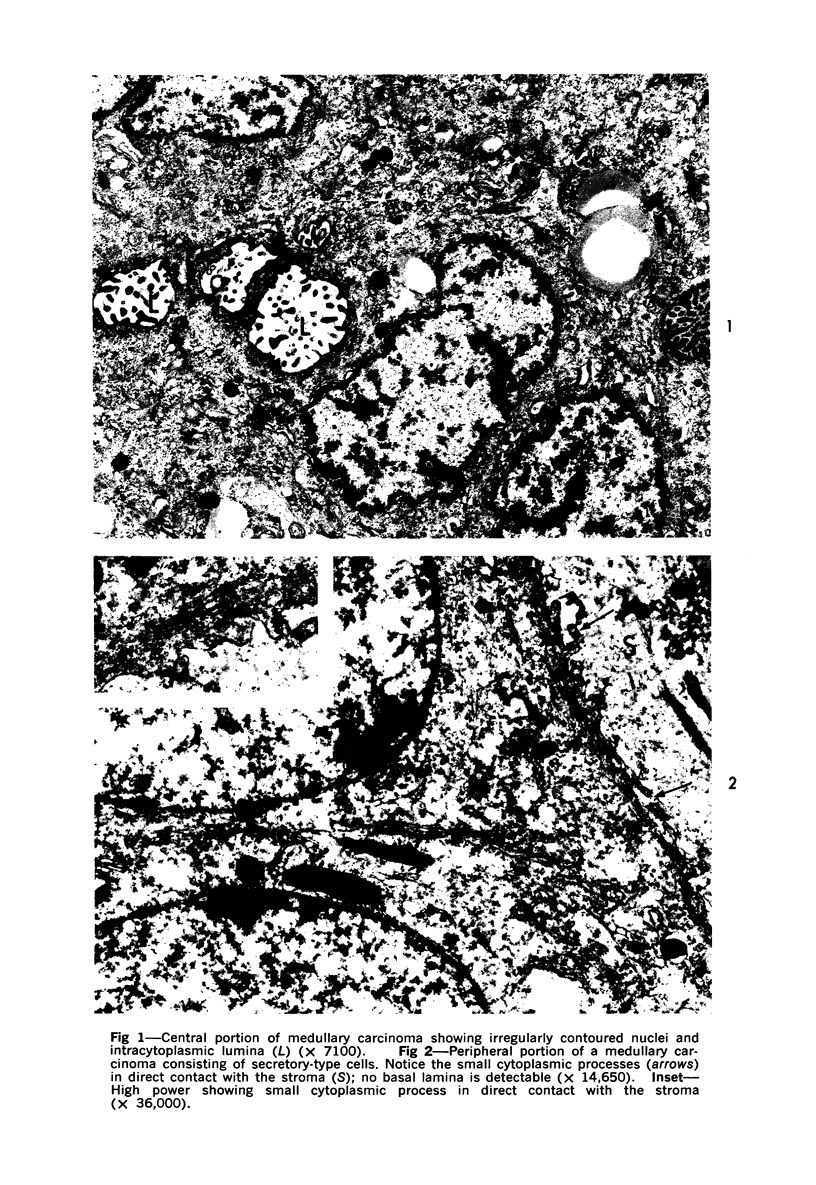
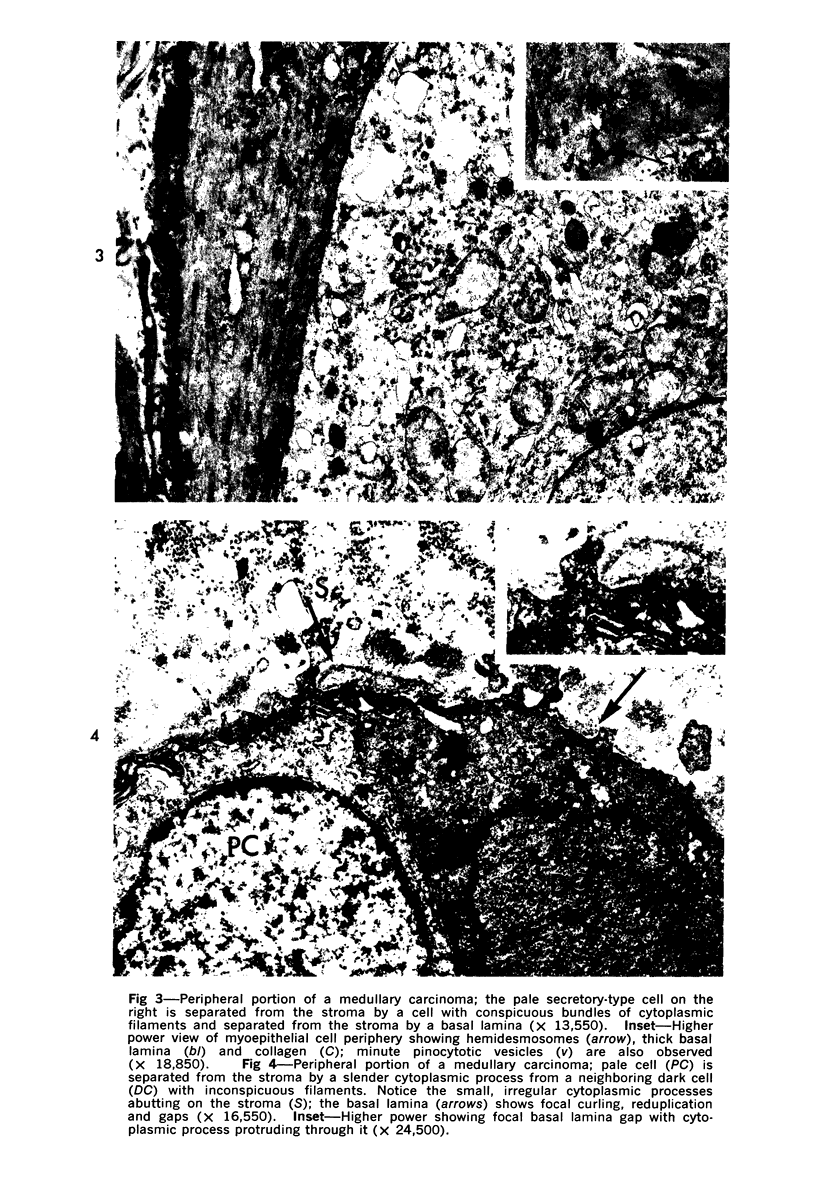
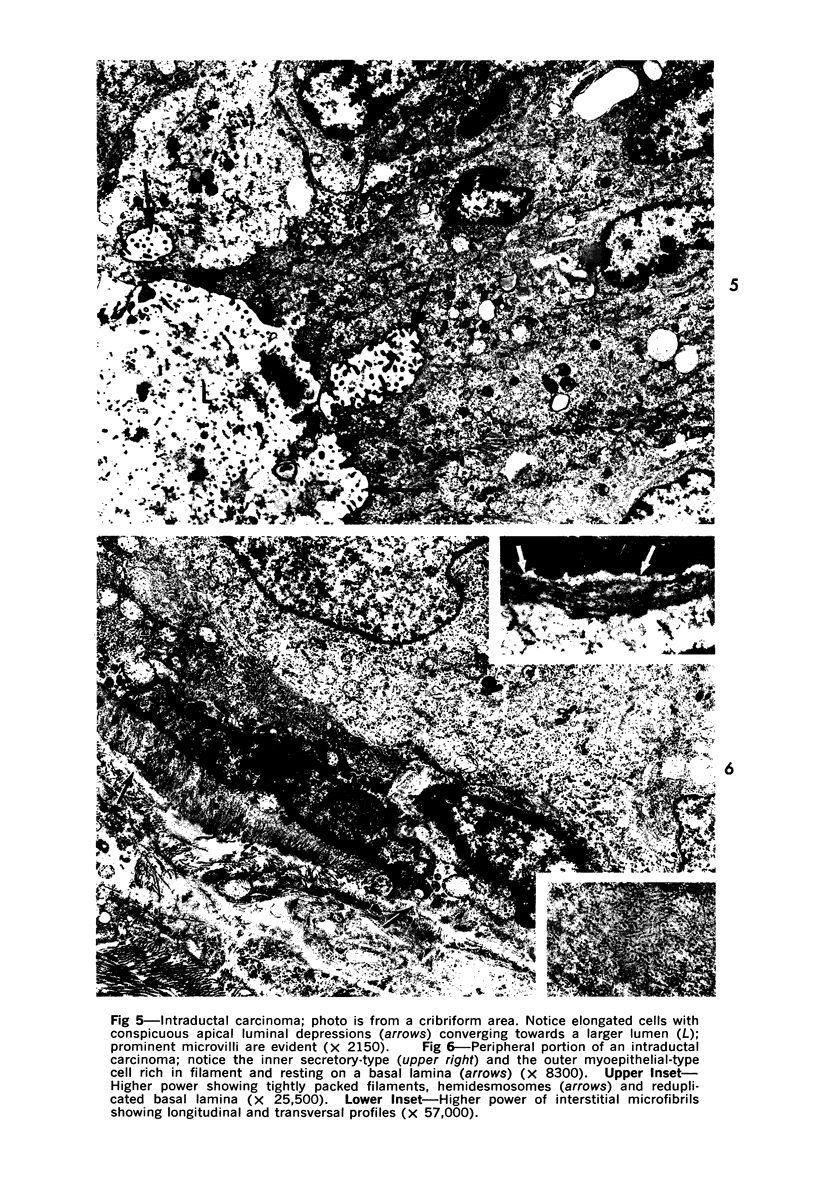
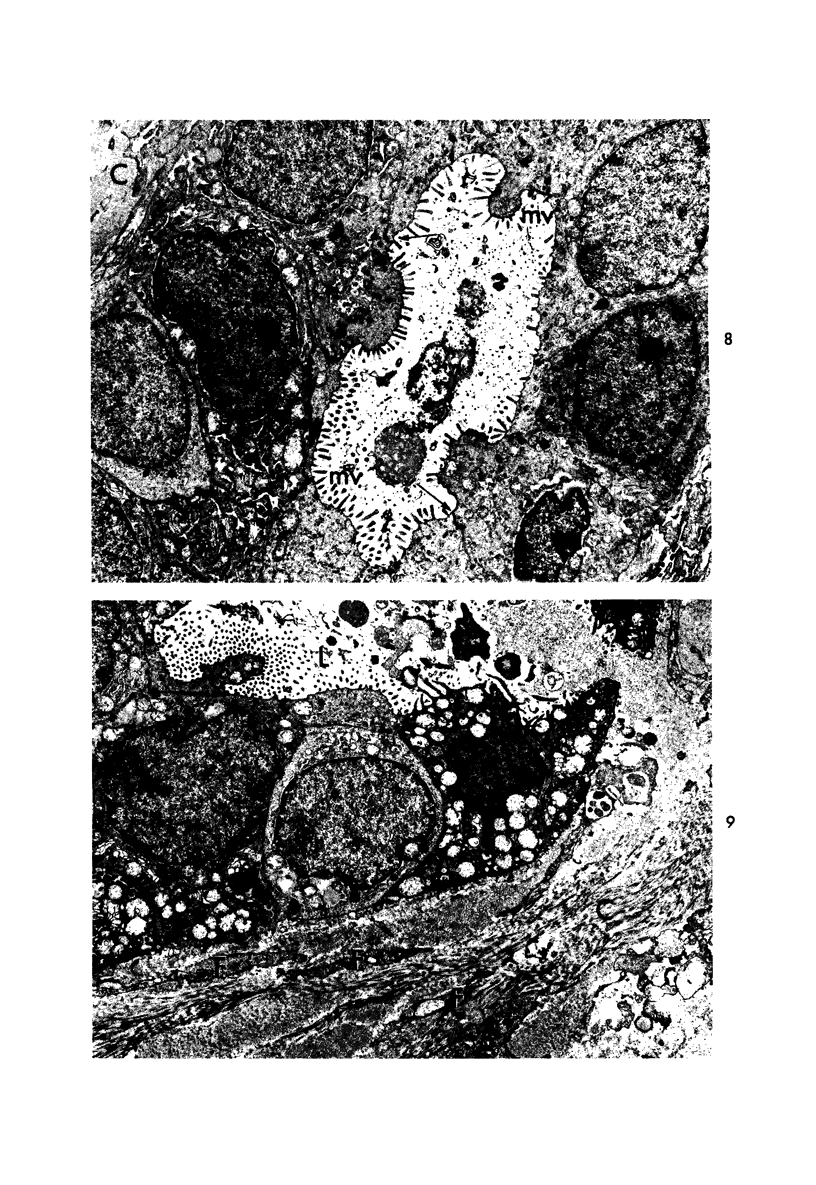
Abstract
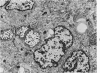
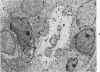
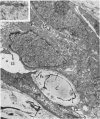
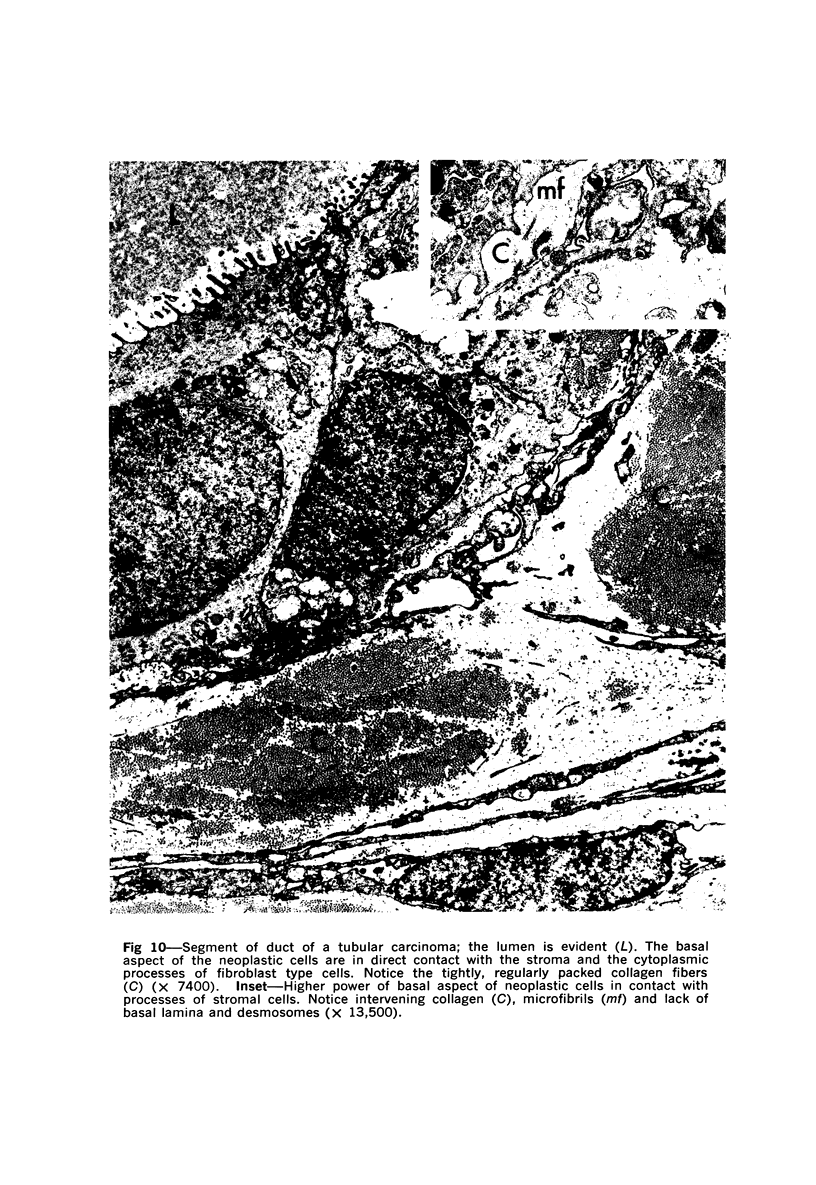
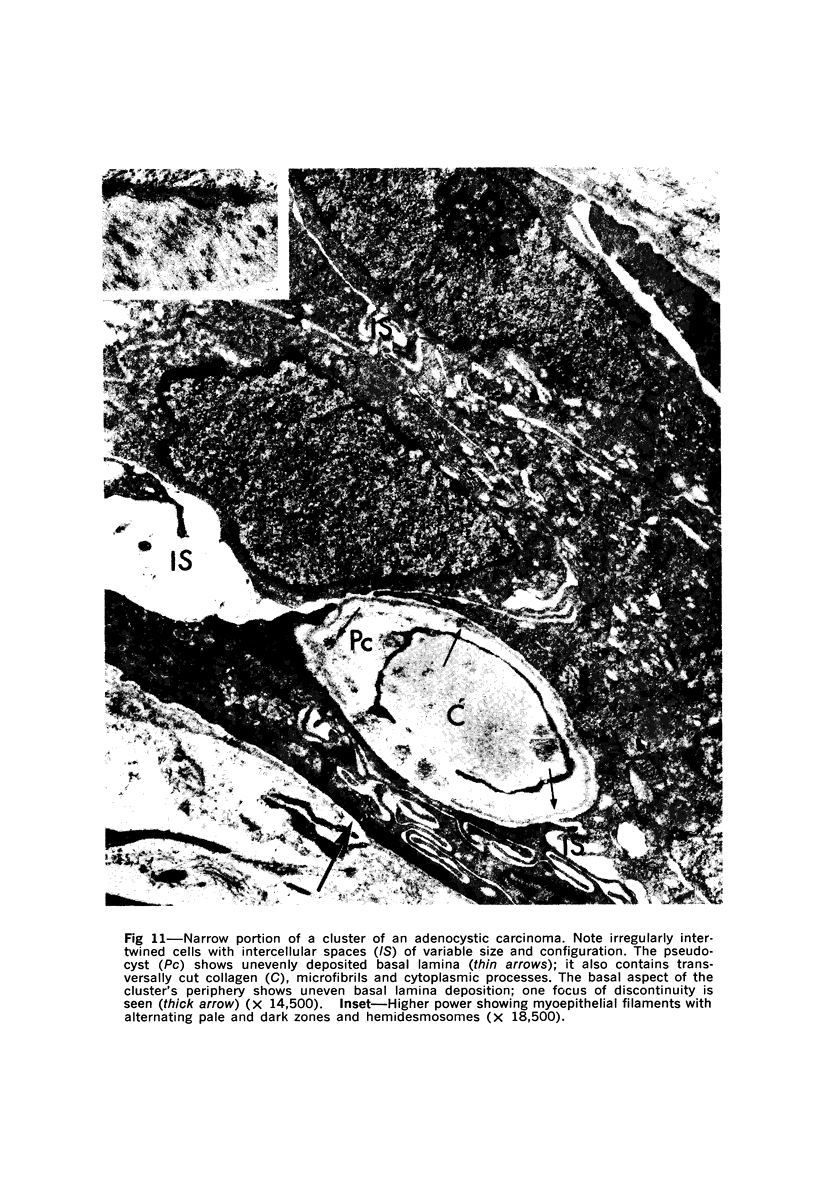
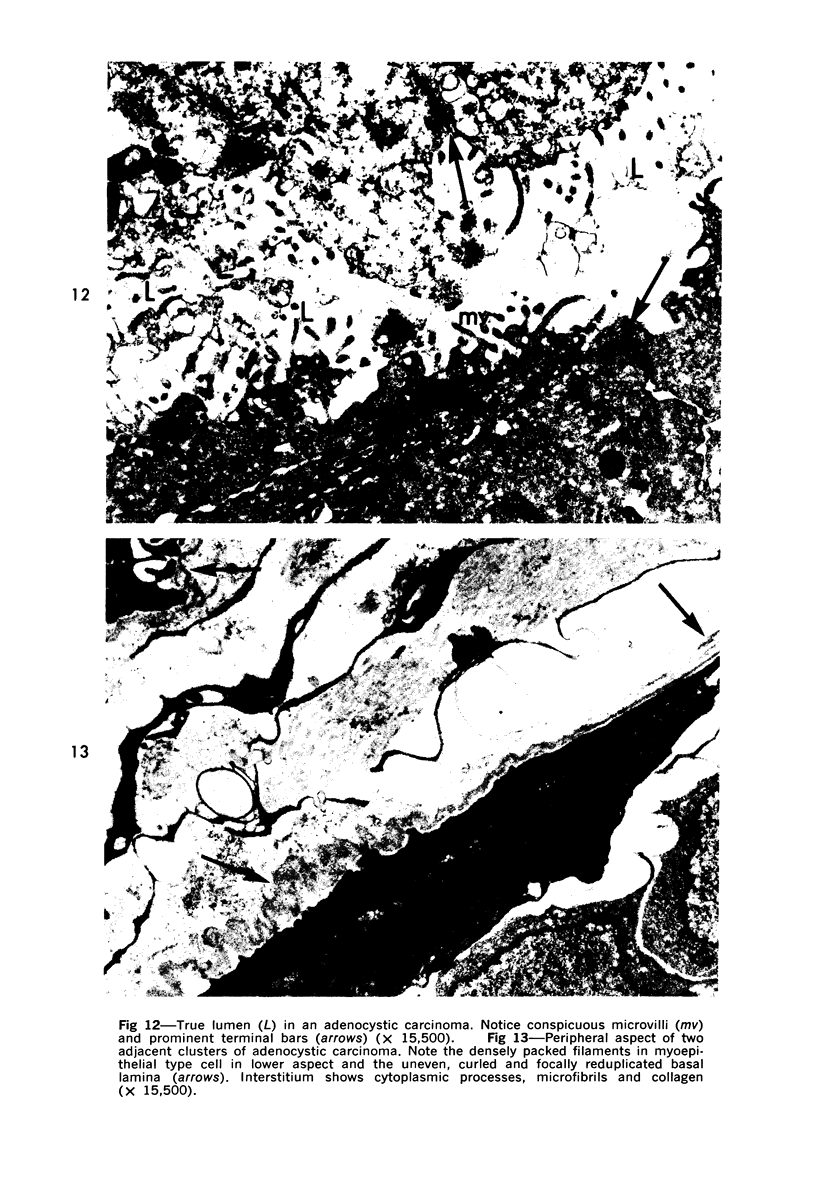
Samples from medullary, intraductal, tubular and adenocystic breast carcinomas were studied ultrastructurally, with emphasis on the patterns of myoepithelial differentiation and basal lamina deposition. Myoepithelial cells, while