe Rey-Bellet with patient selection and diagnosis. Thanks to Ian Craw and Wojtek Krzanowski for discussion of multivariate statistics and Eelco Over for implementation details of the gamma model. This research was supported by the Swiss National Science Foundation (3100-059321.99) and GlaxoSmithKline Ltd. (Worldwide Epidemiology); (EPI40256).
Footnotes
Contributors: Drs. Benson, Leonards, Lothian and Merlo designed the study. Drs. Leonards and Merlo aquired the data, which all authors analyzed. Drs. Benson, Leonards, St. Clair and Merlo wrote the article, and all authors revised it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Philip J. Benson, School of Psychology, College of Life Sciences and Medicine, King's College, University of Aberdeen AB 24 2UB, UK; fax +44-1224-273426; philip.benson@abdn.ac.uk
References
- 1.Dean K, Murray RM. Environmental risk factors for psychosis. Dialogues Clin Neurosci 2005;7:69-80. [DOI] [PMC free article] [PubMed]
- 2.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 2002;25:409-32. [DOI] [PubMed]
- 3.Henquet C, Murray R, Linszen D, et al. The environment and schizophrenia: the role of cannabis use. Schizophr Bull 2005;31:608-12. [DOI] [PubMed]
- 4.van Os J, Hanssen M, Bijl RV, et al. Strauss (1969) revisited: a psychosis continuum in the general population? Schizophr Res 2000; 45:11-20. [DOI] [PubMed]
- 5.McGorry PD, Warner R. Consensus on early intervention in schizophrenia. Schizophr Bull 2002;28:543-4. [DOI] [PubMed]
- 6.Holzman PS. Eye movements and the search for the essence of schizophrenia. Brain Res Brain Res Rev 2000;31:350-6. [DOI] [PubMed]
- 7.Matthysse S, Holzman PS, Gusella JF, et al. Linkage of eye movement dysfunction to chromosome 6p in schizophrenia: additional evidence. Am J Med Genet B Neuropsychiatr Genet 2004;128:30-6. [DOI] [PubMed]
- 8.Schulze K, McDonald C, Frangou S, et al. Hippocampal volume in familial and nonfamilial schizophrenic probands and their unaffected relatives. Biol Psychiatry 2003;53:562-70. [DOI] [PubMed]
- 9.Blackwood DH, St. Clair D, Muir W, et al. Auditory P300 and eye tracking dysfunction in schizophrenic pedigrees. Arch Gen Psychiatry 1991;48:899-909. [DOI] [PubMed]
- 10.Bramon E, Rabe-Hesketh S, Sham P, et al. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res 2004;70:315-29. [DOI] [PubMed]
- 11.Calkins ME, Iacono WG. Eye movement dysfunction in schizophrenia: a heritable characteristic for enhancing phenotype definition. Am J Med Genet 2000;97:72-6. [DOI] [PubMed]
- 12.Takarae Y, Minshew NJ, Luna B, et al. Pursuit eye movements in autism. Brain 2004;127:2584-94. [DOI] [PubMed]
- 13.Bares M, Brázdila M, Kanovský P, et al. The effect of apomorphine administration on smooth pursuit ocular movements in early Parkinsonian patients. Parkinsonism Relat Disord 2003;9:139-44. [DOI] [PubMed]
- 14.Judge J, Knox PC, Caravolas M. Smooth pursuit performance implicates poor magnocellular functioning in developmental dyslexia. Perception 2002;31(S2):178.
- 15.Lencer R, Trillenberg P, Trillenberg-Krecker K, et al. Smooth pursuit deficits in schizophrenia, affective disorder and obsessive– compulsive disorder. Psychol Med 2004;34:451-60. [DOI] [PubMed]
- 16.Sutton S. Fact and artifact in the psychology of schizophrenia. In: Hammer M, Salzinger K, Sutton S, editors. Psychopathology, contributions from the social, behavioral, and biological sciences. New York: John Wiley & Sons; 1973. p. 197-215.
