Abstract
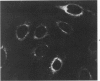
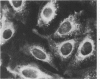
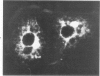
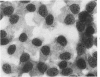
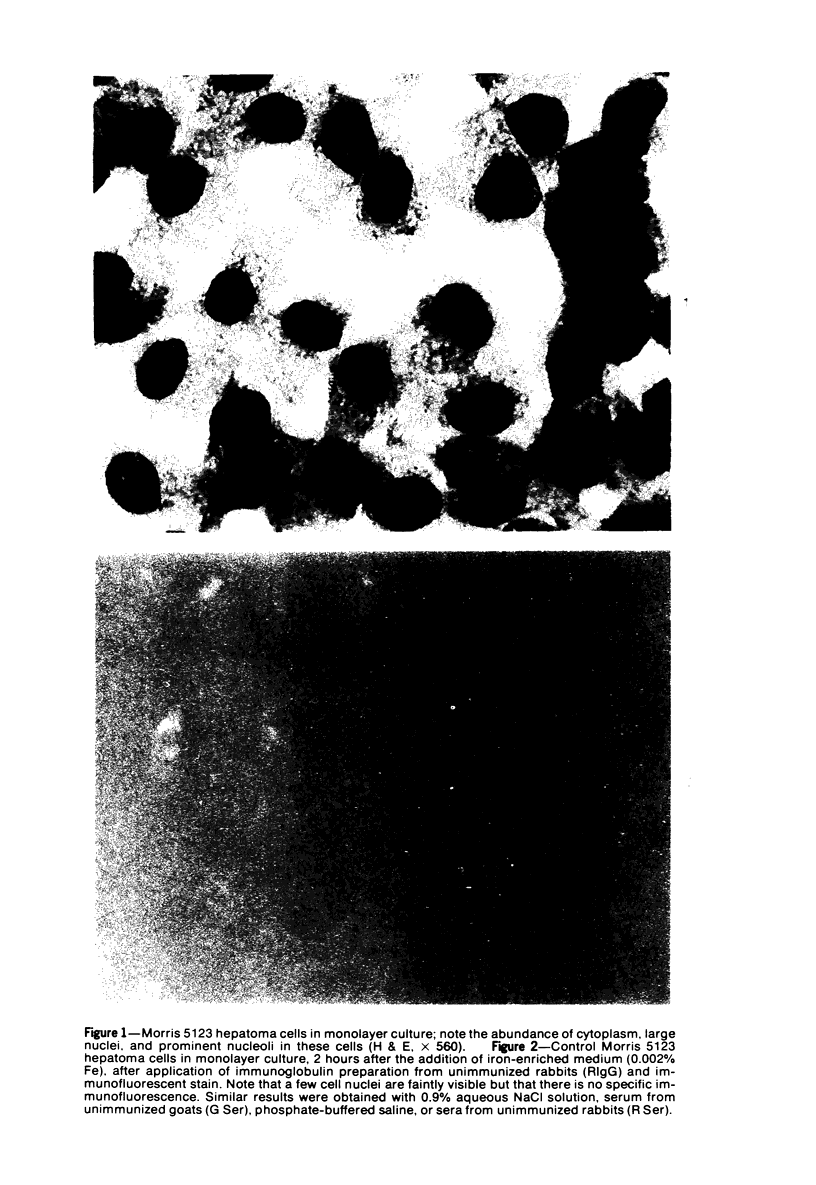
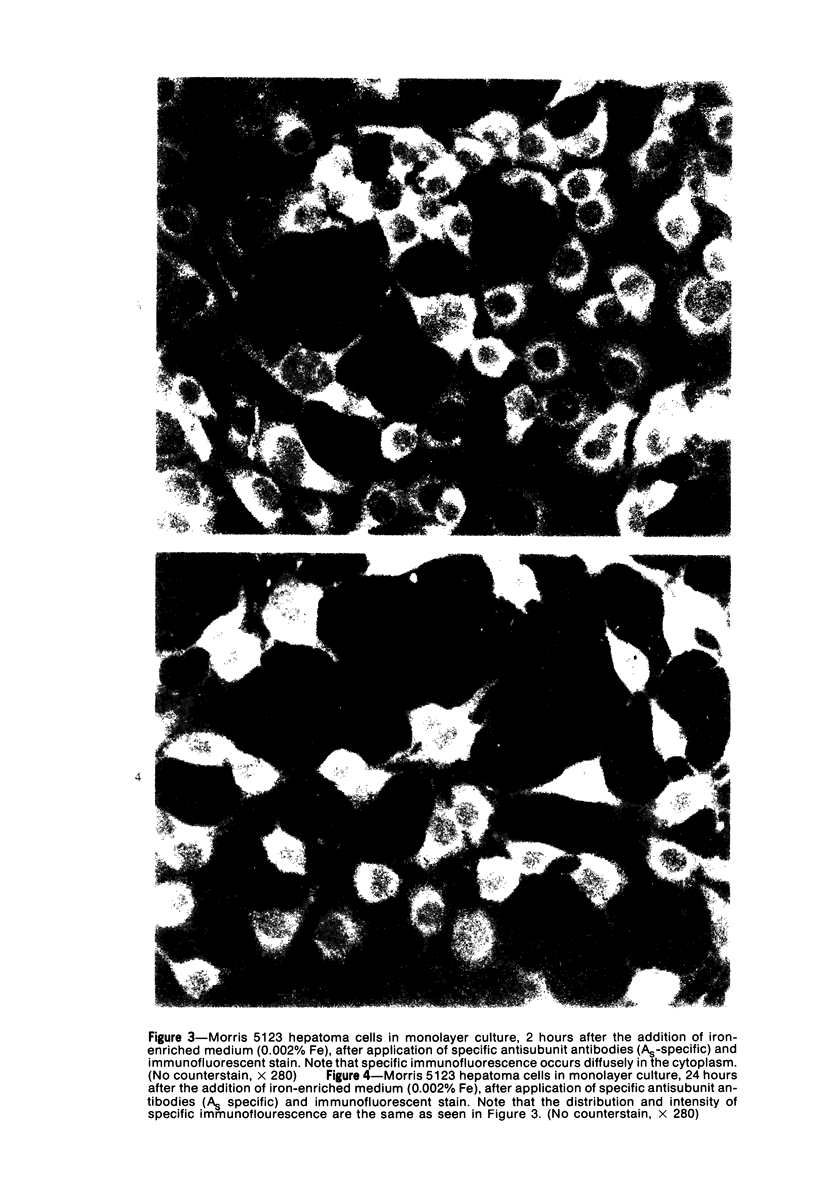
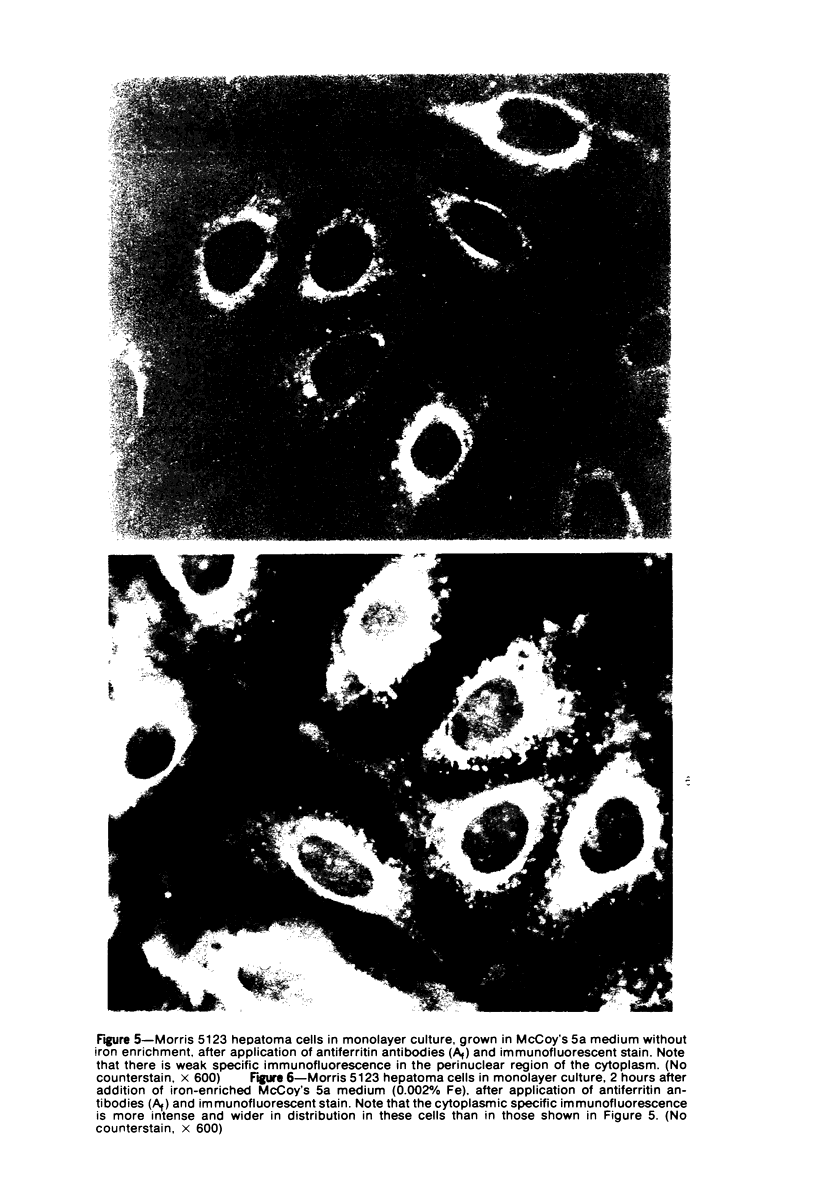
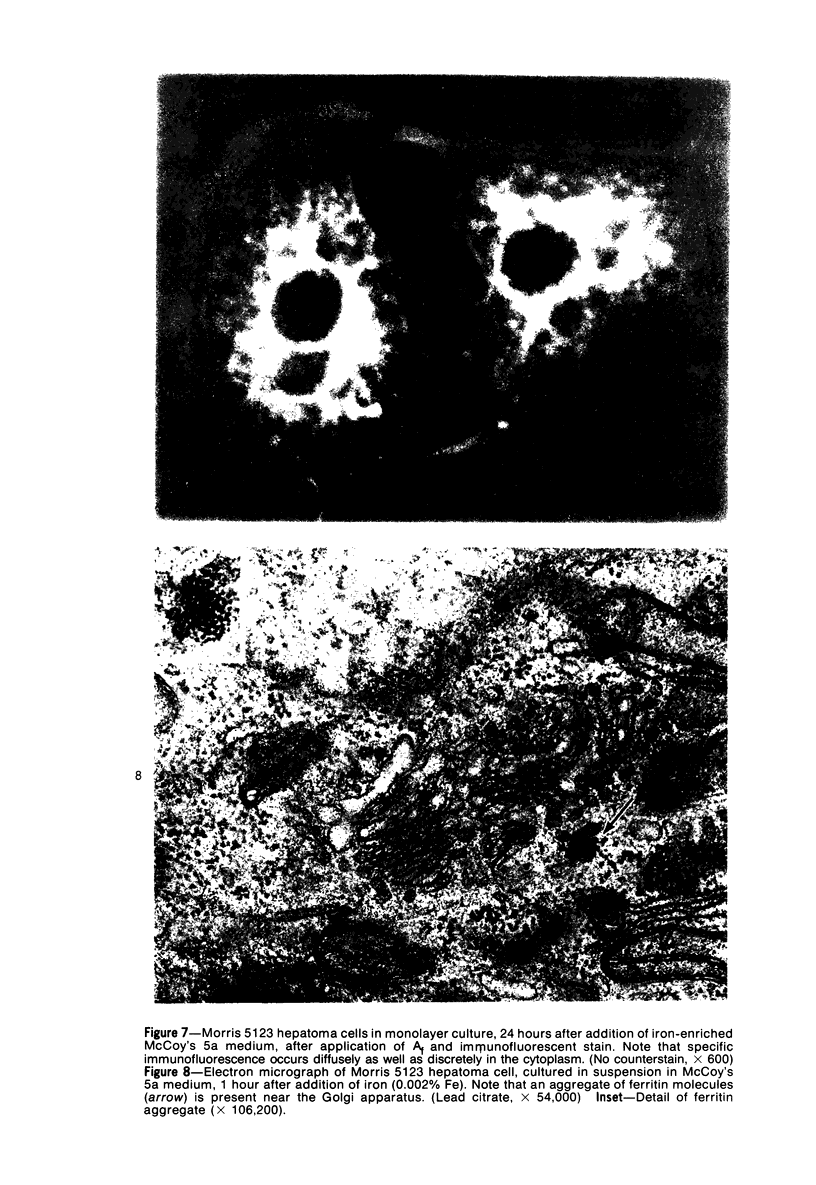
Using precipitating antibodies to ACI rat liver ferritin and to sodium-dodecyl-sulfate-dissociated protein subunits of ACI rat liver ferritin, we have demonstrated the presence of ferritin-positive sites and subunit-positive sites in situ in several rat hepatoma cell lines by immunofluorescence. Hepatoma cells from three transplantable rat hepatomas (Reuber H-139, Reuber H-35, and Morris 5123) were explanted and propagated. Rabbit antibodies specific for either protein subunits of ferritin or ferritin were prepared by affinity chromatography or by dissociation of antibody-antigen complexes with 0.1 M acetic acid followed by differential ultracentrifugation. Explants of Reuber H-139, Reuber H-35, and Morris 5123 hepatoma cells, grown either in ordinary McCoy's 5a medium or in such medium enriched