Abstract
Interaction of intracellular free calcium ([Ca2+]i) and cAMP signaling mechanisms was examined in intact single megakaryocytes by using a combination of single-cell fluorescence microscopy to measure [Ca2+]i and flash photolysis of caged Ca2+, inositol 1,4,5-trisphosphate (IP3), or cAMP to elevate rapidly the concentration of these compounds inside the cell. Photolysis of caged IP3 stimulated Ca2+ release from an IP3-sensitive store. The cAMP-elevating agent carbacyclin inhibited this IP3-induced rise in [Ca2+]i but did not affect the rate of Ca2+ removal from the cytoplasm after photolysis of caged Ca2+. Photolysis of caged cAMP during ADP-induced [Ca2+]i oscillations caused the [Ca2+]i oscillation to transiently cease without affecting the rate of Ca2+ uptake and/or extrusion. We conclude that the principal mechanism of cAMP-dependent inhibition of Ca2+ mobilization in megakaryocytes appears to be by inhibition of IP3-induced Ca2+ release and not by stimulation of Ca2+ removal from the cytoplasm. Two inhibitors of cAMP-dependent protein kinase, a specific peptide inhibitor of the catalytic subunit of cAMP protein kinase and KT5720, blocked the inhibitory effect of carbacyclin, indicating that the inhibition of IP3-induced Ca2+-release by carbacyclin is mediated by cAMP-dependent protein kinase.
Calcium and cAMP are ubiquitous intracellular second messengers involved in transduction of extracellular signals. Extensive evidence suggests that Ca2+ and cAMP commonly cross-talk and modulate each other (1); however, depending on the tissue, cAMP can either potentiate or inhibit the agonist-induced [Ca2+]i elevation. In hepatocytes, pancreatic β cells, rat parotid acinar cells, and articular chondrocytes, cAMP potentiates the agonist-induced [Ca2+]i elevation (2–6). In contrast, in neutrophils, eosinophils, monocytes/macrophages, platelets, megakaryocytes, and smooth muscle, cAMP inhibits the activator-induced elevation of [Ca2+]i (7–10).
The mechanism of the cAMP-dependent regulation of [Ca2+]i is poorly understood but potentially involves activation of cAMP-dependent protein kinase (cAMP-PK), which leads to the phosphorylation of a number of proteins, including the inositol 1,4,5-trisphosphate (IP3) receptor (9, 11–14). However, whether phosphorylation physiologically regulates IP3 receptor function has not been established yet. Several reports obtained from in vitro preparations allow for no consensus as to whether cAMP-dependent phosphorylation of the IP3 receptor potentiates or inhibits its ability to release Ca2+ (14–16).
Megakaryocytes have become a unique model for studying Ca2+ mobilization and its regulation because, as the progenitor of platelets, they share many functional properties with platelets, including responsiveness to various bioactive substances that promote or inhibit platelet activation (17–21). Furthermore, rat megakaryocytes are a convenient model for studying [Ca2+]i signaling in nonexcitable cells because they appear to posses only IP3-sensitive Ca2+ stores, without the presence of IP3-insensitive ryanodine-sensitive Ca2+ pools (18). The large size of megakaryocytes and their relative insensitivity to mechanical stimulation compared with platelets make it possible to introduce into the intact cell caged second messenger molecules that are biologically inert until photolyzed by a flash of ultraviolet light. This approach has proved valuable in studying the process of intracellular signaling in various cell types (22–24).
We have reported (10) that in megakaryocytes agonist-induced [Ca2+]i oscillations are reversibly inhibited by agents that elevate intracellular cAMP (prostacyclin, 3-isobutyl-1-methylxanthine, forskolin, and 8-bromo-cAMP) and cGMP [sodium nitroprusside (SNP) and 8-(4-chlorophenylthio)guanosine 3′,5′-cyclic monophosphate]. In platelets, the elevation of cAMP by the vasodilatory prostaglandins E1 and I2 or by the introduction of cAMP analogues inhibits many platelet responses such as [Ca2+]i elevation, phosphoinositide metabolism, protein phosphorylation, aggregation, and granule exocytosis (for review, see ref. 25). It has been suggested that cAMP may act by inhibiting IP3-induced Ca2+ mobilization and/or by stimulating Ca2+ removal from the cytoplasm (26–30). The present study provides direct evidence that elevation of cAMP inhibits IP3-induced Ca2+ release in an intact cell but does not affect the removal of Ca2+ from the cytoplasm. Furthermore, we also show that this inhibition is mediated by cAMP-PK.
MATERIALS AND METHODS
Cell Preparation and Loading.