prominent in the intraductal and adenocystic carcinomas, were rarely found in the medullary neoplasms and appeared absent in the tubular neoplasms. Parallel to the above, basal laminae were most abundant and evenly deposited in the intraductal and adenocystic tumors, but were infrequent in the medullary tumors and seemingly absent in the tubular carcinomas. Well-defined myoepithelial cells and retention of the capability to synthesize basal lamina are evidence of differentiation in neoplastic cell populations. Thus, their presence in intraductal and adenocystic carcinomas (generally associated with a good prognosis) is not surprising. However, their scarcity or absence in the similarly favorable group of medullary and tubular carcinomas suggests that other factors may also influence the invasive and metastasizing ability of breast carcinomas.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eneroth C. M., Hjertman L., Moberger G., Wersäll J. Ultrastructural characteristics of adenoid cystic carcinoma of salivary glands. Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1968;192(4):358–368. doi: 10.1007/BF00411131. [DOI] [PubMed] [Google Scholar]
- Erlandson R. A., Carstens P. H. Ultrastructure of tubular carcinoma of the breast. Cancer. 1972 Apr;29(4):987–995. doi: 10.1002/1097-0142(197204)29:4<987::aid-cncr2820290446>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Goldenberg V. E., Goldenberg N. S., Sommers S. C. Comparative ultrastructure of atypical ductal hyperplasia, intraductal carcinoma, and infiltrating ductal carcinoma of the breast. Cancer. 1969 Dec;24(6):1152–1169. doi: 10.1002/1097-0142(196912)24:6<1152::aid-cncr2820240614>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Gould N. S., Benditt E. P. Ultrastructural aspects of papillary and sclerosing carcinomas of the thyroid. Cancer. 1972 Jun;29(6):1613–1625. doi: 10.1002/1097-0142(197206)29:6<1613::aid-cncr2820290628>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970;53:161–220. [PubMed] [Google Scholar]
