Abstract
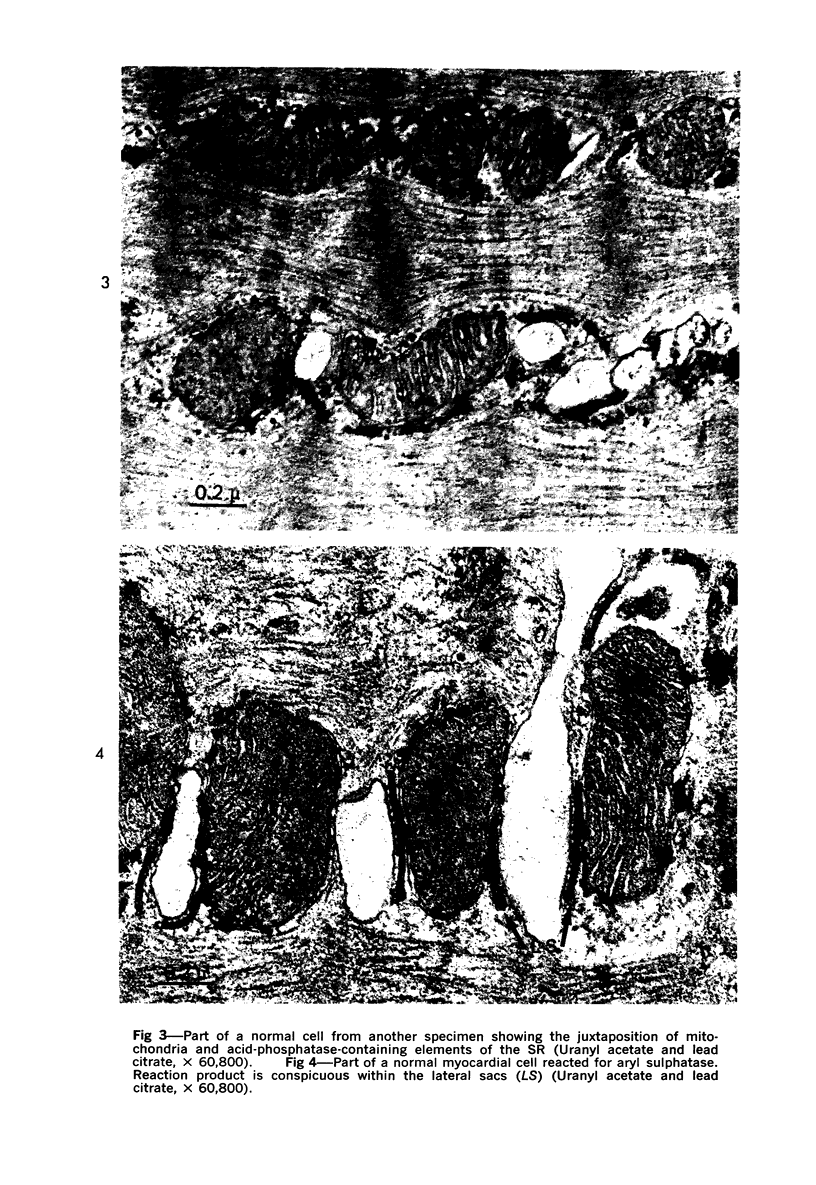
The effect of ischemia on the integrity of myocardial lysosomes was observed 3 1/2 and 24 hours after the production of infarcts in 20 anesthetized closed-chest dogs by electrically induced thrombosis of the left anterior descending coronary artery. Biopsies from normal, marginal and infarcted areas were fixed and incubated to localize the lysosomal enzymes acid phosphatase and aryl sulphatase. Reaction product in normal cells was localized in small circular or oblong profiles between bundles of myofilaments and adjacent to mitochondria. In addition, curvilinear, membrane-bound profiles containing reaction product were found in close apposition to transverse tubules and near the free margins of the myocardial cells. Thus the distribution of elements of the sarcoplasmic reticulum. Additional reaction product was also seen in residual bodies, on myelin figures, and in the few conventional appearing spherical lysosomes. Little or no acid phosphatase or aryl sulphatase reaction product was seen in the sarcoplasmic reticulum of infarcted myocardium. The degree of cellular degeneration correlated with disappearance of enzyme activity from the sarcoplasmic reticulum and included disruption of membranes and loss of mitochondrial matrix and erosion of I but not A bands. Marginal areas showed variable amounts of cellular degeneration. Separation of myofilament bundles and loss of glycogen correlated with the localized disappearance of acid phosphatase and aryl sulphatase activity in marginal tissue. Disruption of mitochondrial and erosion of I bands correlated with extensive loss of these enzymes. The data suggest that degeneration of myocardial cells following ischemic injury is associated with release of endogenous lysosomal enzymes from the sarcoplasmic reticulum.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R., Morris M., Smith J. Histochemistry of lysosomes in rat heart muscle. J Histochem Cytochem. 1967 Oct;15(10):596–599. doi: 10.1177/15.10.596. [DOI] [PubMed] [Google Scholar]
- Arcangeli P., Del Soldato P., Digiesi V., Melani F. Changes in the activities of lysosomal enzymes in striated muscle following ischemia. Life Sci II. 1973 Jan 8;12(1):13–23. doi: 10.1016/0024-3205(73)90175-6. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. II. Cytochemistry and electron microscopy of bone marrow cells. J Cell Biol. 1968 Nov;39(2):299–317. doi: 10.1083/jcb.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing R. J. Reparative processes in heart muscle following myocardial infarction. Cardiology. 1971;56(1):314–324. doi: 10.1159/000169375. [DOI] [PubMed] [Google Scholar]
- Brunk U. T., Ericsson J. L. Cytochemical evidence for the leakage of acid phosphatase through ultrastructurally intact lysosomal membranes. Histochem J. 1972 Nov;4(6):479–491. doi: 10.1007/BF01011128. [DOI] [PubMed] [Google Scholar]
- Brunk U. T., Ericsson J. L. The demonstration of acid phosphatase in vitro cultured tissue cells. Studies on the significance of fixation, tonicity and permeability. Histochem J. 1972 Jul;4(4):349–363. doi: 10.1007/BF01005009. [DOI] [PubMed] [Google Scholar]
- Buchanan W. E., Schwartz T. B. Lysosomal enzyme activity in heart and skeletal muscle of cortisone-treated rats. Am J Physiol. 1967 Apr;212(4):732–736. doi: 10.1152/ajplegacy.1967.212.4.732. [DOI] [PubMed] [Google Scholar]
