Abstract
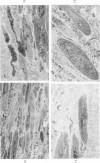
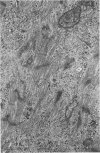
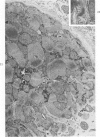
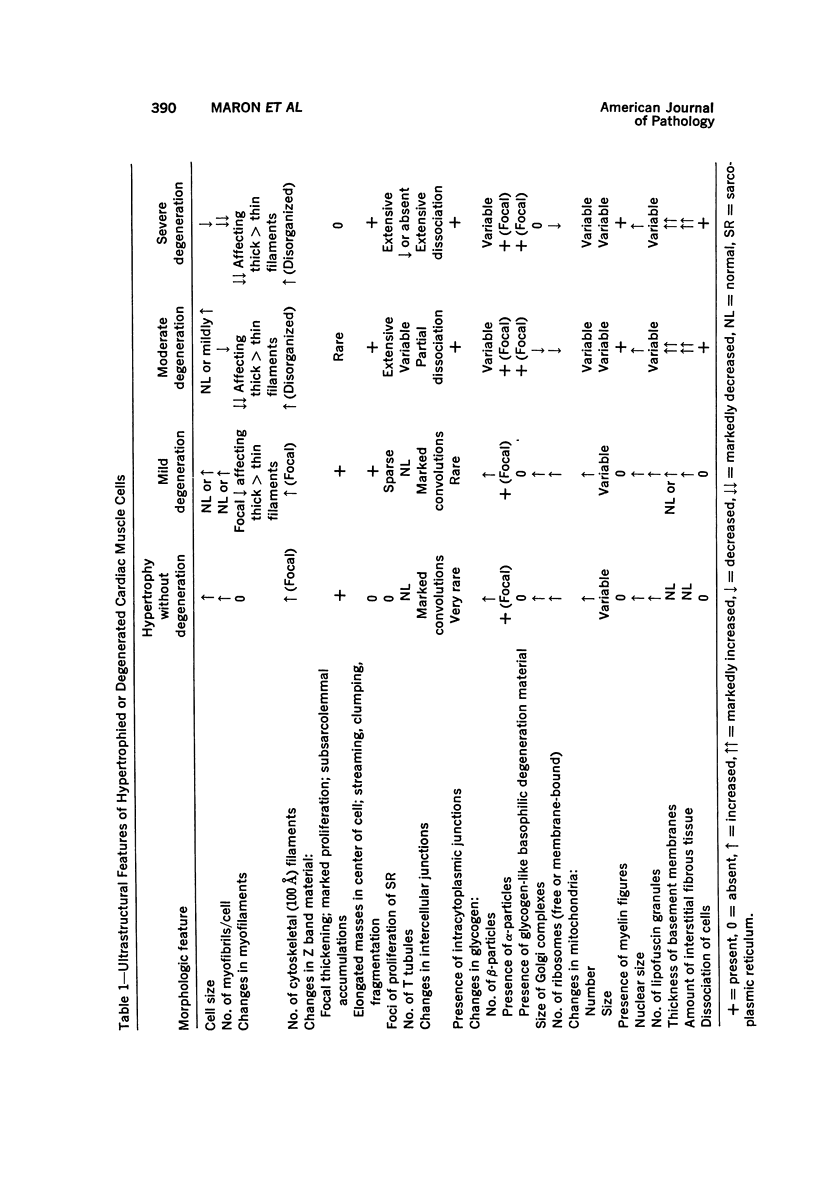
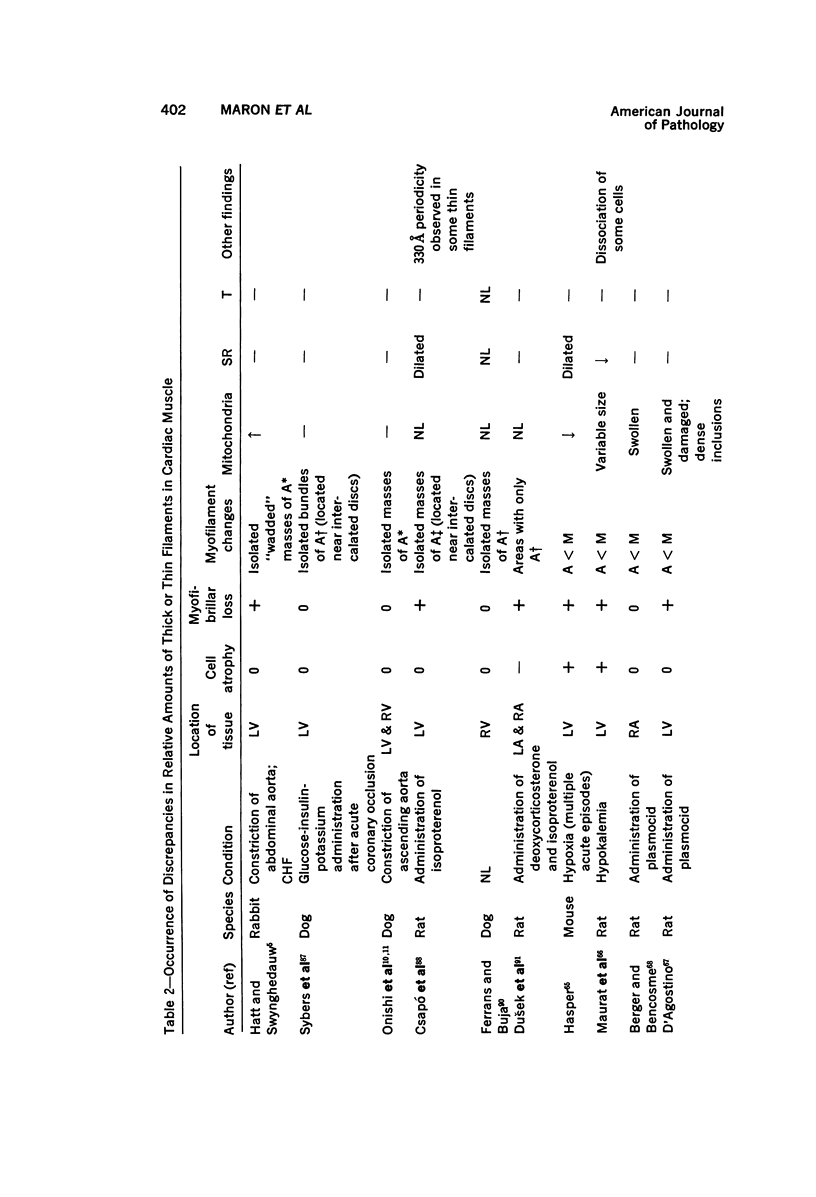
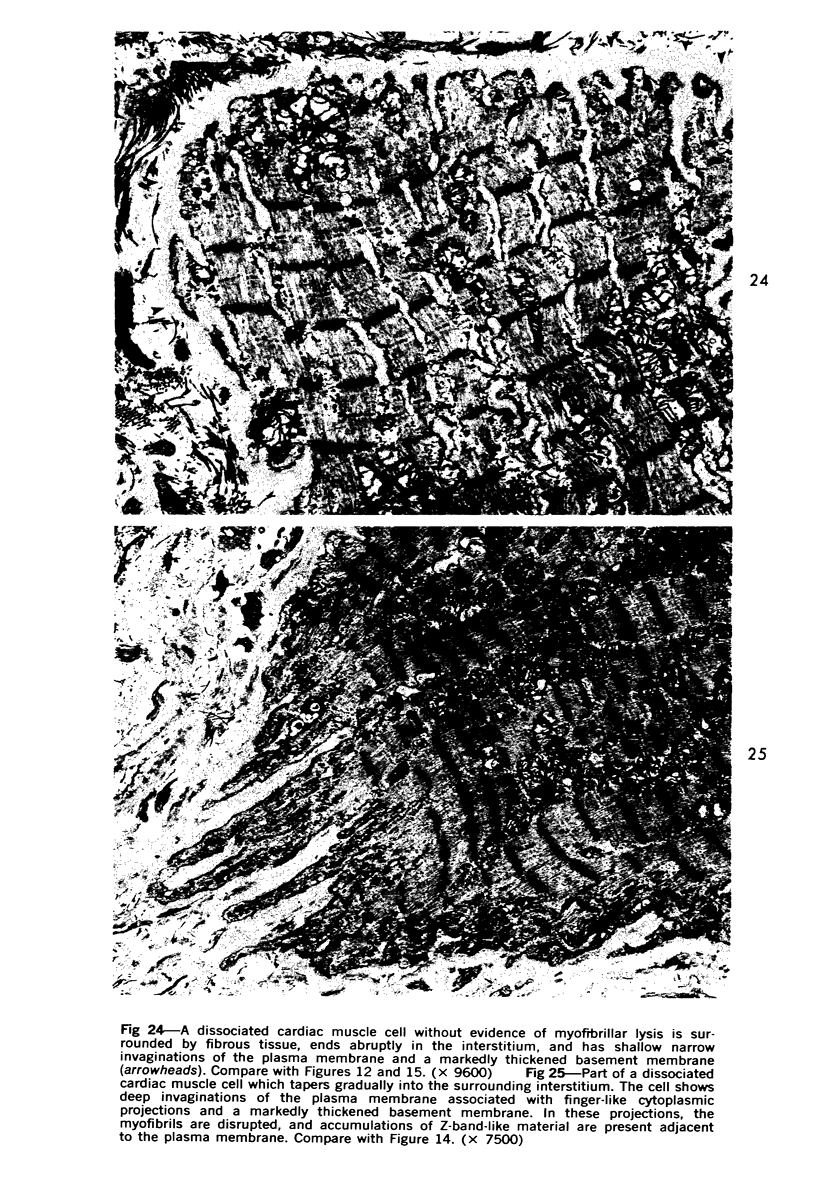
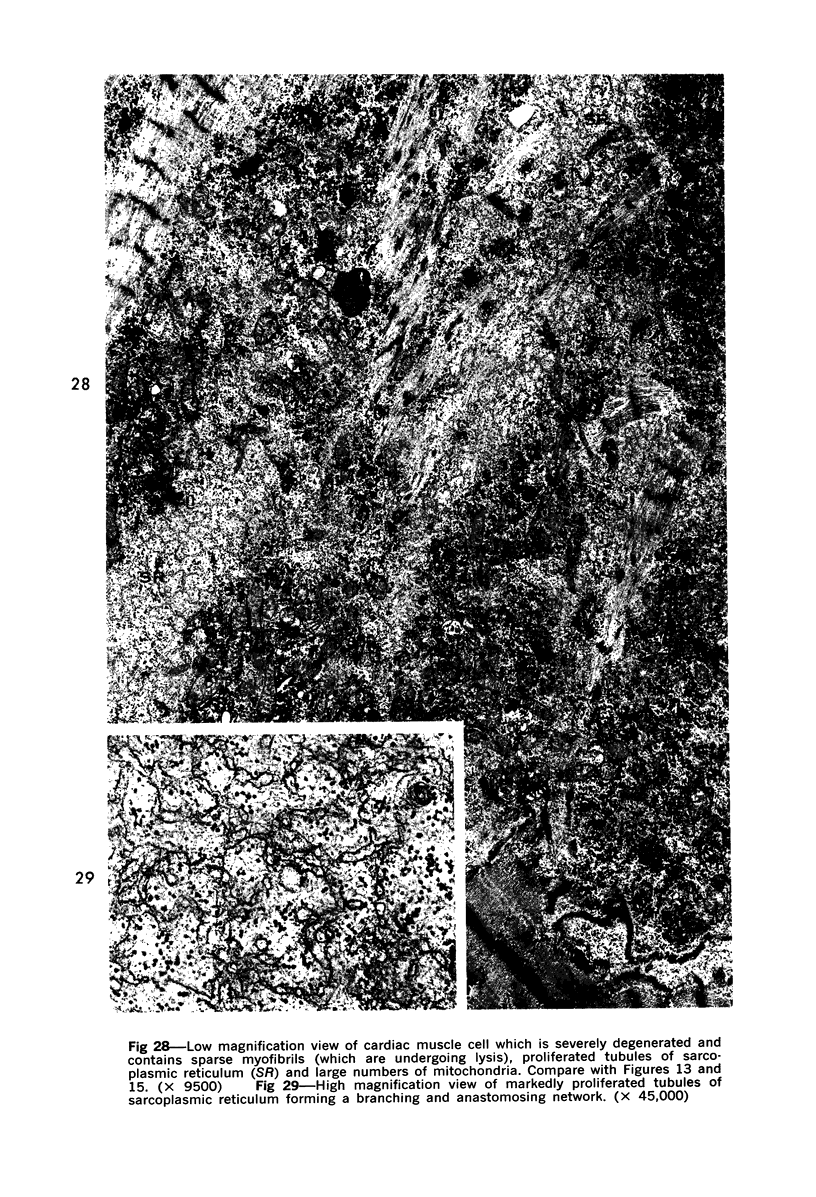
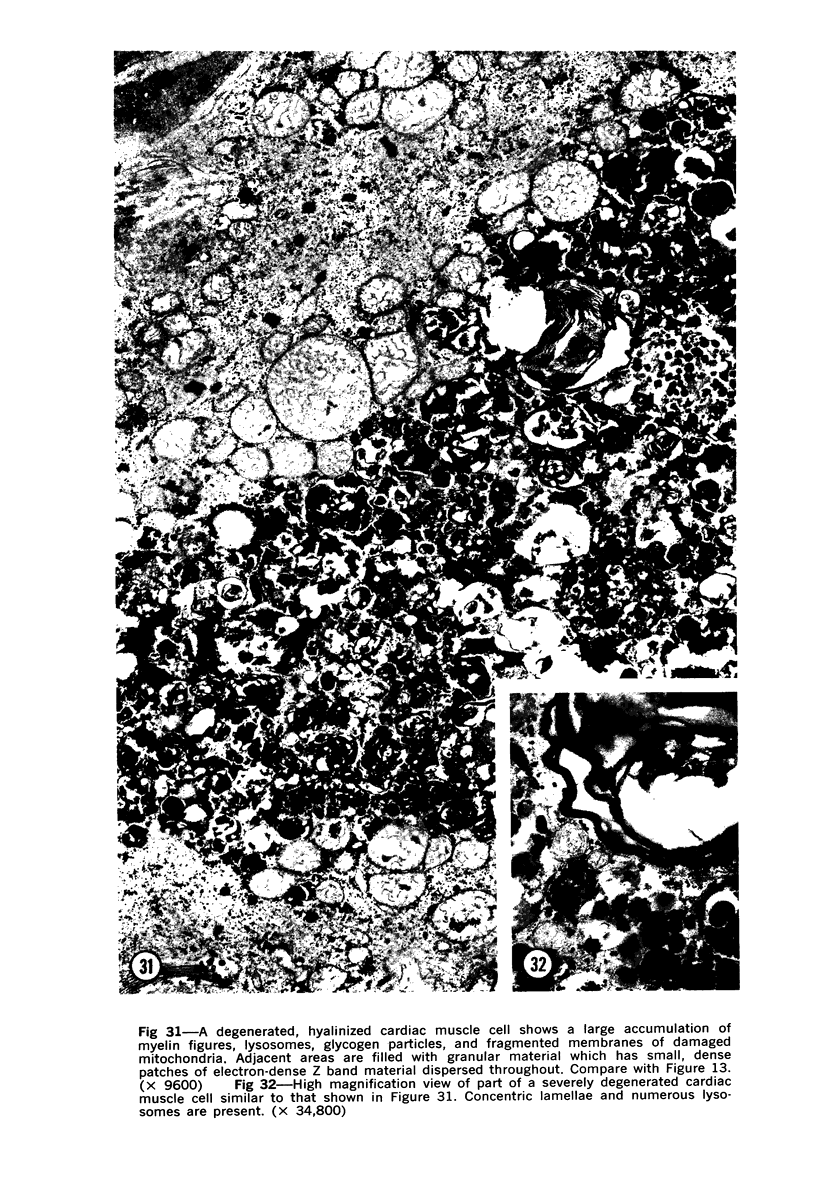
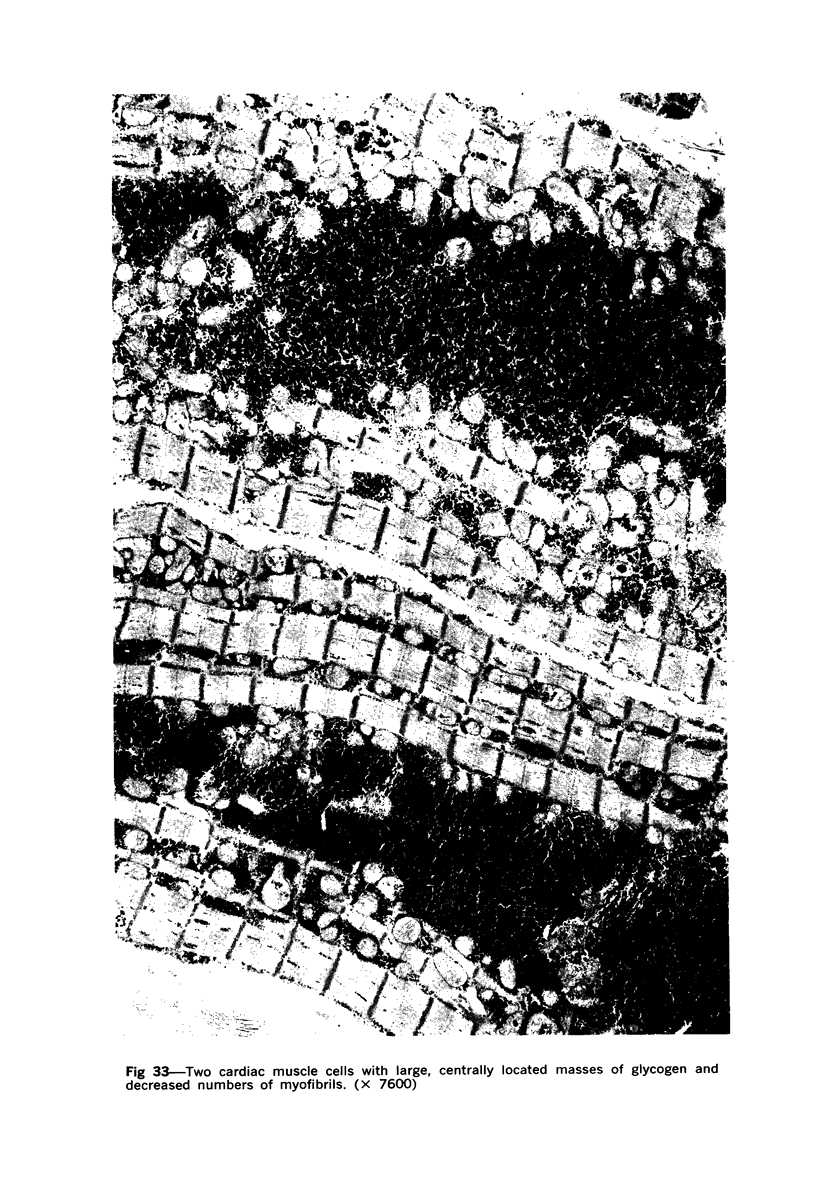
Degenerated cardiac muscle cells were present in hypertrophied ventricular muscle obtained at operation from 12 (38%) of 32 patients with asymmetric septal hypertrophy (hypertrophic cardiomyopathy) or aortic valvular disease. Degenerated cells demonstrated a wide variety of ultrastructural alterations. Mildly altered cells were normal-sized or hypertrophied and showed focal changes, including preferential loss of thick (myosin) filaments, streaming and clumping of Z band material, and proliferation of the tubules of sarcoplasmic reticulum. Moderately and severely degenerated cells were normal-sized or atrophic and showed additional changes, including extensive myofibrillar lysis and loss of T tubules. The appearance of the most severely degenerated cells usually reflected the cytoplasmic organelle (sarcoplasmic reticulum, glycogen, or mitochondria) which underwent proliferation and filled the myofibril-free areas of these cells. Moderately and severely degenerated cells were present in areas of fibrosis, had thickened basement membranes, and had lost their intercellular connections. These observations suggest that degenerated cardiac muscle cells have poor contractile function and may be responsible for impaired cardiac performance in some patients with chronic ventricular hypertrophy.
Full text
PDF













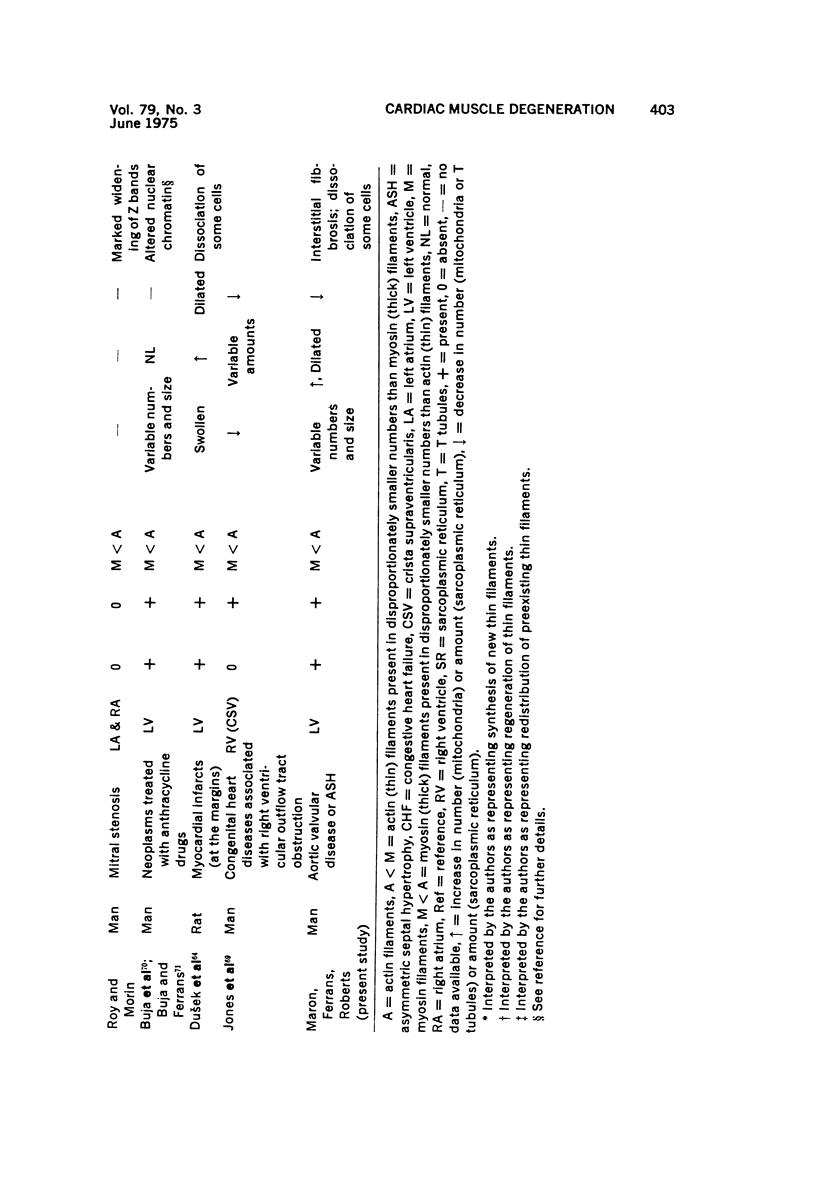
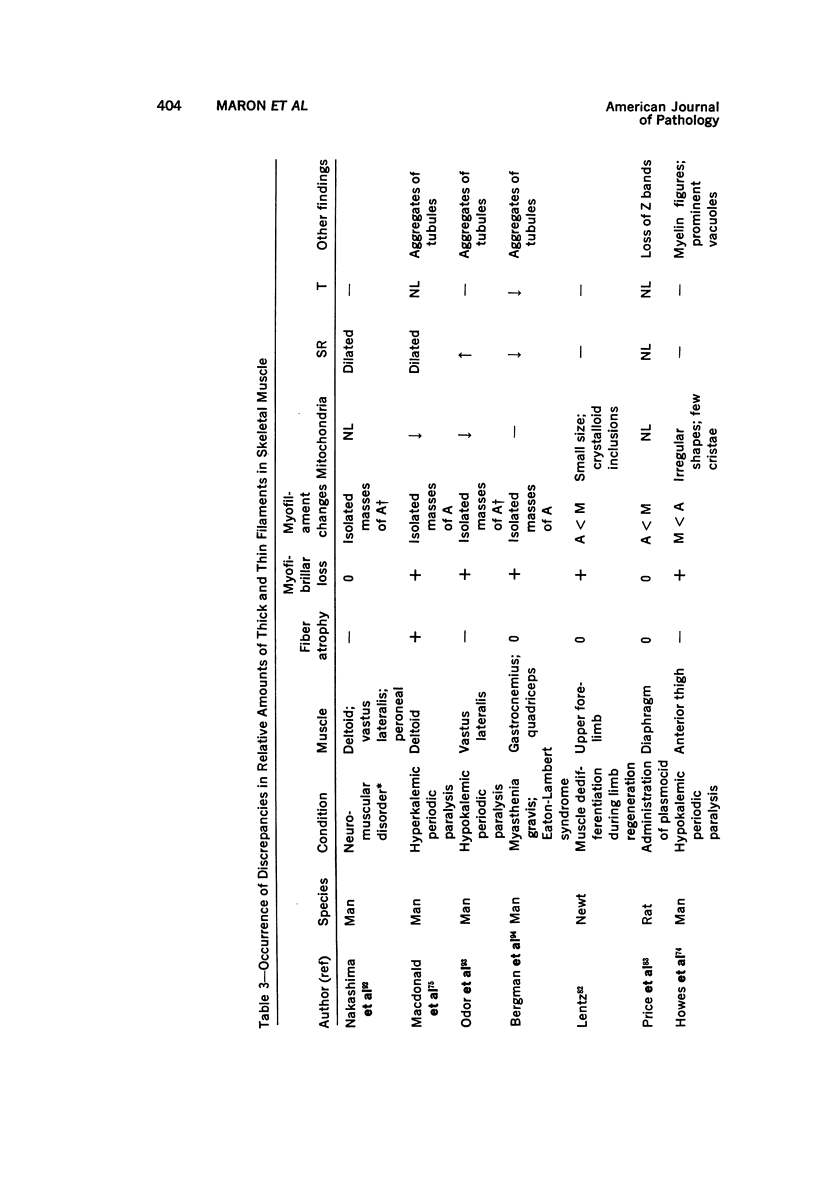
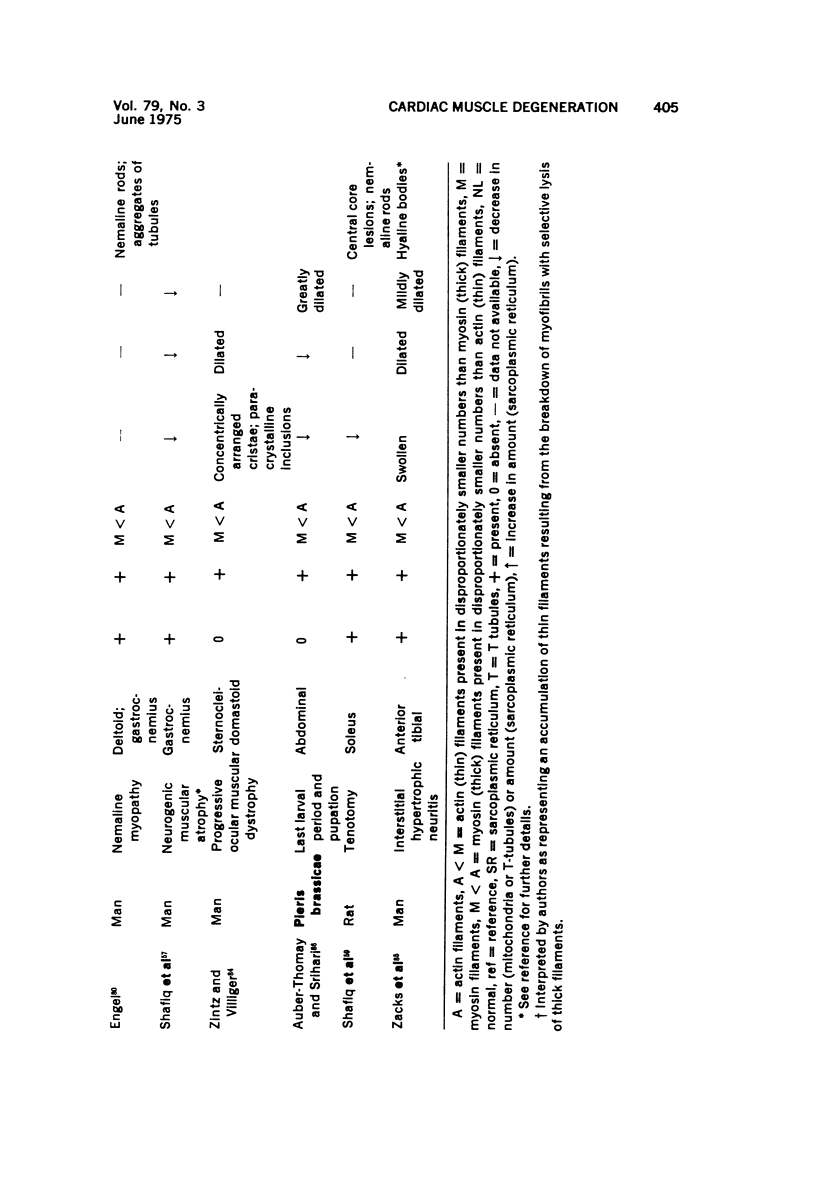














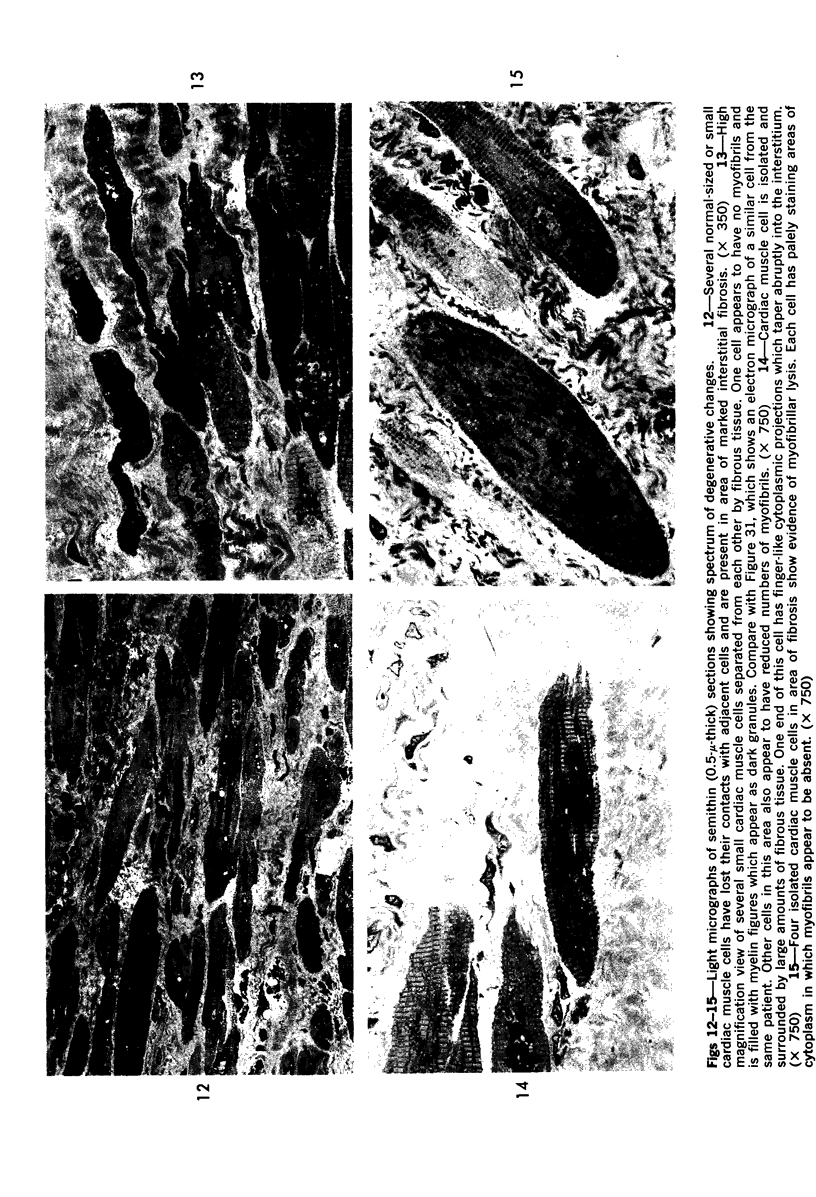
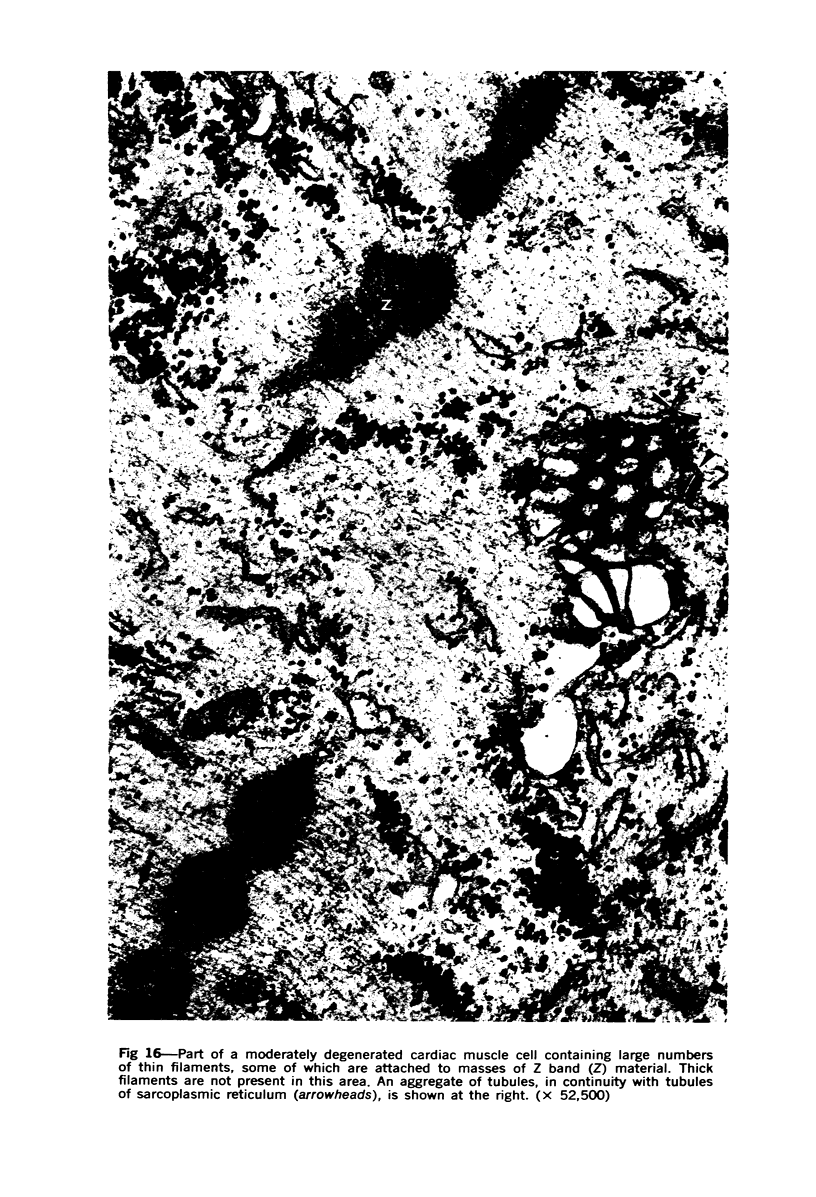
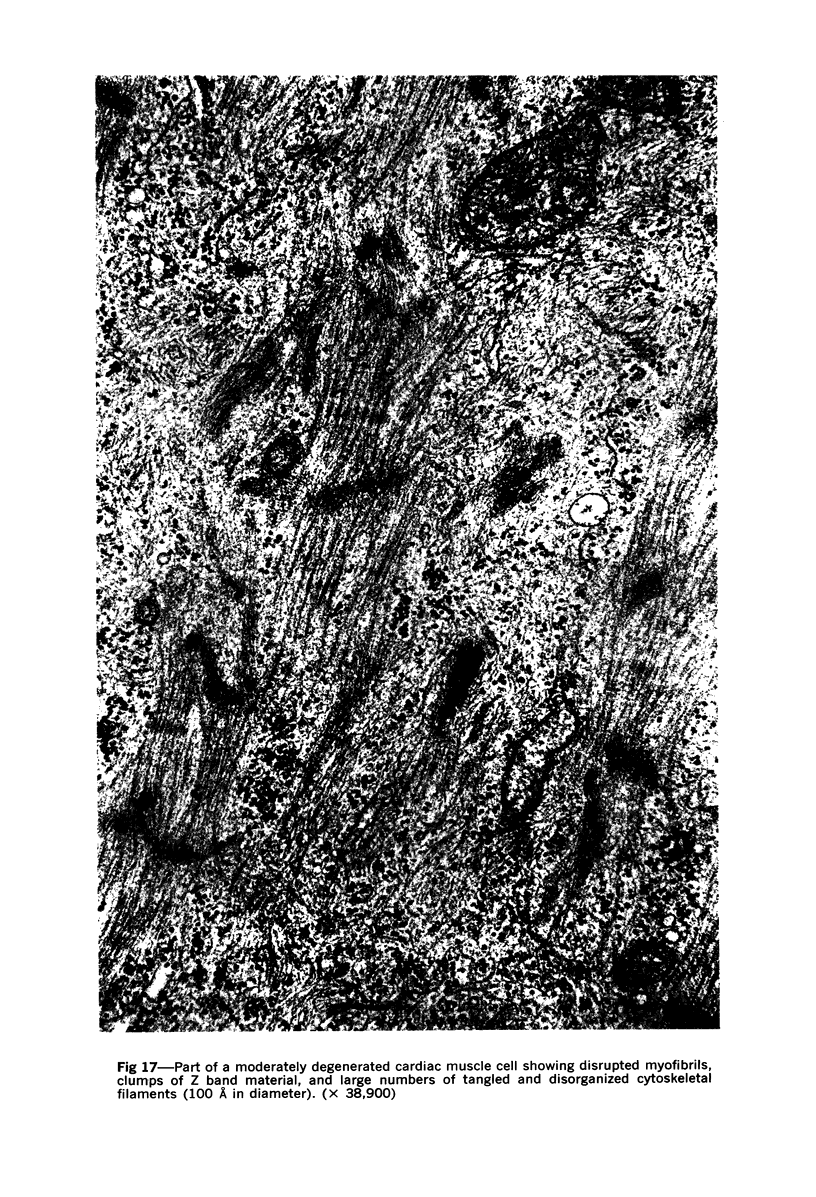
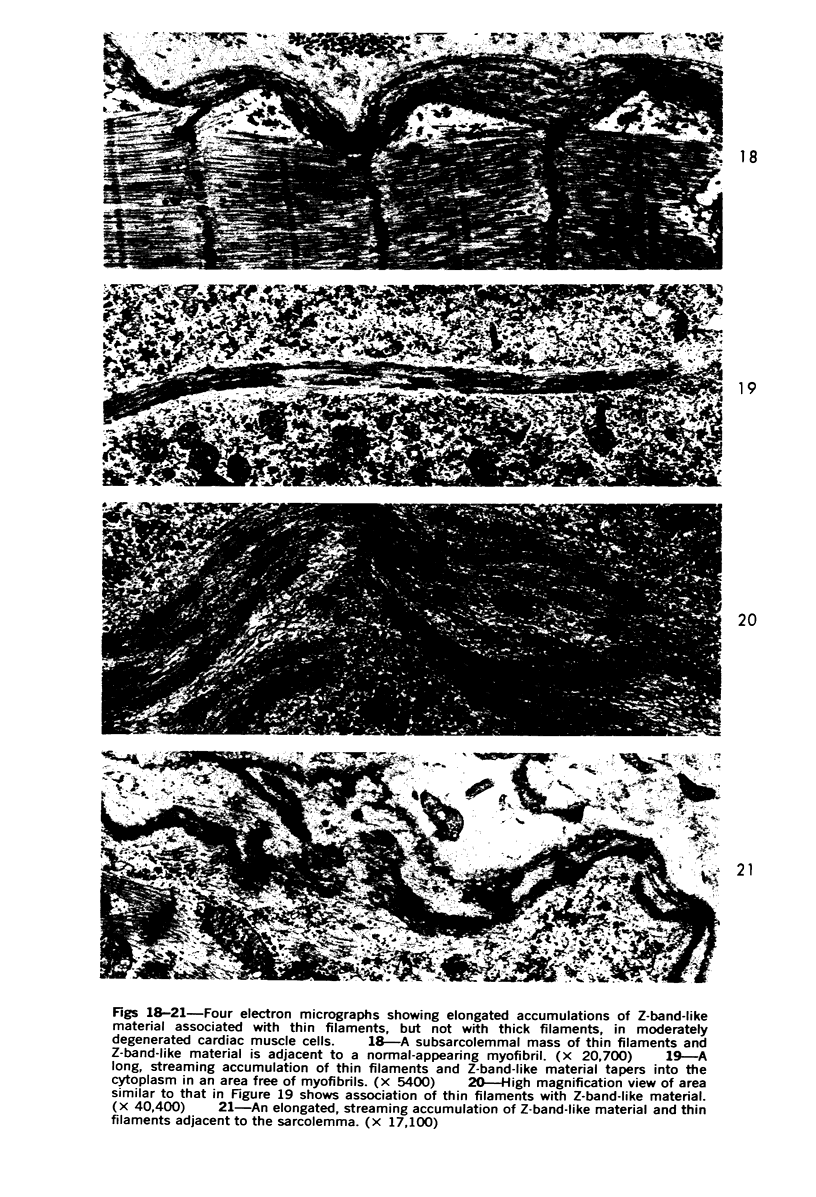
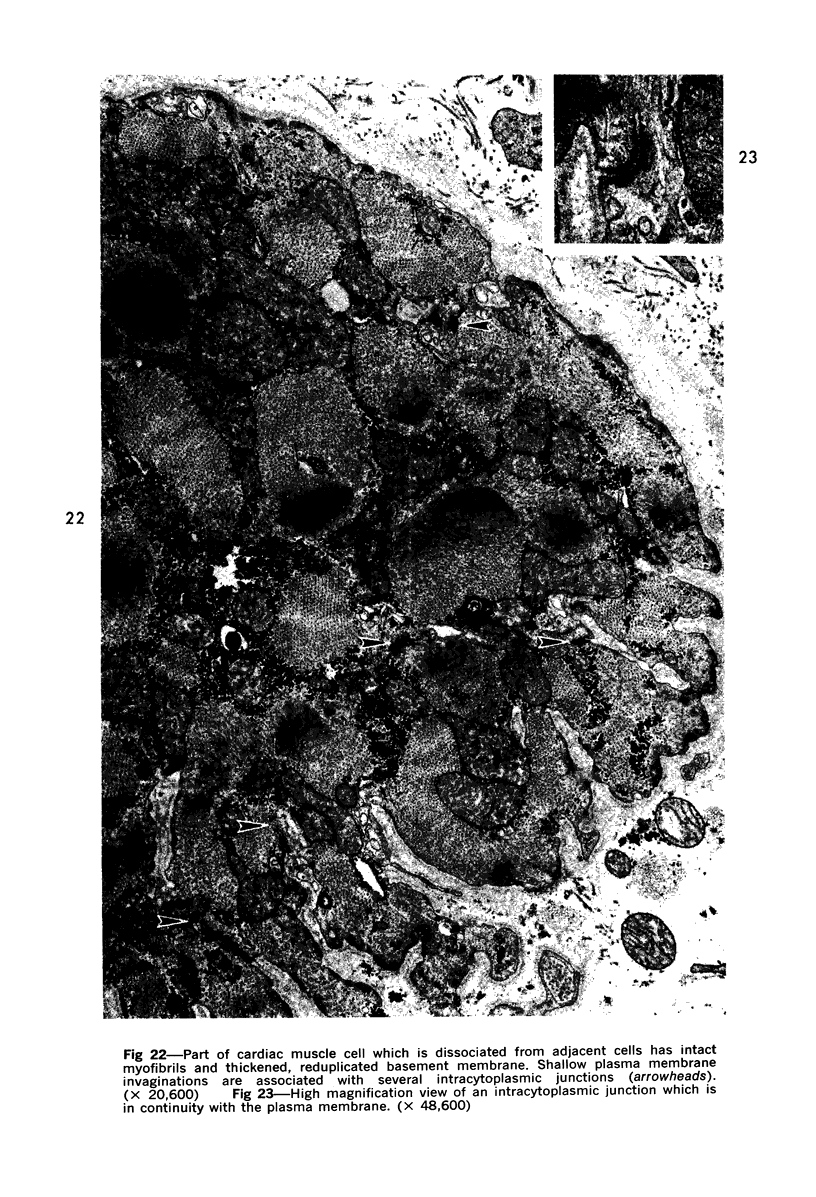

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A., Johnson G. S., Pastan I., Pollard T. D. Isolation and characterization of myosin from cloned mouse fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3693–3697. doi: 10.1073/pnas.69.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelstein R. S., Pollard T. D., Kuehl W. M. Isolation and characterization of myosin and two myosin fragments from human blood platelets. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2703–2707. doi: 10.1073/pnas.68.11.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C. S. Electron microscopic observations in alcoholic heart disease. Br Heart J. 1967 Mar;29(2):200–206. doi: 10.1136/hrt.29.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger C., Chenard J. Quebec beer-drinkers' cardiomyopathy: ultrastructural changes in one case. Can Med Assoc J. 1967 Oct 7;97(15):916–921. [PMC free article] [PubMed] [Google Scholar]