- Koss L. G., Brannan C. D., Ashikari R. Histologic and ultrastructural features of adenoid cystic carcinoma of the breast. Cancer. 1970 Dec;26(6):1271–1279. doi: 10.1002/1097-0142(197012)26:6<1271::aid-cncr2820260614>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud J., Morin J. Ultrastructure d'un épithélioma médullaire de la glande mammaire. Laval Med. 1971 May;42(5):496–507. [PubMed] [Google Scholar]
- Murad T. M., Scharpelli D. G. The ultrastructure of medullary and scirrhous mammary duct carcinoma. Am J Pathol. 1967 Feb;50(2):335–360. [PMC free article] [PubMed] [Google Scholar]
- Ozzello L. Epithelial-stromal junction of normal and dysplastic mammary glands. Cancer. 1970 Mar;25(3):586–600. doi: 10.1002/1097-0142(197003)25:3<586::aid-cncr2820250314>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ozzello L., Sanpitak P. Epithelial-stromal junction of intraductal carcinoma of the breast. Cancer. 1970 Dec;26(6):1186–1198. doi: 10.1002/1097-0142(197012)26:6<1186::aid-cncr2820260603>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Ozzello L. Ultrastructure of intra-epithelial carcinomas of the breast. Cancer. 1971 Dec;28(6):1508–1515. doi: 10.1002/1097-0142(197112)28:6<1508::aid-cncr2820280625>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- Tandler B. Ultrastructure of adenoid cystic carcinoma of salivary gland origin. Lab Invest. 1971 Jun;24(6):504–512. [PubMed] [Google Scholar]
- Thrasher T. V., Richart R. M. An ultrastructural comparison of endometrial adenocarcinoma and normal endometrium. Cancer. 1972 Jun;29(6):1713–1723. doi: 10.1002/1097-0142(197206)29:6<1713::aid-cncr2820290641>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- WELLINGS S. R., ROBERTS P. Electron microscopy of sclerosing adenosis and infiltrating duct carcinoma of the human mammary gland. J Natl Cancer Inst. 1963 Feb;30:269–287. [PubMed] [Google Scholar]