- Canonico P. G., Bird J. W. Lysosomes in skeletal muscle tissue. Zonal centrifugation evidence for multiple cellular sources. J Cell Biol. 1970 May;45(2):321–333. doi: 10.1083/jcb.45.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., BEAUFAY H. Tissue fractionation studies. 10. Influence of ischaemia on the state of some bound enzymes in rat liver. Biochem J. 1959 Dec;73:610–616. doi: 10.1042/bj0730610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S. The cytochemical demonstration of lysosomal aryl sulfatase activity by light and electron microscopy. J Histochem Cytochem. 1965 Jul-Aug;13(6):520–523. doi: 10.1177/13.6.520. [DOI] [PubMed] [Google Scholar]
- Heggtveit H. A. Morphological alterations in the ischaemic heart. Cardiology. 1971;56(1):284–290. doi: 10.1159/000169372. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Gennaro D. E., Fox A. C., Hirsch J., Streuli F., Weissmann G. Colloidal lanthanum as a marker for impaired plasma membrane permeability in ischemic dog myocardium. Am J Pathol. 1975 May;79(2):207–218. [PMC free article] [PubMed] [Google Scholar]
- Hopsu-Havu V. K., Arstila A. U., Helminen H. J., Kalimo H. O. Improvements in the method for the electron microscopic localization of arylsulphatase activity. Histochemie. 1967;8(1):54–64. doi: 10.1007/BF00279874. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Leighty E. G., Stoner C. D., Ressallat M. M., Passananti G. T., Sirak H. D. Effects of acute asphyxia and deep hypothermia on the state of binding of lysosomal acid hydrolases in canine cardiac muscle. Circ Res. 1967 Jul;21(1):59–64. doi: 10.1161/01.res.21.1.59. [DOI] [PubMed] [Google Scholar]
- Nelson B. D. Hepatic lysosome and serum enzyme alterations in rats exposed to high altitude. Am J Physiol. 1966 Sep;211(3):651–655. doi: 10.1152/ajplegacy.1966.211.3.651. [DOI] [PubMed] [Google Scholar]
- Pearce G. W. Electron microscopy in the study of muscular dystrophy. Ann N Y Acad Sci. 1966 Sep 9;138(1):138–150. doi: 10.1111/j.1749-6632.1966.tb41162.x. [DOI] [PubMed] [Google Scholar]
- Ravens K. G., Gudbjarnason S. Changes in the activities of lysosomal enzymes in infarcted canine heart muscle. Circ Res. 1969 Jun;24(6):851–856. doi: 10.1161/01.res.24.6.851. [DOI] [PubMed] [Google Scholar]
- Ricciutti M. A. Lysosomes and myocardial cellular injury. Am J Cardiol. 1972 Oct;30(5):498–502. doi: 10.1016/0002-9149(72)90040-9. [DOI] [PubMed] [Google Scholar]
- Ricciutti M. A. Myocardial lysosome stability in the early stages of acute ischemic injury. Am J Cardiol. 1972 Oct;30(5):492–497. doi: 10.1016/0002-9149(72)90039-2. [DOI] [PubMed] [Google Scholar]
- SHNITKA T. K., NACHLAS M. M. Histochemical alterations in ischemic heart muscle and early myocardial infarction. Am J Pathol. 1963 May;42:507–527. [PMC free article] [PubMed] [Google Scholar]
- Topping T. M., Travis D. F. An electron cytochemical study of mechanisms of lysosomal activity in the rat left ventricular mural myocardium. J Ultrastruct Res. 1974 Jan;46(1):1–22. doi: 10.1016/s0022-5320(74)80018-3. [DOI] [PubMed] [Google Scholar]
- VAN LANCKER J. L., HOLTZER R. L. The release of acid phosphatase and beta-glucuronidase from cytoplasmic granules in the early course of autolysis. Am J Pathol. 1959 May-Jun;35(3):563–573. [PMC free article] [PubMed] [Google Scholar]