- Berger J. M., Bencosme S. A. Divergence in patterns of atrial and ventricular cardiocyte degeneration: studies with plasmocid. J Mol Cell Cardiol. 1971 Mar;2(1):41–50. doi: 10.1016/0022-2828(71)90077-0. [DOI] [PubMed] [Google Scholar]
- Bergman R. A., Johns R. J. Ultrastructural alterations in muscle from patients with myasthenia gravis and Eaton-Lumbert syndrome. Ann N Y Acad Sci. 1971 Sep 15;183:88–122. doi: 10.1111/j.1749-6632.1971.tb30744.x. [DOI] [PubMed] [Google Scholar]
- Bishop S. P., Cole C. R. Ultrastructural changes in the canine myocardium with right ventricular hypertrophy and congestive heart failure. Lab Invest. 1969 Mar;20(3):219–229. [PubMed] [Google Scholar]
- Buja L. M., Ferrans V. J., Maron B. J. Intracytoplasmic junctions in cardiac muscle cells. Am J Pathol. 1974 Mar;74(3):613–647. [PMC free article] [PubMed] [Google Scholar]
- Buja L. M., Ferrans V. J., Mayer R. J., Roberts W. C., Henderson E. S. Cardiac ultrastructural changes induced by daunorubicin therapy. Cancer. 1973 Oct;32(4):771–788. doi: 10.1002/1097-0142(197310)32:4<771::aid-cncr2820320407>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Bulloch R. T., Murphy M. L., Pearce M. B. Fine structural lesions in the myocardium of a beer drinker with reversible heart failure. Am Heart J. 1970 Nov;80(5):629–637. doi: 10.1016/0002-8703(70)90009-8. [DOI] [PubMed] [Google Scholar]
- Colborn G. L., Carsey E., Jr Electron microscopy of the sinoatrial node of the squirrel monkey Saimiri sciureus. J Mol Cell Cardiol. 1972 Oct;4(5):525–536. doi: 10.1016/0022-2828(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Csapó Z., Dusek J., Rona G. Early alterations of the cardiac muscle cells in isoproterenol-induced necrosis. Arch Pathol. 1972 Apr;93(4):356–365. [PubMed] [Google Scholar]
- Csapó Z., Dusek J., Rona G. Peculiar myofilament changes near the intercalated disc in isoproterenol-induced cardiac muscle cell injury. J Mol Cell Cardiol. 1974 Feb;6(1):79–83. doi: 10.1016/0022-2828(74)90009-1. [DOI] [PubMed] [Google Scholar]
- Côté G., Mohiuddin S. M., Roy P. E. Occurrence of Z-band widening in human atrial cells. Exp Mol Pathol. 1970 Dec;13(3):307–318. doi: 10.1016/0014-4800(70)90093-6. [DOI] [PubMed] [Google Scholar]
- D AGOSTINO A. N. AN ELECTRON MICROSCOPIC STUDY OF SKELETAL AND CARDIAC MUSCLE OF THE RAT POISONED BY PLASMOCID. Lab Invest. 1963 Nov;12:1060–1071. [PubMed] [Google Scholar]
