Abstract
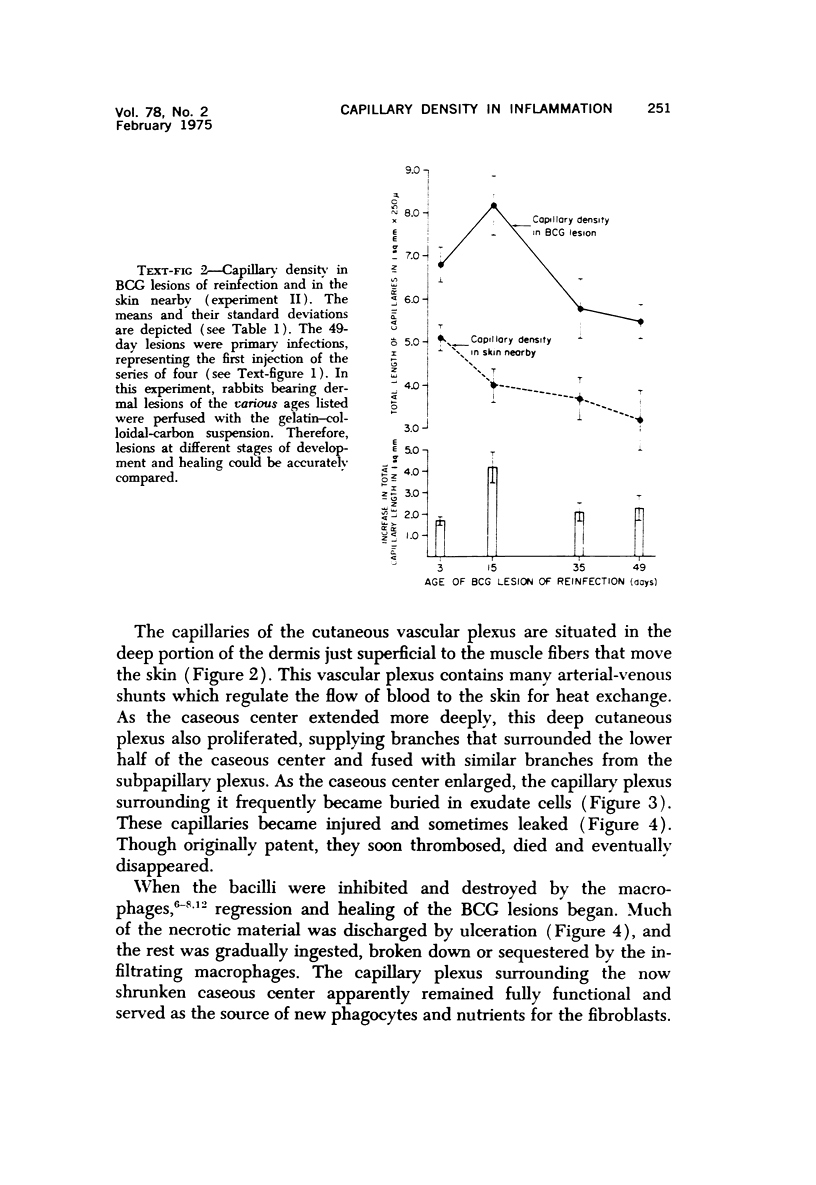
Dermal tuberculous lesions were produced in rabbits by the intradermal injection of BCG. At various times after infection, anesthetized animals were perfused with a gelatin-colloidal carbon medium via the abdominal aorta. The capillary density of the nonnecrotic granulation tissue in the lesions was determined quantitatively by counting the capillaries under an ocular grid of a microscope. The capillary density in normal skin near the lesions was 3.8 plus or minus 0.5 in millimetersof capillary lengths per square millimeter in 250-mu tissue sections. The capillary density of the nonnecrotic tissue in BCGlesions averaged 6.1 plus or minus 0.6 mm/sq mm, an increase of 60%. The capillary density remained more or less constant as the BCG lesions grew and then regressed. The development of delayed hypersensitivity seemed to increase the capillary density, but this increase may have been a response to an extension of the necrosis at the time delayed hypersensitivity developed. Capillary densities in tuberculin reactions resembled those in BCG lesions. In the early stages, the increaseed capillary network of dermal BCG lesions was derived mainly from the subpapillary vascular plexus of the deep dermis supplied branches that surrounded the lower half of the caseous necrotic center and anastomosed with capillaries from the subpapillary plexus supplying the upper half. When the necrotic center extended, nearby capillaries thrombosed and in turn became necrotic. Peripherally, new capillaries formed and anastomosed with existing capillaries. From these vessels, mononuclear phagocytes emigrated, destroyed the tubercle bacilli, and enabled the lesion to heal. In the BCG lesions at all stages of development and healing, the capillary network in the nonnecrotic areas seemed adequate to supply and nourish the defense cells controlling the infection.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandrasekhar S., Shima K., Dannenberg A. M., Kambara T., Fabrikant J. I., Roessler W. G. Radiation, Infection, and Macrophage Function IV. Effect of Radiation on the Proliferative Abilities of Mononuclear Phagocytes in Tuberculous Lesions of Rabbits. Infect Immun. 1971 Feb;3(2):254–259. doi: 10.1128/iai.3.2.254-259.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Ando M., Shima K. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. 