Abstract
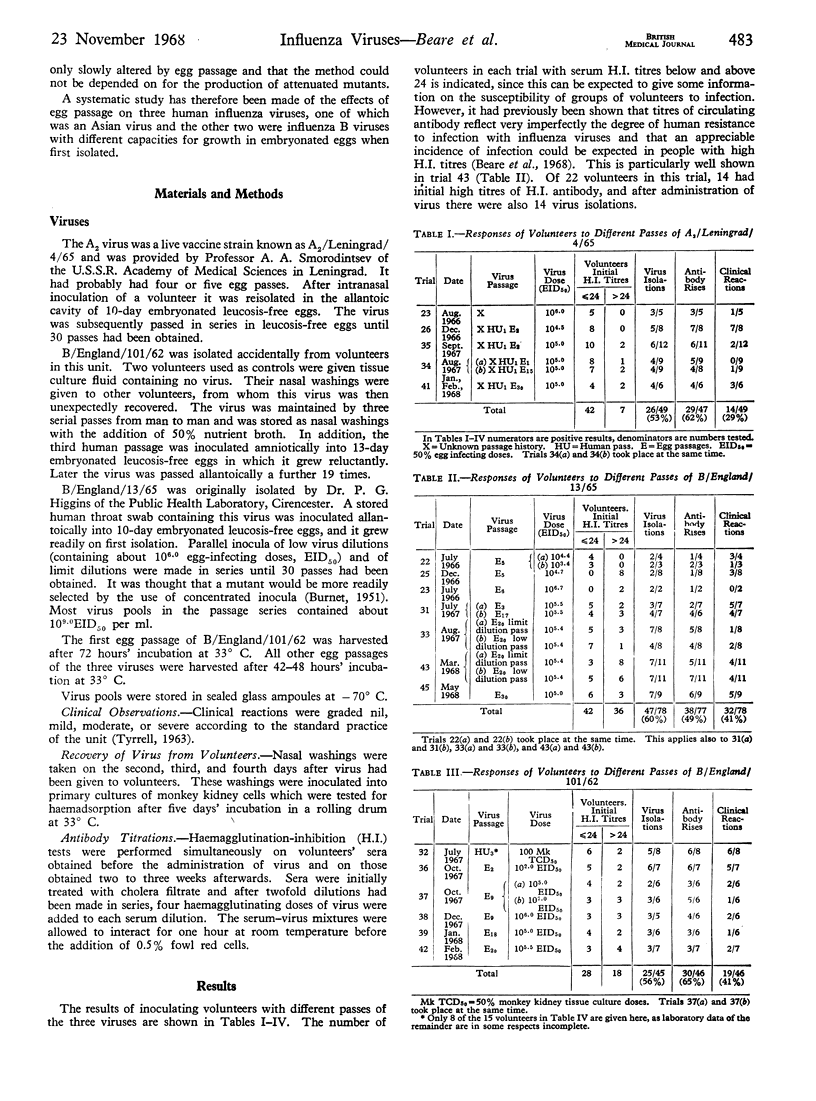
For vaccination live viruses are better than dead ones, but live influenza vaccines are difficult to prepare. One influenza A2 and two influenza B viruses were passed in series in embryonated eggs. At several stages of their passage they were inoculated into volunteers, and their effects assessed by virus isolations, antibody rises, and clinical reactions. The A2 virus and one of the influenza B viruses, both of which had grown readily in embryonated eggs on first isolation, continued to induce human infections and clinical reactions after 30 egg passes. The other influenza B virus acquired enhanced human pathogenicity after three passages from man to man. After adaptation to eggs in which it at first grew reluctantly, its human virulence was appreciably reduced. It underwent no further change during a total of 20 egg passes. There was little convincing evidence of an increased incidence of clinical reactions during the winter seasons, but the numbers of volunteers were too small to draw definite conclusions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- McDonald J. C., Zuckerman A. J., Beare A. S., Tyrrell D. A. Trials of Live Influenza Vaccine in the Royal Air Force. Br Med J. 1962 Apr 14;1(5284):1036–1042. doi: 10.1136/bmj.1.5284.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHDANOV V. M., RITOVA V. V., ORLOVA A. V., SOKOLOVA N. N. The characteristics of influenza-virus strains isolated in 1957. Lancet. 1957 Oct 12;273(6998):735–736. doi: 10.1016/s0140-6736(57)92272-9. [DOI] [PubMed] [Google Scholar]