- Dusek J., Rona G., Kahn D. S. Healing process in the marginal zone of an experimental myocardial infarct. Findings in the surviving cardiac muscle cells. Am J Pathol. 1971 Mar;62(3):321–338. [PMC free article] [PubMed] [Google Scholar]
- Emans J. B., Jones A. L. Hypertrophy of liver cell smooth surfaced reticulum following progesterone administration. J Histochem Cytochem. 1968 Sep;16(9):561–571. doi: 10.1177/16.9.561. [DOI] [PubMed] [Google Scholar]
- Engel A. G. Late-onset rod myopathy (a new syndrome?): light and electron microscopic observations in two cases. Mayo Clin Proc. 1966 Nov;41(11):713–741. [PubMed] [Google Scholar]
- Engel A. G. Ultrastructural reactions in muscle disease. Med Clin North Am. 1968 Jul;52(4):909–931. [PubMed] [Google Scholar]
- FOUTS J. R., ROGERS L. A. MORPHOLOGICAL CHANGES IN THE LIVER ACCOMPANYING STIMULATION OF MICROSOMAL DRUG METABOLIZING ENZYME ACTIVITY BY PHENOBARBITAL, CHLORDANE, BENZPYRENE OR METHYL-CHOLANTHRENE IN RATS. J Pharmacol Exp Ther. 1965 Jan;147:112–119. [PubMed] [Google Scholar]
- FREEMAN J. A., SPURLOCK B. O. A new epoxy embedment for electron microscopy. J Cell Biol. 1962 Jun;13:437–443. doi: 10.1083/jcb.13.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W. The sporadic occurrence in cardiac muscle of anomalous Z bands exhibiting a periodic structure suggestive of tropomyosin. J Cell Biol. 1968 Jan;36(1):266–270. doi: 10.1083/jcb.36.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay F. S., Cooke P. H. Reversible disaggregation of myofilaments in vertebrate smooth muscle. J Cell Biol. 1973 Feb;56(2):399–411. doi: 10.1083/jcb.56.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrans V. J., Buja L. M., Jones M. Ultrastructure and cytochemistry of glycogen in cardiac diseases. Recent Adv Stud Cardiac Struct Metab. 1973;3:97–144. [PubMed] [Google Scholar]
- Ferrans V. J., Massumi R. A., Shugoll G. I., Ali N., Roberts W. C. Ultrastructural studies of myocardial biopsies in 45 patients with obstructive or congestive cardiomyopathy. Recent Adv Stud Cardiac Struct Metab. 1973;2:231–272. [PubMed] [Google Scholar]
- Ferrans V. J., Roberts W. C. Intermyofibrillar and nuclear-myofibrillar connections in human and canine myocardium. An ultrastructural study. J Mol Cell Cardiol. 1973 Jun;5(3):247–257. doi: 10.1016/0022-2828(73)90065-5. [DOI] [PubMed] [Google Scholar]
- Fischman D. A., Meltzer H. Y., Poppei R. W. Disruption of myofibrils in the skeletal muscle of psychotic patients. Arch Gen Psychiatry. 1970 Dec;23(6):503–515. doi: 10.1001/archpsyc.1970.01750060023003. [DOI] [PubMed] [Google Scholar]
- Fisher E. R., Cohn R. E., Danowski T. S. Ultrastructural observations of skeletal muscle in myopathy and neuropathy with special reference to muscular dystrophy. Lab Invest. 1966 Apr;15(4):778–793. [PubMed] [Google Scholar]