- 17.Phillips ML, David AS. Visual scan paths are abnormal in deluded schizophrenics. Neuropsychologia 1997;35:99-105. [DOI] [PubMed]
- 18.Loughland CM, Williams LM, Gordon E. Schizophrenia and affective disorder show different visual scanning behaviour for faces: a trait versus state-based distinction? Biol Psychiatry 2002;52:338-48. [DOI] [PubMed]
- 19.Loughland CM, Williams LM, Harris AW. Visual scanpath dysfunction in first-degree relatives of schizophrenia probands: evidence for a vulnerability marker? Schizophr Res 2004;67:11-21. [DOI] [PubMed]
- 20.Green MJ, Williams LM, Davidson D. Visual scanpaths to threat-related faces in deluded schizophrenia. Psychiatry Res 2003;119:271-85. [DOI] [PubMed]
- 21.Minassian A, Granholm E, Verney S, et al. Visual scanning deficits in schizophrenia and their relationship to executive functioning impairment. Schizophr Res 2005;74:69-79. [DOI] [PubMed]
- 22.Phillips ML, David AS. Abnormal visual scan paths: a psychophysiological marker of delusions in schizophrenia. Schizophr Res 1998;29:235-45. [DOI] [PubMed]
- 23.Gordon E, Coyle S, Anderson J, et al. Eye movement response to a facial stimulus in schizophrenia. Biol Psychiatry 1992;31:626-9. [DOI] [PubMed]
- 24.Williams LM, Loughland CM, Gordon E, et al. Visual scanpaths in schizophrenia: is there a deficit in face recognition? Schizophr Res 1999; 40:189-99. [DOI] [PubMed]
- 25.Loughland CM, Williams LM, Gordon E. Visual scanpaths to positive and negative facial emotions in an outpatient schizophrenia sample. Schizophr Res 2002;55:159-70. [DOI] [PubMed]
- 26.Kojima T, Matsushima E, Ando K, et al. Exploratory eye movements and neuropsychological tests in schizophrenic patients. Schizophr Bull 1992;18:85-94. [DOI] [PubMed]
- 27.Leonards U, Duhoux S, Rey-Bellet P, et al. Do disturbances in social interaction influence visual scene exploration in psychotic patients? Perception 2002;31(Suppl):182.
- 28.Gaebel W, Ulrich G, Frick K. Visuomotor performance of schizophrenic patients and normal controls in a picture viewing task. Biol Psychiatry 1987;22:1227-37. [DOI] [PubMed]
- 29.Rayner K, Pollatsek A. Eye movements and scene perception. Can J Psychol 1992;46:342-76. [DOI] [PubMed]
- 30.Schyns PG, Bonnar L, Gosselin F. Show me the features! Understanding recognition from the use of visual information. Psychol Sci 2002;13:402-9. [DOI] [PubMed]
- 31.van Os J, Verdoux H. Diagnosis and classification of schizophrenia: categories versus dimension, distributions versus disease. In: Murray RM, Jones PB, Susser E, et al., editors. The epidemiology of schizophrenia. Cambridge (UK): Cambridge University Press; 2003. p. 364-410.
- 32.Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry 2005;186:364-6. [DOI] [PubMed]
- 33.Craddock N, O'Donocan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull 2006;32:9-16. [DOI] [PMC free article] [PubMed]
- 34.Arendt M, Rosenberg R, Foldager L, et al. Cannabis-induced psychosis and subsequent schizophrenia-spectrum disorders: follow-up study of 535 incident cases. Br J Psychiatry 2005;187:510-5. [DOI] [PubMed]
- 35.First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV axis I disorders (SCID-I)–clinician version. Washington: American Psychiatric Press; 1997.
- 36.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 37.Andreasen NC. The scale for the assessment of negative symptoms (SANS). Iowa: University of Iowa; 1983.
- 38.Andreasen NC. The scale for the assessment of positive symptoms (SAPS). Iowa: University of Iowa; 1984.
- 39.Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004;24:192-208. [DOI] [PubMed]
- 40.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed., rev. Washington: The Association; 1987.
- 41.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders (SCID-I)–nonpatient version. Washington: American Psychiatric Press; 1997.
- 42.Over EAB, Hooge ITC, Erkelens CJ. A quantitative measure for the uniformity of fixation density: the Voronoi method. Behav Res Methods 2006;38:251-61. [DOI] [PubMed]
- 43.Krzanowski WJ. Between-groups comparison of principal components. J Am Statist Assoc 1979;74:703-7 (correction in 76[376]:1022).
- 44.Trillenberg P, Lencer R, Heide W. Eye movements and psychiatric disease. Curr Opin Neurol 2004;17:43-7. [DOI] [PubMed]
- 45.Huestegge L, Radach R, Kunert H-J, et al. Visual search in long-term cannabis users with early age of onset. In: Hyönä J, Munoz DP, Heide W et al., editors. The brain's eye: neurobiological and clinical aspects of oculomotor research (progress in brain research vol. 140). St. Louis: Elsevier; 2002. p. 377-94. [DOI] [PubMed]
- 46.Olincy A, Johnson LL, Ross RG. Differential effects of cigarette smoking on performance of a smooth pursuit and a saccadic eye movement task in schizophrenia. Psychiatry Res 2003;117:223-36. [DOI] [PubMed]
- 47.Avila MT, Sherr JD, Hong E, et al. Effects of nicotine on leading saccades during smooth pursuit eye movements in smokers and nonsmokers with schizophrenia. Neuropsychopharmacology 2003;28:2184-91. [DOI] [PubMed]
- 48.Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001;178:116-22. [DOI] [PubMed]
- 49.Williams JH, Wellman NA, Rawlins JNP. Cannabis use correlates with schizotypy in healthy people. Addiction 1996;91:869-77. [PubMed]
- 50.Nuñez LA, Gurpegui M. Cannabis-induced psychosis: a cross-sectional comparison with acute schizophrenia. Acta Psychiatr Scand 2002;105:173-8. [DOI] [PubMed]
- 51.Potvin S, Stip E, Roy J-Y. Toxic psychoses as pharmacological models of schizophrenia. Current Psychiatry Reviews 2005;1:23-32.
