Abstract
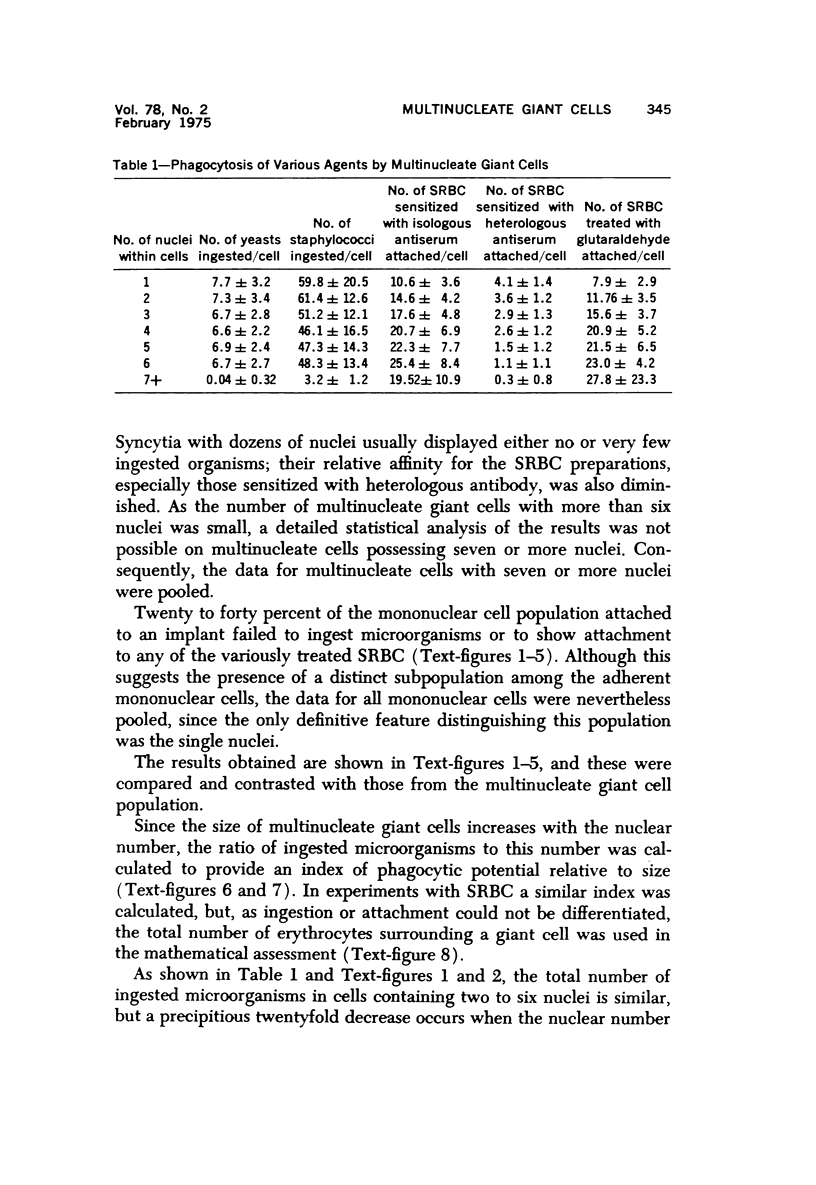
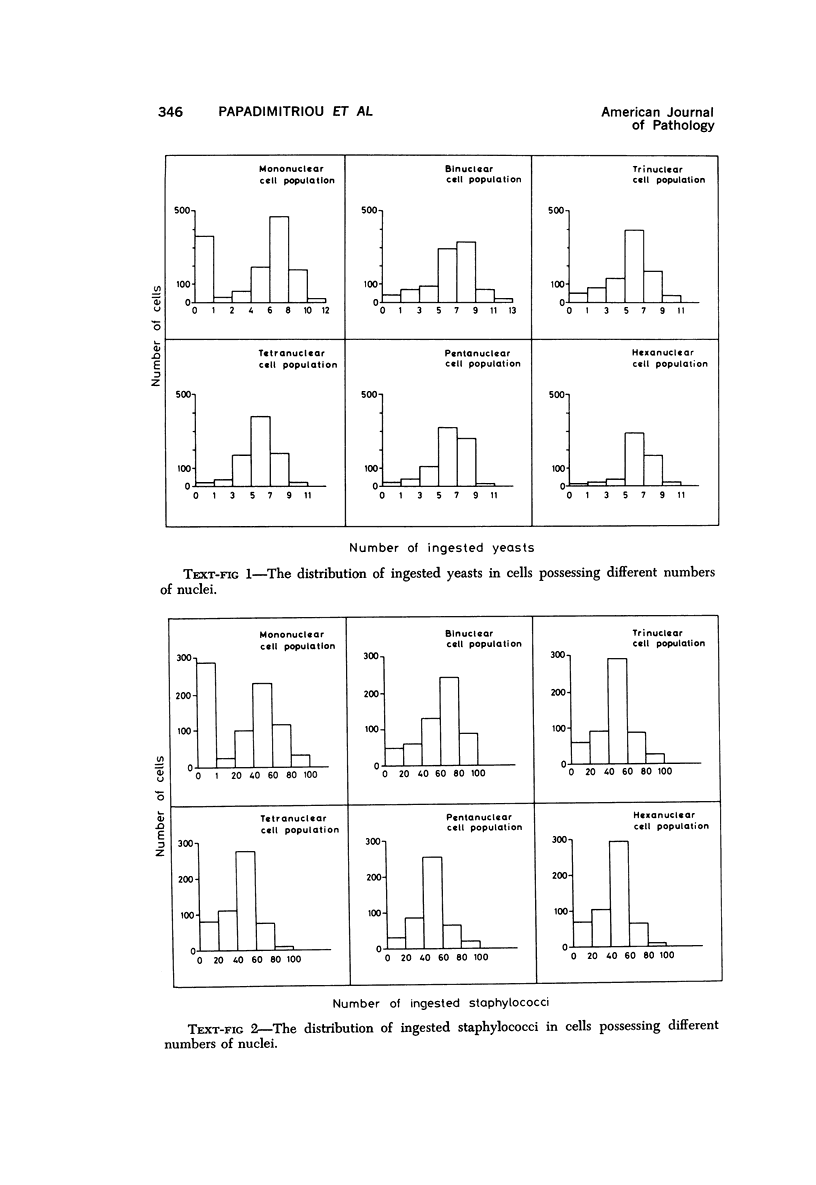
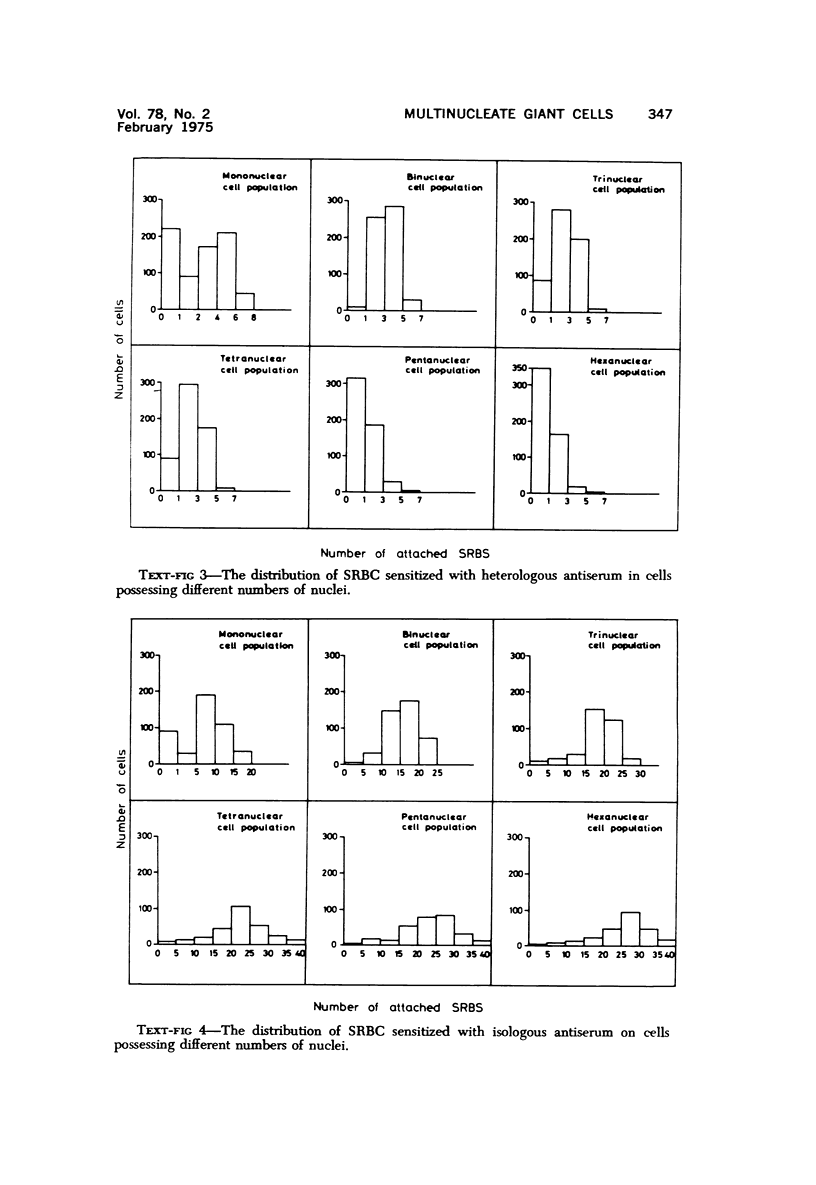
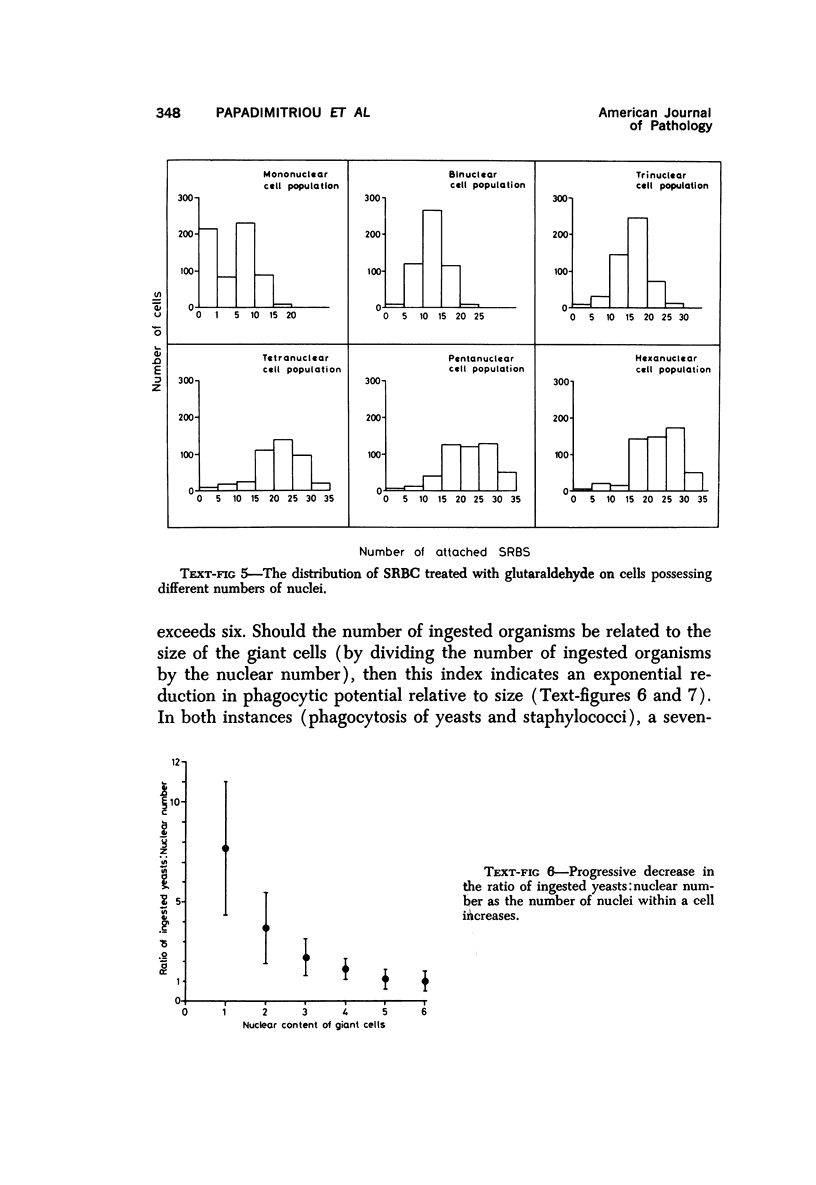
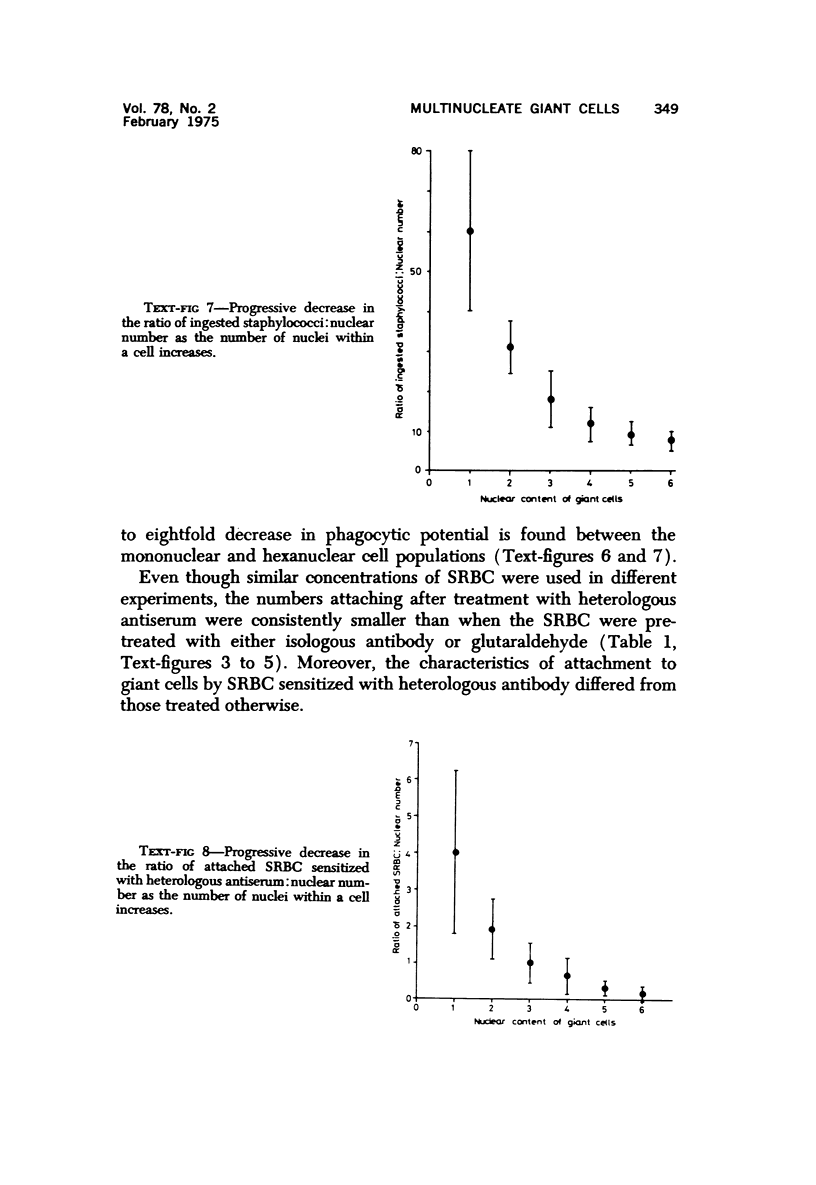
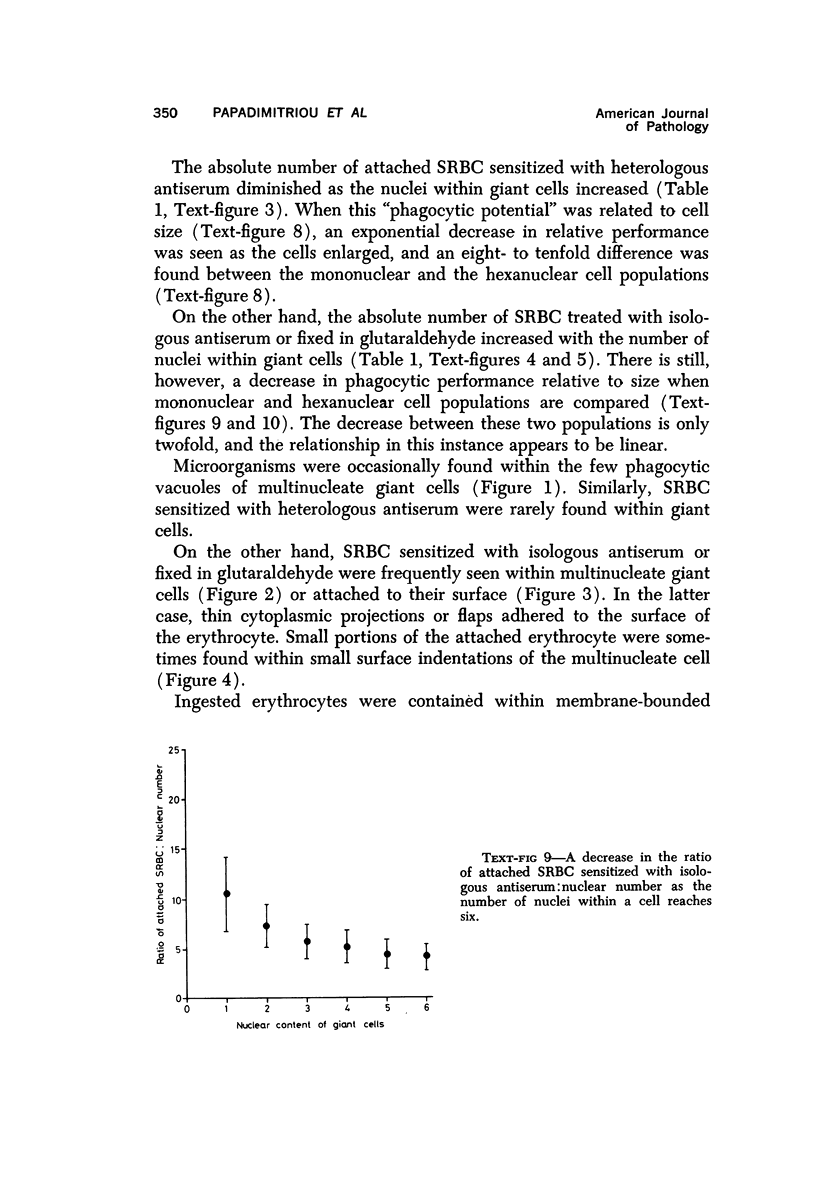
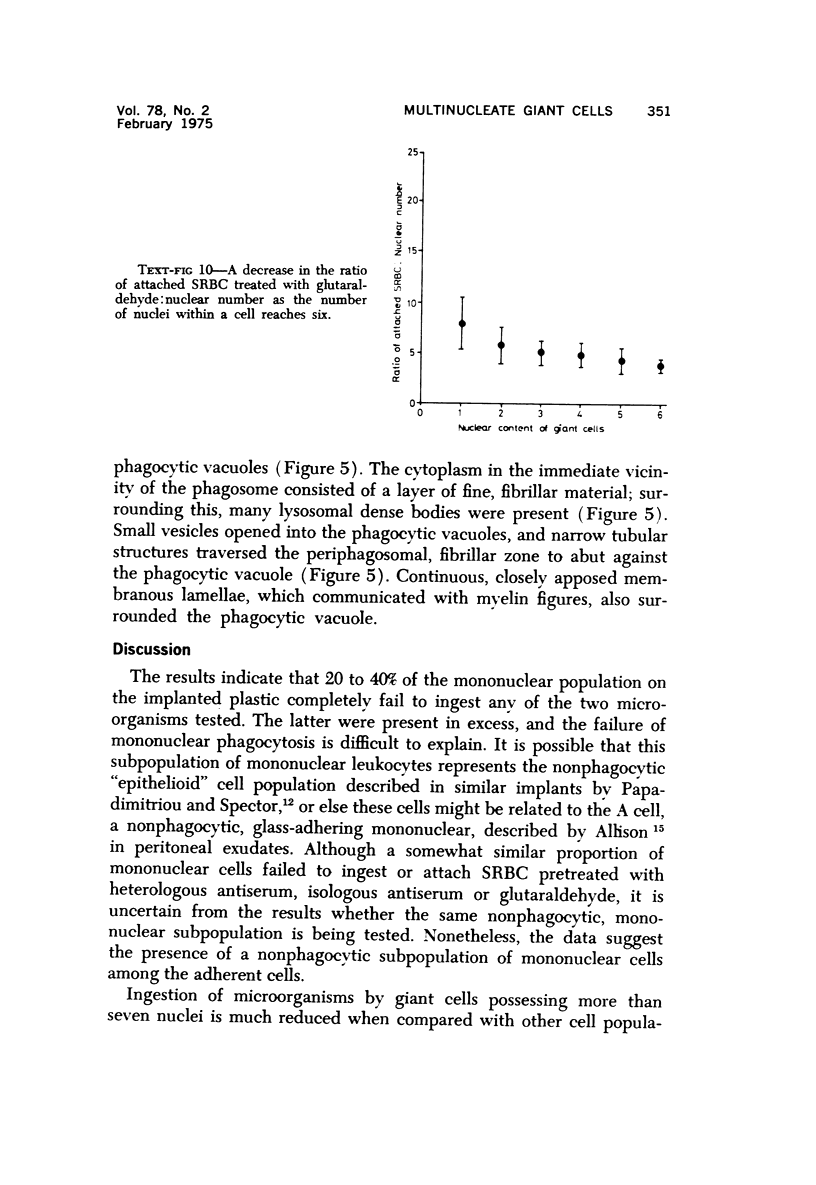
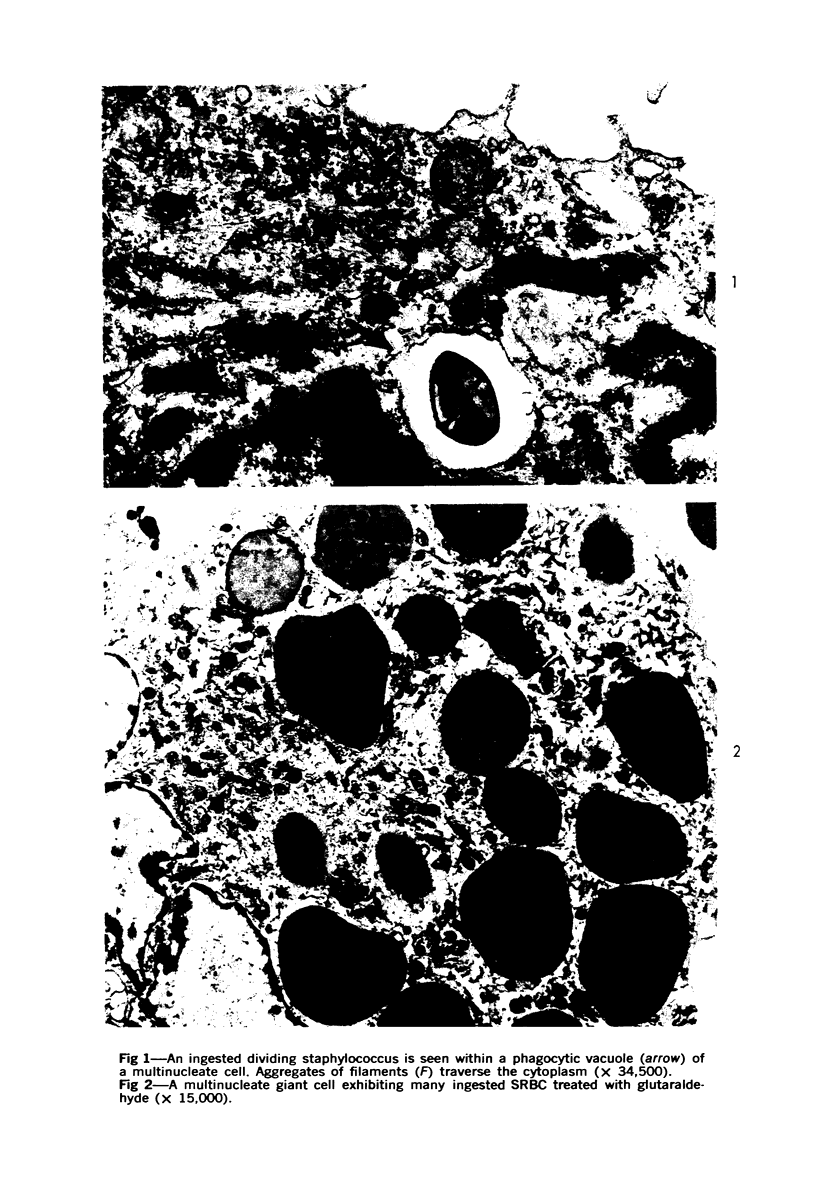
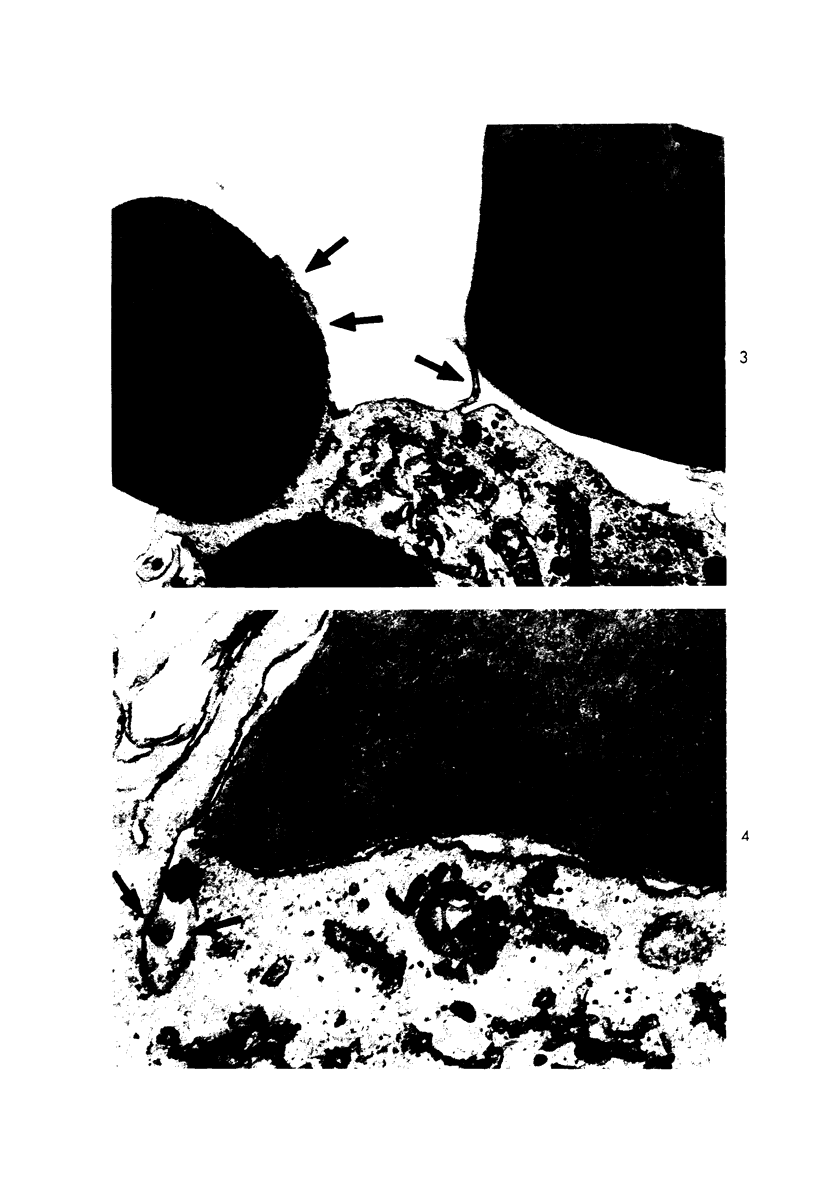