- HASPER B. ULTRAMIKROSKOPISCHE HERZMUSKELVERAENDERUNGEN NACH WIEDERHOLTER HYPOXIE. Beitr Pathol Anat. 1964 Aug;130:321–351. [PubMed] [Google Scholar]
- HIBBS R. G., FERRANS V. J., BLACK W. C., WEILBAECHER D. G., BURCH G. E. ALCOHOLIC CARDIOMYOPATHY; AN ELECTRON MICROSCOPIC STUDY. Am Heart J. 1965 Jun;69:766–779. doi: 10.1016/0002-8703(65)90450-3. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. ELECTRON MICROSCOPE STUDIES ON THE STRUCTURE OF NATURAL AND SYNTHETIC PROTEIN FILAMENTS FROM STRIATED MUSCLE. J Mol Biol. 1963 Sep;7:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- Haese W. H., Maron B. J., Mirowski M., Rowe R. D., Hutchins G. M. Peculiar focal myocardial degeneration and fatal ventricular arrhythmias in a child. N Engl J Med. 1972 Jul 27;287(4):180–181. doi: 10.1056/NEJM197207272870409. [DOI] [PubMed] [Google Scholar]
- Hatt P. Y., Berjal G., Moravec J., Swynghedauw B. Heart failure: an electron microscopic study of the left ventricular papillary muscle in aortic insufficiency in the rabbit. J Mol Cell Cardiol. 1970 Sep;1(3):235–247. doi: 10.1016/0022-2828(70)90004-0. [DOI] [PubMed] [Google Scholar]
- Hatt P. Y., Berjal G., Moravec J., Swynghedauw B. Le myocarde ventriculaire dans l'insuffisance cardiaque expérimentale par insuffisance aortique chez le lapin. Etude au microscope électronique. Arch Mal Coeur Vaiss. 1970 Mar;63(3):383–407. [PubMed] [Google Scholar]
- Hatt P. Y., Ledoux C., Bonvalet J. P. Lyse et synthèse des protéines myocardiques au cours de l'insuffisance cardiaque expérimentale. (Etude au microscope électronique) Arch Mal Coeur Vaiss. 1965 Dec;58(12):1703–1721. [PubMed] [Google Scholar]
- Howes E. L., Jr, Price H. M., Blumberg J. M., Pearson C. M. Hypokalemic periodic paralysis. Electromicroscopic changes in the sarcoplasm. Neurology. 1966 Mar;16(3):242–256. doi: 10.1212/wnl.16.3.242. [DOI] [PubMed] [Google Scholar]
- Hudgson P., Gardner-Medwin D., Fulthorpe J. J., Walton J. N. Nemaline myopathy. Neurology. 1967 Dec;17(12):1125–1142. doi: 10.1212/wnl.17.12.1125. [DOI] [PubMed] [Google Scholar]
- Hughes J. T., Brownell B. Ultrastructure of muscle in Werdnig-Hoffmann disease. J Neurol Sci. 1969 Mar-Apr;8(2):361–379. doi: 10.1016/0022-510x(69)90118-x. [DOI] [PubMed] [Google Scholar]
- Hutterer F., Schaffner F., Klion F. M., Popper H. Hypertrophic, hypoactive smooth endoplasmic reticulum: a sensitive indicator of hepatotoxicity exemplified by dieldrin. Science. 1968 Sep 6;161(3845):1017–1019. doi: 10.1126/science.161.3845.1017. [DOI] [PubMed] [Google Scholar]