Megakaryocytes were obtained from adult Wistar rats as described (10). After filtration through a 75-μm nylon mesh to eliminate large masses of cells, the bone marrow suspension was centrifuged and washed twice before incubation in standard external solution (140 mM NaCl/5 mM KCl/1 mM MgCl2/2 mM CaCl2/10 mM glucose/10 mM Hepes, pH 7.4, supplemented with 0.1% BSA). Megakaryocytes were clearly distinguished from other bone marrow cells on the basis of their large size (25–50 μm) and multilobular nucleus (18, 31). All experiments were done within 2–6 h after preparation at room temperature (23°C–25°C).
“Cell-permeant” 4,5-dimethoxy-2-nitrobenzyl-caged cAMP and tetrakis(acetoxymethyl) esters of o-nitrophenyl EGTA (caged calcium) and Oregon Green 488 BAPTA-1 (OGB488) were dissolved in dimethyl sulfoxide (DMSO), stored at −20°C, and applied at 50–100, 10–30, and 2.5–5 μM, respectively. For intracellular loading, megakaryocytes were resuspended in standard external solution, containing either caged cAMP or caged calcium (the final concentration of DMSO was less than 0.1%), and incubated for at least 2 h. The cells were then transferred onto glass coverslips and were additionally incubated with OGB488/tetrakis(acetoxymethyl) for 30 min. The coverslips with adherent cells were then washed several times with the standard external solution and kept in the dark until use to avoid unwanted photolysis of the caged compounds. For experiments with the cAMP-PK inhibitor KT5720, megakaryocytes were preincubated in standard external solution containing 10 μM KT5720 for 2–3 h.
Agonist Application.
Activators and inhibitors (ADP, carbacyclin, SNP, or the mixture of ADP with one of the others) were dissolved in the standard external solution and applied directly to single megakaryocytes by using a DAD-6 computer-controlled local-superfusion system (ALA Scientific Instruments, Westbury, NY). The output tube of the micromanifold (100-μm inside diameter) was placed approximately 200 μm from the cell and the puff pressure was adjusted to achieve rapid agonist application while avoiding any mechanical disturbance of the cell. The time delay for arrival of agonists at the cell was measured and accounted for in the figures.
Caged IP3, a peptide inhibitor of cAMP-PK (IP20), and the cell-impermeant hexapotassium salt of OGB488 were included in the intrapipette solution at 100, 100, and 200 μM, respectively (intrapipette solution: 20 mM KCl/120 mM potassium glutamate/1 mM MgCl2/2 mM Na-GTP/10 mM Hepes, pH 7.3). Standard whole-cell patch-clamp recording techniques were used to voltage clamp and internally dialyze single megakaryocytes. Membrane current was monitored by using an Axopatch-1D patch clamp amplifier (Axon Instruments, Foster City, CA). Recording pipettes were pulled from 1.5-mm borosilicate glass (product 7052; Garner Glass, Claremont, CA) by using a two-stage Narishige PB-7 vertical puller and then fire-polished on a Narishige MF-9 microforge. Pipette resistances were between 1 and 5 MΩ, and the holding potential was −50 mV. For most cells, 5–6 min was required for the OGB488 fluorescence signal to equilibrate in the patch-clamped cell. The baseline fluorescence in cells, loaded with OGB488, hexapotassium salt, was 1,000–3,500 counts per ms, similar to that from cells loaded with OGB488/tetrakis(acetoxymethyl) ester (600–2,000 counts per ms).
Fluorescence Measurement and Flash Photolysis.
Megakaryocytes were viewed through a coverslip forming the bottom of the recording chamber by using a Nikon Diaphot microscope equipped with a Nikon Fluor ×100 1.3 numerical aperture oil-immersion objective. Single-cell fluorometry was accomplished by using an Ionoptix photon-counting fluorescence subsystem (designed by D. Tillotson, Ionoptix, Milton, MA) as described (10), except that in these experiments we used OGB488 as the Ca2+ indicator instead of Fura-2. For OGB488, excitation light was delivered from one channel of the chopper-based dual excitation 75-W xenon arc light source via the light guide. Excitation and emission were centered at 485 and 535 nm, respectively. For caged cAMP and caged IP3 photolysis, pulses of ultraviolet light (290–370 nm) were applied to the cell through the second channel of the dual excitation light source. Caged calcium photolysis was produced either by the second channel of the dual excitation light source or by a 1-ms flash from an XF-10 high-intensity xenon flash lamp (Hi-Tech Scientific, Salisbury, U.K.), focused through a 280- to 360-nm wide-band filter from about 2.5 cm above the cell to produce a 2- to 3-mm spot. The flash energy was regulated by controlling the charging voltage of the capacitor bank used to fire the flash lamp. Fluorescence intensity was measured on-line by using the pia program (Ionoptix). The time resolution was set at 0.1 s by averaging three points to obtain a better signal-to-noise ratio.