Multinucleate giant cells were collected by subcutaneous implantation of plastic films into mice. The attached cells were challanged in vitro with staphylococci, yeasts and sheep erythrocytes treated with either glutaraldehyde or isologous or heterologous antiserum. Cells containing more than seven nuclei rarely phagocytized yeasts or staphylococci, and the uptake and ingestion of sheep erythrocytes treated with heterologous antiserum was equally infrequent. Many sheep erythrocytes treated with isologous antiserum or glutaraldehyde attached to giant cells. When the adherent erythrocytes were related to the increased size of the multinucleate cell by dividing the number attaching by the number of nuclei in the giant cell, a progressive relative reduction was demonstrated as the nuclear content increased. It is suggested that these phenomena are due to the loss of surface receptors subsequent to fusion during the formation of multinucleate cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Immunity and immunopathology in virus infections. Ann Inst Pasteur (Paris) 1972 Oct;123(4):585–608. [PubMed] [Google Scholar]
- Gillman T., Wright L. J. Probable in vivo origin of multi-nucleated giant cells from circulating mononuclears. Nature. 1966 Jan 15;209(5020):263–265. doi: 10.1038/209263a0. [DOI] [PubMed] [Google Scholar]
- Papadimitriou J. M. Detection of macrophage receptors for heterologous IgG by scanning and transmission electron microscopy. J Pathol. 1973 Jul;110(3):213–220. doi: 10.1002/path.1711100304. [DOI] [PubMed] [Google Scholar]
- Papadimitriou J. M. Direct embedding in epoxy resin of cells attached to cellophane. Exp Cell Res. 1972 