with iron (0.002% Fe), gave positive immunofluorescence for subunits as well as ferritin. Exposure of a clonal strain of Morris 5123 hepatoma cells to iron-enriched culture medium for varying lengths of time of up to 24 hours resulted in progressive increase in the quantity of ferritin-specific immunofluorescent cytoplasmic material, which was at first present diffusely, and later in clumps. By contrast, during the initial 24-hour period, subunit-specific immunofluorescence remained at relatively low intensity, with diffuse distribution through the cytoplasma. Our findings indicate a) the presence, in the cytoplasm, of the three kinds of hepatoma cells, of unassembled or only partly assembled subunits of fragments of subunits as well as of ferritin, and b) rapid assembly of the protein subunits into apoferritin and ferritin after administration of iron, so that the concentration of subunits in the cytoplasm was not significantly increased.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arstila A. U., Bradford W. D., Kinney T. D., Trump B. F. Iron metabolism and cell membranes. II. The relationship of ferritin to the cytocavitary network in rat hepatic parenchymal cells. Am J Pathol. 1970 Mar;58(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J. C., Lee S. S., Schlesinger K. J., Richter G. W. Detection of protein subunits of ferritin in situ in cells by immunofluorescence. Am J Pathol. 1974 Jun;75(3):473–487. [PMC free article] [PubMed] [Google Scholar]
- PITOT H. C., PERAINO C., MORSE P. A., Jr, POTTER V. R. HEPATOMAS IN TISSUE CULTURE COMPARED WITH ADAPTING LIVER IN VIVO. Natl Cancer Inst Monogr. 1964 Apr;13:229–245. [PubMed] [Google Scholar]
- Puro D. G., Richter G. W. Ferritin synthesis by free and membrane-bound (poly)ribosomes of rat liver. Proc Soc Exp Biol Med. 1971 Nov;138(2):399–403. doi: 10.3181/00379727-138-35906. [DOI] [PubMed] [Google Scholar]
- RICHTER G. W. Activation of ferritin synthesis and induction of changes in fine structure in HeLa cells in vitro: implications for protein synthesis. Nature. 1961 Apr 29;190:413–415. doi: 10.1038/190413a0. [DOI] [PubMed] [Google Scholar]