Chemicals.
The tetrakis(acetoxymethyl) ester and hexapotassium salt of OGB488 and 4,5-dimethoxy-2-nitrobenzyl-caged cAMP were obtained from Molecular Probes. Caged IP3, the tetrakis(acetoxymethyl) ester of o-nitrophenyl EGTA and KT5720 were from Calbiochem. All other reagents were purchased from Sigma.
RESULTS
cAMP Inhibits IP3-Induced Ca2+ Release in Intact Rat Megakaryocytes but Does Not Affect the Removal of Ca2+ from the Cytoplasm.
To determine whether cAMP inhibits IP3-induced Ca2+ release, we first performed the experiment shown in Fig. 1. Caged IP3 and OGB488 were introduced into megakaryocytes by inclusion in the internal solution inside a whole-cell patch-clamp pipette. Photolysis of caged IP3 stimulated Ca2+ release from an IP3-sensitive store. Because of variability in cell sensitivity to IP3, for every cell after equilibration of the intracellular and the pipette solution, the duration of the UV pulse was adjusted to produce an IP3-induced rise in [Ca2+]i similar in magnitude and time course to that resulting from the agonist application. In control experiments, we were able to use up to 10–12 IP3-releasing UV flashes without any significant degradation of the [Ca2+]i response (data not shown). [Ca2+]i spikes, produced by IP3-releasing flashes, in cells bathed at least 1 h in Ca2+-free solution [containing 1 mM bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate] were similar to those obtained from megakaryocytes bathed in standard external solution, indicating that these IP3-induced [Ca2+]i spikes were the result of IP3-induced Ca2+-release and not Ca2+ influx (n = 4 cells, data not shown).
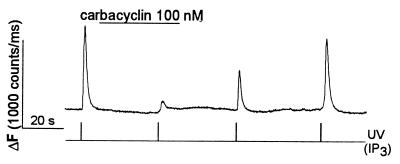
Figure 1.
Carbacyclin inhibits the IP3-induced rise in [Ca2+]i in megakaryocytes. Caged IP3 (100 μM) and OGB488 (200 μM) were included in the patch pipette solution. In this and all other figures, changes in [Ca2+]i were monitored by measuring OGB488 fluorescence intensity and are expressed as ΔF (counts per ms). IP3 was released by a UV pulse from the chopper-based dual-excitation light source, at the times indicated. Carbacyclin (100 nM) was applied to the cell via the local superfusion system.
To elevate cAMP, we used carbacyclin, a chemically stable analog of prostacyclin (32) that is known to elevate intracellular cAMP through a Gs protein-dependent activation of adenylate cyclase (for review, see ref. 33). Previous experiments have demonstrated that prostacyclin inhibits agonist-induced [Ca2+]i oscillations in megakaryocytes (10). As shown in Fig. 1, application of carbacyclin (100 nM), reversibly inhibited the IP3-induced rise in [Ca2+]i (n = 11 cells). Application of higher concentrations of carbacyclin (1–10 μM) caused irreversible inhibition of the IP3-induced rise in [Ca2+]i (n = 4 cells, data not shown).
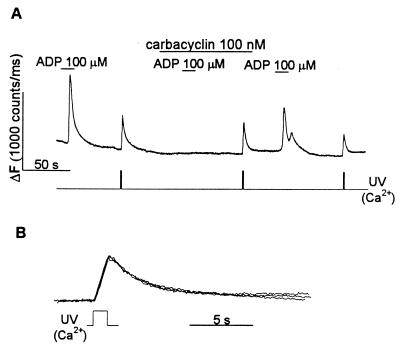
To investigate whether the inhibition of the IP3-induced rise in [Ca2+]i by carbacyclin involves the stimulation of Ca2+ uptake and/or extrusion, we used caged Ca2+ for the experiment in Fig. 2. The time course of the fall in [Ca2+]i, after the flash-induced rise in [Ca2+]i, should reflect the activity of Ca2+ sequestration and/or extrusion mechanisms. Ca2+ was photoreleased after a short application of ADP, which served as a positive control for the inhibitory effect of carbacyclin. ADP has been shown to mobilize Ca2+ from the intracellular stores in platelets (34, 35) and megakaryocytes (17, 21), presumably through the formation of IP3 that occurs in many other cell types (36). Fig. 2A shows that a brief application of ADP usually stimulates a single or biphasic [Ca2+]i spike and that carbacyclin reversibly inhibited the ADP-induced rise in [Ca2+]i. As can be seen in Fig. 2B, photoreleased [Ca2+]i declined with the same rate before, during, and after carbacyclin application. Results similar to those in Fig. 2 were obtained in seven other cells with carbacyclin and four other cells with prostacyclin.