- James T. N. Cardiac conduction system: fetal and postnatal development. Am J Cardiol. 1970 Feb;25(2):213–226. doi: 10.1016/0002-9149(70)90581-3. [DOI] [PubMed] [Google Scholar]
- James T. N., Sherf L., Fine G., Morales A. R. Comparative ultrastructure of the sinus node in man and dog. Circulation. 1966 Jul;34(1):139–163. doi: 10.1161/01.cir.34.1.139. [DOI] [PubMed] [Google Scholar]
- James T. N., Sherf L. Ultrastructure of myocardial cells. Am J Cardiol. 1968 Sep;22(3):389–416. doi: 10.1016/0002-9149(68)90124-0. [DOI] [PubMed] [Google Scholar]
- James T. N., Sherf L. Ultrastructure of the human atrioventricular node. Circulation. 1968 Jun;37(6):1049–1070. doi: 10.1161/01.cir.37.6.1049. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Fawcett D. W. Hypertrophy of the agranular endoplasmic reticulum in hamster liver induced by phenobarbital (with a review on the functions of this organelle in liver). J Histochem Cytochem. 1966 Mar;14(3):215–232. doi: 10.1177/14.3.215. [DOI] [PubMed] [Google Scholar]
- Jones M., Ferrans V. J., Morrow A. G., Roberts W. C. Ultrastructure of crista supraventricularis muscle in patients with congenital heart diseases associated with right ventricular outflow tract obstruction. Circulation. 1975 Jan;51(1):39–67. doi: 10.1161/01.cir.51.1.39. [DOI] [PubMed] [Google Scholar]
- Kawamura K., James T. N. Comparative ultrastructure of cellular junctions in working myocardium and the conduction system under normal and pathologic conditions. J Mol Cell Cardiol. 1971 Sep;3(1):31–60. doi: 10.1016/0022-2828(71)90031-9. [DOI] [PubMed] [Google Scholar]
- Klinkerfuss G. H., Haugh M. J. Disuse atrophy of muscle; histochemistry and electron microscopy. Arch Neurol. 1970 Apr;22(4):309–320. doi: 10.1001/archneur.1970.00480220023005. [DOI] [PubMed] [Google Scholar]
- Legato M. J. Sarcomerogenesis in human myocardium. J Mol Cell Cardiol. 1970 Dec;1(4):425–437. doi: 10.1016/0022-2828(70)90039-8. [DOI] [PubMed] [Google Scholar]
- Lentz T. L. Cytological studies of muscle dedifferentiation and differentiation during limb regeneration of the newt Triturus. Am J Anat. 1969 Apr;124(4):447–479. doi: 10.1002/aja.1001240404. [DOI] [PubMed] [Google Scholar]
- Locke M., Krishnan N. Hot alcoholic phosphotungstic acid and uranyl acetate as routine stains for thick and thin sections. J Cell Biol. 1971 Aug;50(2):550–557. doi: 10.1083/jcb.50.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEERSON F. Z. Compensatory hyperfunction of the heart and cardiac insufficiency. Circ Res. 1962 Mar;10:250–258. doi: 10.1161/01.res.10.3.250. [DOI] [PubMed] [Google Scholar]
- MEERSON F. Z., ZALETAYEVA T. A., LAGUTCHEV S. S., PSHENNIKOVA M. G. STRUCTURE AND MASS OF MITOCHONDRIA IN THE PROCESS OF COMPENSATORY HYPERFUNCTION AND HYPERTROPHY OF THE HEART. Exp Cell Res. 1964 Dec;36:568–578. doi: 10.1016/0014-4827(64)90313-1. [DOI] [PubMed] [Google Scholar]