Feb;70(2):449–452. doi: 10.1016/0014-4827(72)90161-9. [DOI] [PubMed] [Google Scholar]
- Papadimitriou J. M., Finlay-Jones J. M., Walters M. N. Surface characteristics of macrophages, epithelioid and giant cells using scanning electron microscopy. Exp Cell Res. 1973 Feb;76(2):353–362. doi: 10.1016/0014-4827(73)90387-x. [DOI] [PubMed] [Google Scholar]
- Papadimitriou J. M., Sforsina D., Papaelias L. Kinetics of multinucleate giant cell formation and their modification by various agents in foreign body reactions. Am J Pathol. 1973 Nov;73(2):349–364. [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., Spector W. G. The origin, properties and fate of epithelioid cells. J Pathol. 1971 Nov;105(3):187–203. doi: 10.1002/path.1711050305. [DOI] [PubMed] [Google Scholar]
- Spector W. G., Lykke A. W. The cellular evolution of inflammatory granulomata. J Pathol Bacteriol. 1966 Jul;92(1):163–167. doi: 10.1002/path.1700920117. [DOI] [PubMed] [Google Scholar]
- Sutton J. S., Weiss L. Transformation of monocytes in tissue culture into macrophages, epithelioid cells, and multinucleated giant cells. An electron microscope study. J Cell Biol. 1966 Feb;28(2):303–332. doi: 10.1083/jcb.28.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]