Figure 2.
Carbacyclin reversibly inhibits Ca2+ mobilization by ADP without affecting the rate of Ca2+ removal from the cytoplasm. (A) Carbacyclin at 100 nM reversibly inhibited ADP-induced Ca2+ mobilization. The decrease in the peak amplitude of the [Ca2+]i spikes results from a decrease in the amount of unphotolyzed caged-Ca2+ remaining in the cell after the preceding flash. Ca2+ was released by a UV pulse at the times indicated. Carbacyclin (100 nM) and ADP (100 μM) were applied as shown. (B) Carbacyclin does not alter the time course of the fall in [Ca2+]i, after photolysis of caged Ca2+. Normalized [Ca2+]i spikes resulting from photorelease of caged Ca2+ (in A) are shown superimposed on an expanded time scale.
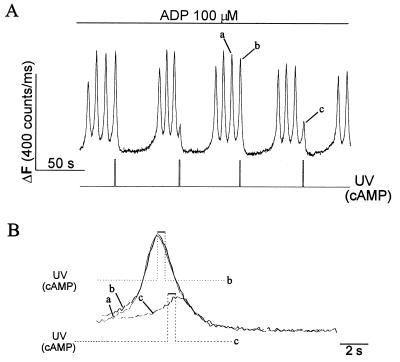
The experiment shown in Fig. 3 provides an independent test of whether or not elevation of cAMP stimulates Ca2+ removal from the cytoplasm. In Fig. 3A, a more prolonged application of 100 μM ADP induces a long-lasting [Ca2+]i oscillation (also see Fig. 5 below). In these experiments, we photoreleased cAMP from caged cAMP to inhibit rapidly these ADP-induced [Ca2+]i oscillations (n = 9 cells). We have reported (10) that the falling phase of each [Ca2+]i spike that makes up the agonist-induced [Ca2+]i oscillation in megakaryocytes results from Ca2+ uptake and/or extrusion. Elevation of cAMP after a [Ca2+]i spike reached maximum did not cause any change in the time course of the falling phase of the spike but delayed development of the next [Ca2+]i spike, thereby reversibly inhibiting the [Ca2+]i oscillation (Fig. 3A, spike b). When cAMP was released during the rising phase of a [Ca2+]i spike before it reached its peak, further Ca2+ release was inhibited and the [Ca2+]i spike aborted (Fig. 3A, spike c). In Fig. 3B, the [Ca2+]i spikes (Fig. 3A, labeled a, b, and c) are shown superimposed on an expanded time scale. It can be seen that cAMP inhibits [Ca2+]i oscillations without affecting the falling phase of the [Ca2+]i spike.
Figure 3.
Elevation of cAMP causes ADP-induced [Ca2+]i oscillations to transiently cease without affecting the kinetics of the falling phase of the individual [Ca2+]i spikes. Megakaryocytes were loaded with caged cAMP and OGB488/tetrakis(acetoxymethyl) ester. (A) [Ca2+]i oscillations were induced by 100 μM ADP as shown. cAMP was released by a 0.5-s UV pulse at the times indicated. (B) The [Ca2+]i spikes labeled a, b, and c in A are shown superimposed on an expanded time scale. The stepped lines indicate the time of occurrence of the UV flashes (cAMP release) during spikes b (dotted line) and c (broken line).
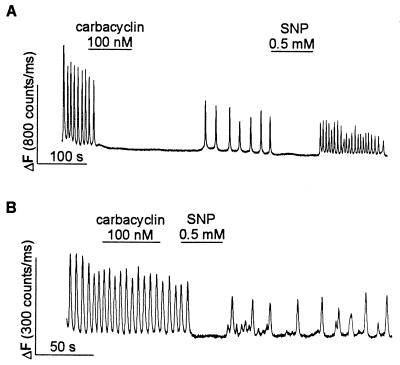
Figure 5.
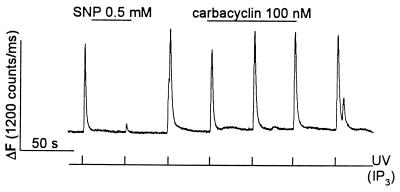
KT5720 prevents inhibition of ADP-induced [Ca2+]i oscillations by carbacyclin but not by SNP. Megakaryocytes were loaded with OGB488/tetrakis(acetoxymethyl) ester. [Ca2+]i oscillations were induced by continuous application of 100 μM ADP. (A) In a control cell both carbacyclin (100 nM) and SNP (0.5 mM) reversibly inhibit the ADP-induced [Ca2+]i oscillation. (B) In a cell pretreated with 10 μM KT5720 for 2–3 h, carbacyclin does not inhibit the ADP-induced [Ca2+]i oscillation, whereas SNP still inhibits the oscillation.