- Macdonald R. D., Rewcastle N. B., Humphrey J. G. Myopathy of hypokalemic periodic paralysis. An electron microscopic study. Arch Neurol. 1969 Jun;20(6):565–585. doi: 10.1001/archneur.1969.00480120011001. [DOI] [PubMed] [Google Scholar]
- Macdonald R. D., Rewcastle N. B., Humphrey J. G. The myopathy of hyperkalemic periodic paralysis. An electron microscopic study. Arch Neurol. 1968 Sep;19(3):274–283. doi: 10.1001/archneur.1968.00480030052005. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Ferrans V. J. Aggregates of tubules in human cardiac muscle cells. J Mol Cell Cardiol. 1974 Jun;6(3):249–264. doi: 10.1016/0022-2828(74)90054-6. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Ferrans V. J., Henry W. L., Clark C. E., Redwood D. R., Roberts W. C., Morrow A. G., Epstein S. E. Differences in distribution of myocardial abnormalities in patients with obstructive and nonobstructive asymmetric septal hypertrophy (ASH). Light and electron microscopic findings. Circulation. 1974 Sep;50(3):436–446. doi: 10.1161/01.cir.50.3.436. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Ferrans V. J. The occurrence of alpha-glycogen in humans. J Mol Cell Cardiol. 1974 Feb;6(1):85–89. doi: 10.1016/0022-2828(74)90010-8. [DOI] [PubMed] [Google Scholar]
- Mastaglia F. L., Walton J. N. An electron microscopic study of skeletal muscle from cases of the Kugelberg-Welander syndrome. Acta Neuropathol. 1971;17(3):201–219. doi: 10.1007/BF00685054. [DOI] [PubMed] [Google Scholar]
- Maurat J. P., Mercier J. N., Ledoux C., Hatt P. Y. Le myocarde dans les délétions expérimentales en potassium chez le rat. Etude au microscope électronique. Arch Mal Coeur Vaiss. 1965 Jul;58(7):1004–1021. [PubMed] [Google Scholar]
- Meerson F. Z. The myocardium in hyperfunction, hypertrophy and heart failure. Circ Res. 1969 Jul;25(1 Suppl):1–163. [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Electron-microscopic structure of denervated skeletal muscle. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):253–269. doi: 10.1098/rspb.1969.0091. [DOI] [PubMed] [Google Scholar]
- Milhorat A. T., Shafiq S. A., Goldstone L. Changes in muscle structure in dystrophic patients, carriers and normal siblings seen by electron microscopy; correlation with levels of serum creatinephosphokinase (CPK). Ann N Y Acad Sci. 1966 Sep 9;138(1):246–292. doi: 10.1111/j.1749-6632.1966.tb41170.x. [DOI] [PubMed] [Google Scholar]
- Mintz G., González-Angulo A., Fraga A. Ultrastructure of muscle in polymyositis. Am J Med. 1968 Feb;44(2):216–224. doi: 10.1016/0002-9343(68)90153-8. [DOI] [PubMed] [Google Scholar]