3. The turnover of macrophages and its relation to their activation and antimicrobial immunity in primary BCG lesions and those of reinfection. J Immunol. 1972 Nov;109(5):1109–1121. [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P. G., Yamamoto I., Mellins H. Z. Blood microcirculation in the lymph node during the primary immune response. J Exp Med. 1972 Oct 1;136(4):697–714. doi: 10.1084/jem.136.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919 May 20;52(6):409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIE M. B., ZAPPASODI P., CARDONA-LYNCH E., DANNENBERG A. M., Jr The response to the intracutaneous inoculation of BCG as an index of native resistance to tuberculosis. J Immunol. 1952 Apr;68(4):369–387. [PubMed] [Google Scholar]
- Ljungqvist A., Almgård L. E. The vascular reaction in the free skin allo- and autografts. A stereomicro-angiographic and histological study in the rabbit. Acta Pathol Microbiol Scand. 1966;68(4):553–562. doi: 10.1111/apm.1966.68.4.553. [DOI] [PubMed] [Google Scholar]
- Nyman S., Lindhe J., Zederfeldt B. The vascularity of wounded areas in estradiol and progesterone treated female rabbits. A microangiographic study. Acta Chir Scand. 1971;137(7):631–637. [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. Participation of mononuclear phagocytes in chronic inflammatory diseases. J Reticuloendothel Soc. 1974 May;15(5):413–438. [PubMed] [Google Scholar]
- Rojas-Espinosa O., Dannenberg A. M., Jr, Sternberger L. A., Tsuda T. The role of cathepsin D in the pathogenesis of tuberculosis. A histochemical study employing unlabeled antibodies and the peroxidase-antiperoxidase complex. Am J Pathol. 1974 Jan;74(1):1–17. [PMC free article] [PubMed] [Google Scholar]
- SCHOEFL G. I. STUDIES ON INFLAMMATION. III. GROWING CAPILLARIES: THEIR STRUCTURE AND PERMEABILITY. Virchows Arch Pathol Anat Physiol Klin Med. 1963 Nov 8;337:97–141. [PubMed] [Google Scholar]
- Shima K., Dannenberg A. M., Jr, Ando M., Chandrasekhar S., Seluzicki J. A., Fabrikant J. I. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. I. Studies involving their incorporation of tritiated thymidine and their content of lysosomal enzymes and bacilli. Am J Pathol. 1972 Apr;67(1):159–180. [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells F. R. A comparison of the cellular and vascular responses in delayed hypersensitivity to tuberculin, of rat and guinea-pig dorsal skin. Br J Exp Pathol. 1972 Jun;53(3):277–288. [PMC free article] [PubMed] [Google Scholar]