Thus, the results in Figs. 1–3 indicate that the principal mechanism of cAMP-dependent inhibition of Ca2+ mobilization in megakaryocytes is by inhibition of IP3-induced Ca2+ release and not by stimulation of Ca2+ removal from the cytoplasm.
The Inhibition of IP3-Induced Ca2+ Release by cAMP Is Mediated by cAMP-PK.
If the inhibition of IP3-induced Ca2+-release by cAMP is mediated by cAMP-PK, it should be possible to block the inhibitory effect of cAMP by inhibiting cAMP-PK. When 100 μM IP20, a specific peptide inhibitor of the catalytic subunit of cAMP-PK (37), was added to the pipette solution containing caged IP3 and OGB488, carbacyclin failed to inhibit IP3-induced Ca2+-release (n = 6 cells; Fig. 4). As a control for the specificity of IP20 action we used SNP, a nitric oxide donor that increases the intracellular level of cGMP through the activation of guanylyl cyclase (for review, see ref. 38). Previous experiments have demonstrated that SNP reversibly inhibits agonist-induced [Ca2+]i oscillations in megakaryocytes (10). If the effect of cGMP is mediated by cGMP-dependent protein kinase (39), it would be expected that IP20 would not prevent the inhibitory effect of SNP. As shown in Fig. 4, SNP still reversibly inhibits IP3-induced Ca2+ release in the presence of IP20. Similar results with SNP were seen in 12 other cells without IP20 in the pipette (data not shown).
Figure 4.
IP20 prevents inhibition of IP3-induced Ca2+ release by carbacyclin but not by SNP. IP20 (100 μM), caged IP3 (100 μM), and OGB488 (200 μM) were included in the intrapipette solution. IP3 was released by UV flashes as indicated. Carbacyclin (100 nM) and SNP (0.5 mM) were applied to the cell as indicated.
In Fig. 5 we also tested the ability of carbacyclin to inhibit ADP-induced [Ca2+]i oscillations in cells pretreated with the cell-permeant selective cAMP-PK inhibitor KT5720 (40). In these experiments a long-lasting [Ca2+]i oscillation was induced by continuous application of ADP. In control cells (Fig. 5A), these oscillations were routinely inhibited by carbacyclin and by SNP applied at the lowest effective concentrations (100 nM and 0.5 mM, respectively) established in preliminary experiments. In cells pretreated with 10 μM KT5720 for 2–3 h (Fig. 5B), carbacyclin no longer inhibited [Ca2+]i oscillations, but SNP still reversibly blocked the [Ca2+]i oscillations (n = 6 cells). In summary, the results in Figs. 4 and 5 indicate that inhibition of IP3-induced Ca2+ release by carbacyclin is mediated by cAMP-PK.
DISCUSSION
In our experiments, the rate of Ca2+ removal from the cytoplasm after photolytic release of Ca2+ was not affected by the cAMP-elevating agent carbacyclin. Likewise, photolytic elevation of cAMP during ADP-induced [Ca2+]i oscillations did not affect the rate of the falling phase of the Ca2+ spike, which has been shown to reflect the rate of Ca2+ removal from the cytoplasm (10). In summary, we did not find any evidence that elevated cAMP stimulates Ca2+ sequestration and/or extrusion in intact megakaryocytes.
The regulation of both intracellular and plasma membrane Ca2+ATPases by cAMP in platelets is still under investigation. It has been reported that cAMP increases the rate and maximal extent of Ca2+ uptake by the dense tubules (29) and stimulates Ca2+ extrusion in quin-2-overloaded platelets by increasing Vmax (30). It was also suggested that cAMP can stimulate Ca2+ uptake by platelets and a 22-kDa membrane protein was implicated in this process as a functional analog of phospholamban (for reviews, see refs. 41 and 42). Subsequent studies identified the 22-kDa protein as rap1B and showed that it was not involved in regulation of the intracellular Ca2+ ATPase (43–45). Moreover, a number of other studies did not find any effect of cAMP-elevating agents on Ca2+ uptake and/or extrusion (27, 46).