- 52.Hall W, Degenhardt L. Is there a specific ‚cannabis psychosis'? In: Castle DJ, Murray R, editors. Marijuana and madness. Cambridge (UK): Cambridge University Press; 2004. p. 89-100.
- 53.Ploner CJ, Tschirch A, Ostendorf F, et al. Oculomotor effects of d-9-tetrahydrocannabinol in humans: implications for the functional neuroanatomy of the brain cannabinoid system. Cereb Cortex 2002;12:1016-23. [DOI] [PubMed]
- 54.Glass M, Dragunow M, Faull RLM. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiological study in the fetal, neonatal and adult human brain. Neuroscience 1997;77:299-318. [DOI] [PubMed]
- 55.Braff DL, Silverton L, Saccuzzo DP, et al. Impaired speed of visual information processing in marijuana intoxication. Am J Psychiatry 1981;138:613-7. [DOI] [PubMed]
- 56.Eichenbaum H, Cohen NJ. Representation in the hippocampus: what do hippocampal neurons code? Trends Neurosci 1988;11:244-8. [DOI] [PubMed]
- 57.Sañudo-Peña MC, Tsou K, Romero J, et al. Role of the superior colliculus in the motor effects of cannabinoids and dopamine. Brain Res 2000;853:207-14. [DOI] [PubMed]
- 58.Dean B, Sundram S, Bradbury R, et al. Studies on [3H]CP-55940 bindings in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience 2001;103:9-15. [DOI] [PubMed]
- 59.Andreasson S, Alleback P, Engstrom A, et al. Cannabis and schizophrenia: a longitudinal study of Swedish conscripts. Lancet 1987;2:1483-6. [DOI] [PubMed]
- 60.Thornicroft G. Cannabis and psychosis. Is there epidemiological evidence for an association? Br J Psychiatry 1990;157:25-33. [DOI] [PubMed]
- 61.Arseneault L, Cannon M, Poulton R, et al. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ 2002;325:1212-3. [DOI] [PMC free article] [PubMed]
- 62.Zammit S, Allebeck P, Andreasson S, et al. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ 2002;325:1199-203. [DOI] [PMC free article] [PubMed]
- 63.Giuffrida A, Leweke FM, Gerth CW, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology 2004;29:2108-14. [DOI] [PubMed]
- 64.Miller P, Lawrie SM, Hodges A, et al. Genetic liability, illicit drug use, life stress and psychotic symptoms: preliminary findings from the Edinburgh study of people at high risk for schizophrenia. Soc Psychiatry Psychiatr Epidemiol 2001;36:338-42. [DOI] [PubMed]
- 65.Barrett SL, Bell R, Watson D, et al. Effects of amisulpride, risperidone and chlorpromazine on auditory and visual latent inhibition, prepulse inhibition, executive function and eye movements in healthy volunteers. J Psychopharmacol 2004;18:156-72. [DOI] [PubMed]
- 66.Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature 1994;371:511-3. [DOI] [PubMed]
- 67.Penn DL, Combs DR, Ritchie M, et al. Emotion recognition in schizophrenia: further investigation of generalized versus specific deficit models. J Abnorm Psychol 2000;109:512-6. [PubMed]
- 68.Green MF, Oliver B, Crawley JN, et al. Social cognition in schizophrenia: recommendations from the Measurement and Treatment Research to Improve Cognition in Schizophrenia New Approaches Conference. Schizophr Bull 2005;31:882-7. [DOI] [PubMed]
- 69.Gooding DC, Mohapatra L, Shea HB. Temporal stability of saccadic task performance in schizophrenia and bipolar patients. Psychol Med 2004;34:921-32. [DOI] [PubMed]
- 70.Paus T. Two modes of central gaze fixation maintenance and oculomotor distractability in schizophrenics. Schizophr Res 1991;5:145-52. [DOI] [PubMed]
- 71.Gooding DC, Grabowski JA, Hendershot CS. Fixation stability in schizophrenia, bipolar, and control subjects. Psychiatry Res 2000;97:119-28. [DOI] [PubMed]