- RICHTER G. W. The cellular transformation of injected colloidal iron complexes into ferritin and hemosiderin in experimental animals; a study with the aid of electron microscopy. J Exp Med. 1959 Feb 1;109(2):197–216. doi: 10.1084/jem.109.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M. Biosynthesis of serum proteins and ferritin by free and attached ribosomes of rat liver. J Biol Chem. 1969 Aug 25;244(16):4308–4315. [PubMed] [Google Scholar]
- Richter G. W., Lee J. C. A study of two types of ferritin from rat hepatomas. Cancer Res. 1970 Mar;30(3):880–888. [PubMed] [Google Scholar]
- Richter G. W. Serological specificity of antibodies to protein subunits of horse spleen ferritin. Biochim Biophys Acta. 1972 Feb 29;257(2):471–481. doi: 10.1016/0005-2795(72)90300-5. [DOI] [PubMed] [Google Scholar]
- Schenk E. A., Churukian C. J. Immunofluorescence counterstains. J Histochem Cytochem. 1974 Oct;22(10):962–966. doi: 10.1177/22.10.962. [DOI] [PubMed] [Google Scholar]
- UMANA R., DOUNCE A. L. RELATIONSHIP OF RAT LIVER CATHEPSINS TO AUTOLYTIC DEGRADATION OF PROTEINS OF THE LIVER CELL NUCLEUS DURING ISOLATION. INTRACELLULAR DISTRIBUTION AND PROPERTIES OF THE CATHEPSINS. Exp Cell Res. 1964 Jul;35:277–292. doi: 10.1016/0014-4827(64)90095-3. [DOI] [PubMed] [Google Scholar]