It is known that, for example, in cardiomyocytes the intracellular Ca2+ pump can be regulated by cAMP, which acts via cAMP-PK. cAMP-PK phosphorylates the regulatory protein phospholamban, resulting in dissociation of phospholamban from the Ca2+ATPase, thus augmenting its ATPase activity (for review, see ref. 47). ATPases of the sarco-endoplasmic reticulum calcium ATPase 1 and 2 classes can bind phospholamban (for review, see ref. 48), and sarco-endoplasmic reticulum calcium ATPase 2b is expressed in platelets (49). However, the key issue is whether or not the tissue of interest contains the regulatory protein phospholamban or an analogous protein.
The results presented herein and our earlier work (10) demonstrate directly that in intact megakaryocytes inhibition of calcium mobilization by prostacyclin is mediated by a cAMP-dependent inhibition of IP3-induced Ca2+ release. Moreover, our findings also imply that cAMP-PK is required for inhibition of IP3-induced Ca2+ release, although it is not clear whether this inhibition requires phosphorylation of only the IP3 receptor or phosphorylation of other proteins is involved.
Phosphorylation of the IP3 receptor by cAMP-PK has been reported in several preparations (9, 11–14); however, whether phosphorylation of the IP3 receptor potentiates or inhibits Ca2+ release remains unclear. In cerebellar membranes, phosphorylation of the IP3 receptor by cAMP-PK diminished the potency of IP3 in releasing Ca2+ (15) but also augmented the total amount of Ca2+ that could be released by IP3. On the other hand, experiments on hepatocytes suggest that hormones that elevate cAMP increase the sensitivity of calcium stores to IP3 and that this effect appears to be mediated by cAMP-PK phosphorylation of the IP3 receptor (2). Recently, it was reported that the platelet IP3 receptor can be phosphorylated by cAMP-PK and by endogenous membrane-bound kinases and that the additional phosphorylation by cAMP-PK inhibits the rate of IP3-mediated Ca2+ release (14). These differences in experimental findings might be explained by heterogeneity of the IP3 receptors in different preparations or by the presence in these preparations of another protein that is phosphorylated by cAMP-PK and that regulates IP3 receptor function.
The existence of several genes (50, 51) and alternative splicing of mRNAs from a single gene (52, 53) have been reported to generate several subtypes of the IP3 receptor. These subtypes of the IP3 receptor are expressed to various degrees in different tissues (50), and more than one subtype may even be found localized to the same intracellular Ca2+ pool (16). Heterogeneity of IP3 receptors in both IP3 binding and Ca2+ releasing properties has also been suggested (54, 55). Finally, cAMP-PK may act differently on different IP3 receptor subtypes. It has been found that type 1 and 2 IP3 receptors have different coupling domains, which in the type 1 IP3 receptor contains the cAMP-dependent phosphorylation site, suggesting that the two receptors may be subject to different types of regulation (51).
To avoid dealing with the heterogeneity of IP3 receptor subtypes obtained from a single preparation, immunoaffinity-purified type 1 IP3 receptors from a mouse cerebellar microsomal fraction were reconstituted into the lipid vesicles (16). In this preparation, the phosphorylation of the type 1 IP3 receptor by cAMP-PK caused a 20% increase in IP3-induced 45Ca2+ influx into the proteoliposomes, and this effect was prevented by a cAMP-PK inhibitor. However, the longer type 1a IP3 receptor of neurons is phosphorylated at two sites by cAMP-PK, compared with the type 1b receptor from peripheral tissues, which is almost exclusively phosphorylated at one site (52). At present, we know of no direct evidence that phosphorylation of the IP3 receptor regulates IP3-induced Ca2+ release in intact cells.
Acknowledgments
We thank Drs. R. Sha’afi and L. Jaffe for valuable comments on this manuscript and Dr. R. Zucker for helpful advice when we were initiating this project.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: IP3, inositol 1,4,5-trisphosphate; cAMP-PK, cAMP-dependent protein kinase; OGB488, Oregon Green 488 BAPTA-1; SNP, sodium nitroprusside; IP20, peptide inhibitor of cAMP-dependent protein kinase; [Ca2+]i, intracellular Ca2+ concentration.