- Nakashima N., Tamura Z., Okamoto S., Goto H. Inclusion bodies in human neuromuscular disorder. Arch Neurol. 1970 Mar;22(3):270–278. doi: 10.1001/archneur.1970.00480210080010. [DOI] [PubMed] [Google Scholar]
- Novi A. M. Beitrag zur Feinstruktur des Herzmuskels bei experimenteller Herzhypertrophie. Beitr Pathol Anat. 1968;137(1):19–50. [PubMed] [Google Scholar]
- Odor D. L., Patel A. N., Pearce L. A. Familial hypokalemic periodic paralysis with permanent myopathy. A clinical and ultrastructural study. J Neuropathol Exp Neurol. 1967 Jan;26(1):98–114. doi: 10.1097/00005072-196701000-00008. [DOI] [PubMed] [Google Scholar]
- Onishi S., Büchner F., Zittel R., Thermann M. Das elektronenmikroskopische Bild der Herzmuskelzelle des Hundes bei experimenteller Herzhypertrophie in der Anpassungsphase. Beitr Pathol Anat. 1969;139(1):94–114. [PubMed] [Google Scholar]
- PRICE H., PEASE D. C., PEARSON C. M. Selective actin filament and Z-band degeneration induced by plasmocid. An electron microscopic study. Lab Invest. 1962 Jul;11:549–562. [PubMed] [Google Scholar]
- REWCASTLE N. B., HUMPHREY J. G. VACUOLAR MYOPATHY: CLINICAL, HISTOCHEMICAL, AND MICROSCOPIC STUDY. Arch Neurol. 1965 Jun;12:570–582. doi: 10.1001/archneur.1965.00460300018003. [DOI] [PubMed] [Google Scholar]
- RICHTER G. W., KELLNER A. Hypertrophy of the human heart at the level of fine structure. An analysis and two postulates. J Cell Biol. 1963 Jul;18:195–206. doi: 10.1083/jcb.18.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Myosin-like tactoids in trypsin-treated blood platelets. J Cell Biol. 1971 Sep;50(3):900–904. doi: 10.1083/jcb.50.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R. W., Morton D. J., Weidemann J. F. Irregular Z bands occurring in rat soleus muscles. J Ultrastruct Res. 1971 Jul;36(1):205–210. doi: 10.1016/s0022-5320(71)80098-9. [DOI] [PubMed] [Google Scholar]
- Roy P. E., Dorais J., Morin P. J. Les foyers de nécrose intracelulaire focale dans le muscle cardiaque humain. Ann Anat Pathol (Paris) 1972 Jan-Mar;17(1):39–51. [PubMed] [Google Scholar]
- SCHLESINGER M. J., REINER L. Focal myocytolysis of the heart. Am J Pathol. 1955 May-Jun;31(3):443–459. [PMC free article] [PubMed] [Google Scholar]
- Santa T. Fine structure of the human skeletal muscle in myopathy. Arch Neurol. 1969 May;20(5):479–489. doi: 10.1001/archneur.1969.00480110043004. [DOI] [PubMed] [Google Scholar]
- Schrodt G. R., Walker S. M. Ultrastructure of membranes in denervation atrophy. Am J Pathol. 1966 Jul;49(1):33–51. [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Sordahl L. A., Entman M. L., Allen J. C., Reddy Y. S., Goldstein M. A., Luchi R. J., Wyborny L. E. Abnormal biochemistry in myocardial failure. Am J Cardiol. 1973 Sep 20;32(4):407–422. doi: 10.1016/s0002-9149(73)80031-1. [DOI] [PubMed] [Google Scholar]