References
- 1.Tsunoda Y. Biochim Biophys Acta. 1993;1154:105–156. doi: 10.1016/0304-4157(93)90008-c. [DOI] [PubMed] [Google Scholar]
- 2.Burgess G M, Bird G S J, Obie J F, Putney J W., Jr J Biol Chem. 1991;266:4772–4781. [PubMed] [Google Scholar]
- 3.Sunchez-Bueno A, Marrero I, Cobbold P. Biochem J. 1993;291:163–168. doi: 10.1042/bj2910163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fournier L, Whitfield J F, Schwartz J-L, Begin-Heick N. J Biol Chem. 1993;269:1120–1124. [PubMed] [Google Scholar]
- 5.Rubin R P, Adolf M A. J Pharmacol Exp Ther. 1994;268:600–606. [PubMed] [Google Scholar]
- 6.D’Andrea P, Paschini V, Vittur F. Biochem J. 1996;318:569–573. doi: 10.1042/bj3180569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira M M, Gristwood R M, Cooper N, Hellewell P G. Trends Pharmacol Sci. 1997;18:164–169. doi: 10.1016/s0165-6147(97)01049-3. [DOI] [PubMed] [Google Scholar]
- 8.Feinstein M B, Egan J J, Sha’afi R I, White J. Biochem Biophys Res Commun. 1983;113:598–604. doi: 10.1016/0006-291x(83)91768-0. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Latif A A. Proc Soc Exp Biol Med. 1996;211:163–177. doi: 10.3181/00379727-211-43959b. [DOI] [PubMed] [Google Scholar]
- 10.Tertyshnikova S, Fein A. Cell Calcium. 1997;21:331–344. doi: 10.1016/s0143-4160(97)90026-9. [DOI] [PubMed] [Google Scholar]
- 11.Ferris C D, Cameron A M, Bredt D S, Huganir R L, Snyder S H. Biochem Biophys Res Commun. 1991;175:192–198. doi: 10.1016/s0006-291x(05)81219-7. [DOI] [PubMed] [Google Scholar]
- 12.Joseph S K, Ryan S V. J Biol Chem. 1993;268:23059–23065. [PubMed] [Google Scholar]
- 13.El-Daher S S, Eigenthaler M, Walter U, Furuichi T, Miyawaki A, Mikoshiba K, Kakkar V V, Authi K S. Thromb Haemostasis. 1996;76:1063–1071. [PubMed] [Google Scholar]
- 14.Quinton T M, Brown K D, Dean W L. Biochemistry. 1996;35:6865–6871. doi: 10.1021/bi960128m. [DOI] [PubMed] [Google Scholar]
- 15.Supattapone S, Danoff S, Theibert A, Joseph S K, Steiner J, Snyder S H. Proc Natl Acad Sci USA. 1988;85:8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakade S, Rhee S K, Hamanaka H, Mikoshiba K. J Biol Chem. 1994;269:6735–6742. [PubMed] [Google Scholar]
- 17.Uneyama C, Uneyama H, Akaike N. J Physiol. 1993;470:731–749. doi: 10.1113/jphysiol.1993.sp019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uneyama H, Uneyama C, Akaike N. J Biol Chem. 1993;268:168–174. [PubMed] [Google Scholar]
- 19.Somasundaram B, Mahaut-Smith M P. J Physiol. 1994;480:225–231. doi: 10.1113/jphysiol.1994.sp020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somasundaram B, Mahaut-Smith M P. J Biol Chem. 1995;270:16638–16644. doi: 10.1074/jbc.270.28.16638. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda M, Kurokawa K, Maruyama Y. J Physiol. 1992;447:711–728. doi: 10.1113/jphysiol.1992.sp019025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams S R, Tsien R Y. Annu Rev Physiol. 1993;55:755–784. doi: 10.1146/annurev.ph.55.030193.003543. [DOI] [PubMed] [Google Scholar]
- 23.Zucker R. In: Methods in Cell Biology. Nuccitelli R, editor. San Diego: Academic; 1994. pp. 31–63. [Google Scholar]
- 24.Lev-Ram V, Jiang T, Wood J, Lawrence D S, Tsien R Y. Neuron. 1997;18:1025–1038. doi: 10.1016/s0896-6273(00)80340-2. [DOI] [PubMed] [Google Scholar]
- 25.Walter U, Eigenthaler M, Geiger J, Reinhard M. Adv Exp Med Biol. 1993;344:237–249. doi: 10.1007/978-1-4615-2994-1_19. [DOI] [PubMed] [Google Scholar]
- 26.Tohmatsu T, Nishida A, Nagao S, Nakashima S, Nozawa Y. Biochim Biophys Acta. 1989;1013:190–193. doi: 10.1016/0167-4889(89)90048-7. [DOI] [PubMed] [Google Scholar]
- 27.Cavallini L, Coassin M, Borean A, Alexandre A. J Biol Chem. 1996;271:5545–5551. doi: 10.1074/jbc.271.10.5545. [DOI] [PubMed] [Google Scholar]
- 28.Kaser-Glanzmann R, Jakabova M, George J N, Luscher E F. Biochim Biophys Acta. 1977;466:429–440. doi: 10.1016/0005-2736(77)90336-4. [DOI] [PubMed] [Google Scholar]
- 29.Tao J, Johansson J S, Haynes D H. Biochim Biophys Acta. 1992;1105:29–39. doi: 10.1016/0005-2736(92)90159-j. [DOI] [PubMed] [Google Scholar]
- 30.Johansson J S, Nied L E, Haynes D H. Biochim Biophys Acta. 1992;1105:19–28. doi: 10.1016/0005-2736(92)90158-i. [DOI] [PubMed] [Google Scholar]
- 31.Kapural L, Fein A. Biochim Biophys Acta. 1997;1326:319–328. doi: 10.1016/s0005-2736(97)00035-7. [DOI] [PubMed] [Google Scholar]
- 32.Whittle B J, Moncada S, Whiting F, Vane J R. Prostaglandins. 1980;19:605–627. doi: 10.1016/s0090-6980(80)80010-4. [DOI] [PubMed] [Google Scholar]
- 33.Wise H, Jones R L. Trends Pharmacol Sci. 1996;17:17–21. doi: 10.1016/0165-6147(96)81565-3. [DOI] [PubMed] [Google Scholar]
- 34.Sage S O, Rink T J. J Biol Chem. 1987;262:16364–16369. [PubMed] [Google Scholar]
- 35.MacKenzie A B, Mahaut-Smith M P, Sage S O. J Biol Chem. 1996;271:2879–2881. doi: 10.1074/jbc.271.6.2879. [DOI] [PubMed] [Google Scholar]
- 36.Berridge M J. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 37.Cheng H C, Kemp B E, Pearson R B, Smith A J, Misconi L, Van Patten S M, Walsh D A. J Biol Chem. 1986;261:989–992. [PubMed] [Google Scholar]
- 38.Moncada S, Palmer R M J, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 39.Moro M A, Russel R J, Cellek S, Lizasoain I, Su Y, Darley-Usmar V M, Radomski M W, Moncada S. Proc Natl Acad Sci USA. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- 41.Dean W L. Cell Calcium. 1989;10:289–297. doi: 10.1016/0143-4160(89)90055-9. [DOI] [PubMed] [Google Scholar]
- 42.Authi K S. In: Mechanisms of Platelet Activation and Control. Authi K S, Watson S P, Kakkar V V, editors. New York: Plenum; 1993. pp. 83–104. [Google Scholar]
- 43.Siess W, Grunberg B, Luber K. In: Mechanisms of Platelet Activation and Control. Authi K S, Watson S P, Kakkar V V, editors. New York: Plenum; 1993. pp. 229–235. [Google Scholar]
- 44.Fisher T H, White G C. Biochem Biophys Res Commun. 1990;159:644–650. doi: 10.1016/0006-291x(89)90043-0. [DOI] [PubMed] [Google Scholar]
- 45.O’Rourke F, Zavoico G B, Feinstein M F. Biochem J. 1989;257:715–721. doi: 10.1042/bj2570715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rink T J, Sage S O. J Physiol. 1987;393:513–524. doi: 10.1113/jphysiol.1987.sp016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tada M, Toyofuku T. J Cardiac Failure. 1996;2:S77–S85. doi: 10.1016/s1071-9164(96)80062-5. [DOI] [PubMed] [Google Scholar]
- 48.Grover A K, Khan I. Cell Calcium. 1992;13:9–17. doi: 10.1016/0143-4160(92)90025-n. [DOI] [PubMed] [Google Scholar]
- 49.Enouf J, Bredoux R, Papp B, Djaffar I, Lompre A M, Kieffer N, Gayet O, Clemetson K, Wuytack F, Rosa J P. Biochem J. 1992;286:135–140. doi: 10.1042/bj2860135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross C A, Danoff S K, Schell M J, Snyder S H, Ullrich A. Proc Natl Acad Sci USA. 1992;89:4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sudhof T C, Newton C L, Atcher B T, III, Ushkaryov Y A, Mignery G A. EMBO J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danoff S K, Ferris C D, Donath C, Fisher G, Munemitsu S, Ullrich A, Snyder S H, Ross C A. Proc Natl Acad Sci USA. 1991;88:2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mignery G A, Newton C L, Archer B T, III, Sudhof T S. J Biol Chem. 1990;265:12679–12685. [PubMed] [Google Scholar]
- 54.Pietri F, Hilly M, Mauger J-P. J Biol Chem. 1990;265:17478–17485. [PubMed] [Google Scholar]
- 55.Ferris C D, Cameron A M, Huganir R L, Snyder S H. Nature (London) 1992;356:350–352. doi: 10.1038/356350a0. [DOI] [PubMed] [Google Scholar]