- Shafiq S. A., Gorycki M. A., Asiedu S. A., Milhorat A. T. Tenotomy. Effect on the fine structure of the soleus of the rat. Arch Neurol. 1969 Jun;20(6):625–633. doi: 10.1001/archneur.1969.00480120071006. [DOI] [PubMed] [Google Scholar]
- Shafiq S. A., Milhorat A. T., Gorycki M. A. Fine structure of human muscle in neurogenic atrophy. Neurology. 1967 Oct;17(10):934–948. doi: 10.1212/wnl.17.10.934. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative study of Purkinje fibers and ventricular fibers. J Cell Biol. 1968 Mar;36(3):497–526. doi: 10.1083/jcb.36.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative ultrastructural study with special reference to frog and chicken hearts. Z Zellforsch Mikrosk Anat. 1969;98(3):437–468. [PubMed] [Google Scholar]
- Thornell L. E. Ultrastructural variations of Z bands in cow Purkinje fibres. J Mol Cell Cardiol. 1973 Oct;5(5):409–417. doi: 10.1016/0022-2828(73)90011-4. [DOI] [PubMed] [Google Scholar]
- Van Noorden S., Olsen E. G., Pearse A. G. Hypertrophic obstructive cardiomyopathy, a histological, histochemical, and ultrastructural study of biopsy material. Cardiovasc Res. 1971 Jan;5(1):118–131. doi: 10.1093/cvr/5.1.118. [DOI] [PubMed] [Google Scholar]
- Virágh S., Challice C. E. Variations in filamentous and fibrillar organization, and associated sarcolemmal structures, in cells of the normal mammalian heart. J Ultrastruct Res. 1969 Sep;28(5):321–334. doi: 10.1016/s0022-5320(69)80025-0. [DOI] [PubMed] [Google Scholar]
- WALKER S. M., SCHRODT G. R., TRUONG X. T., WALL E. J. ELECTRON MICROSCOPE STUDY OF SARCOPLASMIC RETICULUM AND MYOFILAMENTS OF TENOTOMIZED RAT MUSCLE. Am J Phys Med. 1965 Aug;44:176–192. [PubMed] [Google Scholar]
- Wechsler W. Comparative electron microscopic studies on various forms of muscle atrophies and dystrophies in animals and man. Ann N Y Acad Sci. 1966 Sep 9;138(1):113–137. doi: 10.1111/j.1749-6632.1966.tb41161.x. [DOI] [PubMed] [Google Scholar]
- Zacks S. I., Lipshutz H., Markind S., Elliott F. A. "Hyaline degeneration" in severely atrophic muscle. Histochemical and electron microscopic observations. Brain. 1968 Sep;91(3):463–472. doi: 10.1093/brain/91.3.463. [DOI] [PubMed] [Google Scholar]
- Zintz R., Villiger W. Elektronenmikroskopische Befunde bei 3 Fällen von chronisch progressiver okulärer Muskeldystrophie. Ophthalmologica. 1967;153(6):439–459. doi: 10.1159/000305086. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Grusky G. The actin and myosin filaments of human and bovine blood platelets. J Clin Invest. 1972 Feb;51(2):419–430. doi: 10.1172/JCI106828. [DOI] [PMC free article] [PubMed] [Google Scholar]