Abstract
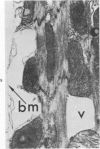
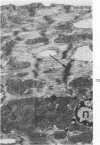
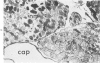
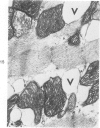
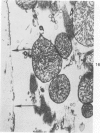
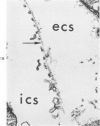
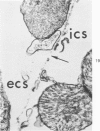
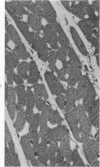
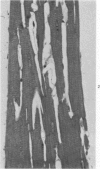
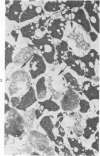
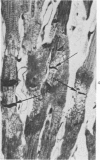
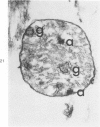
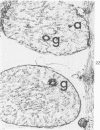
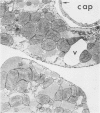
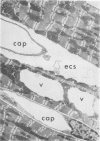
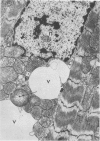
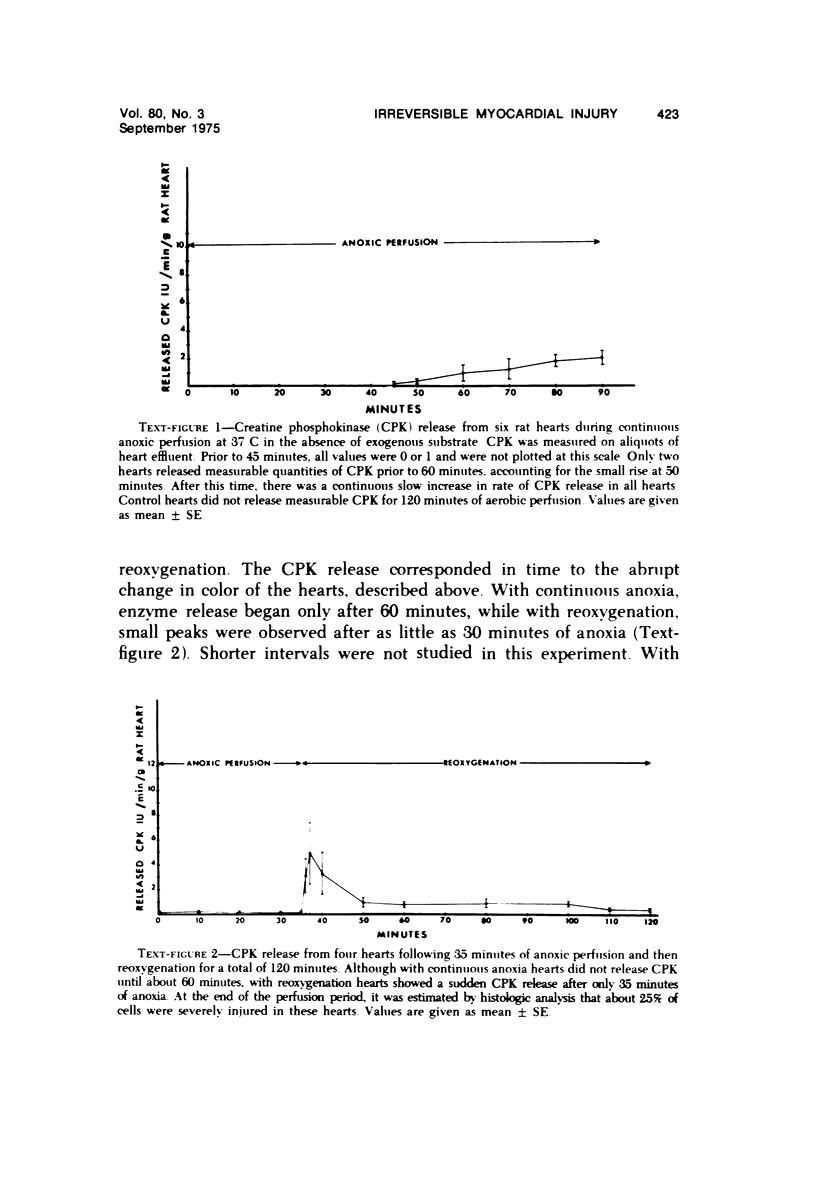
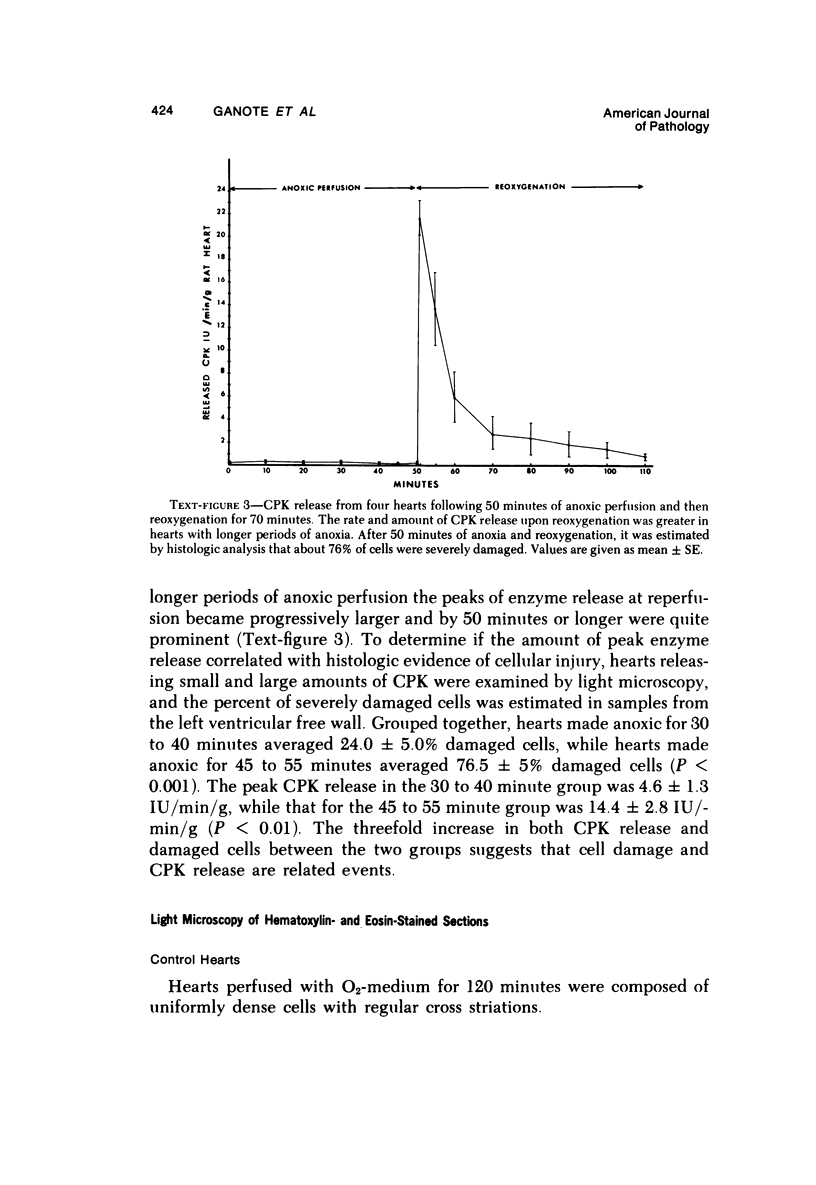
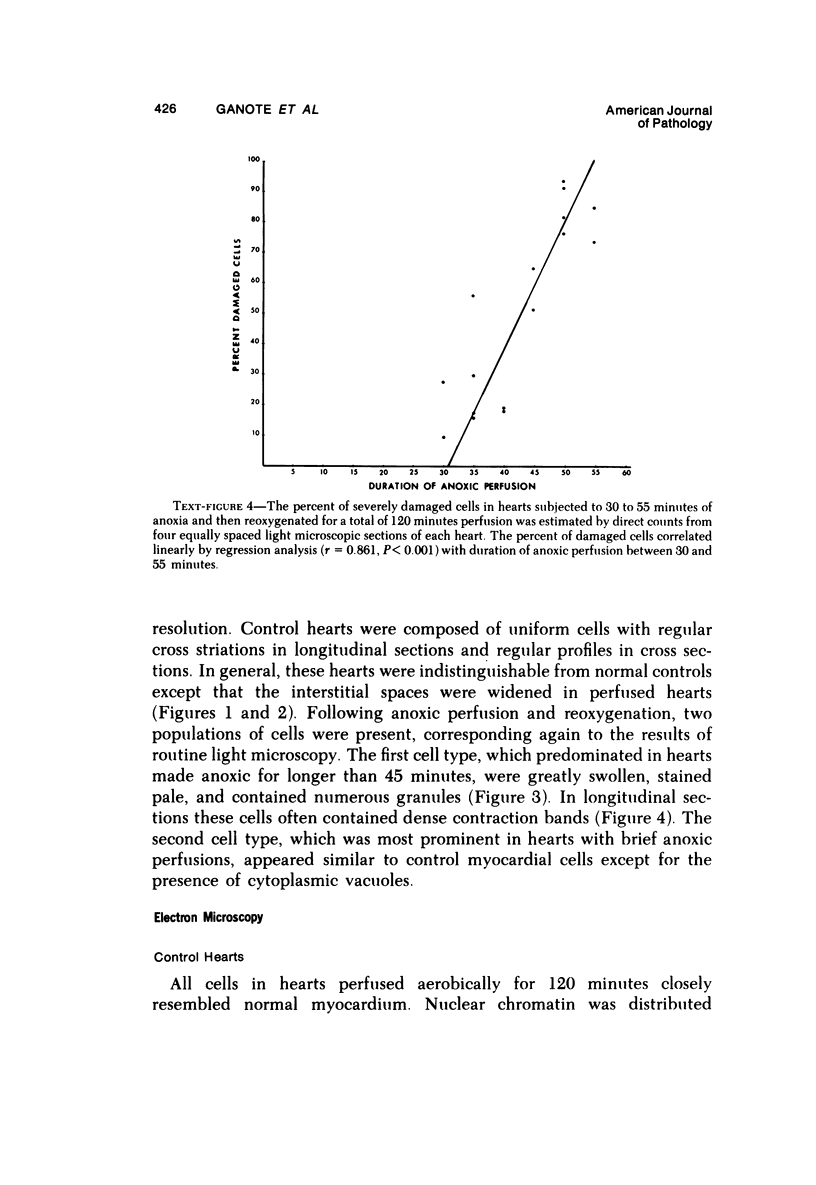
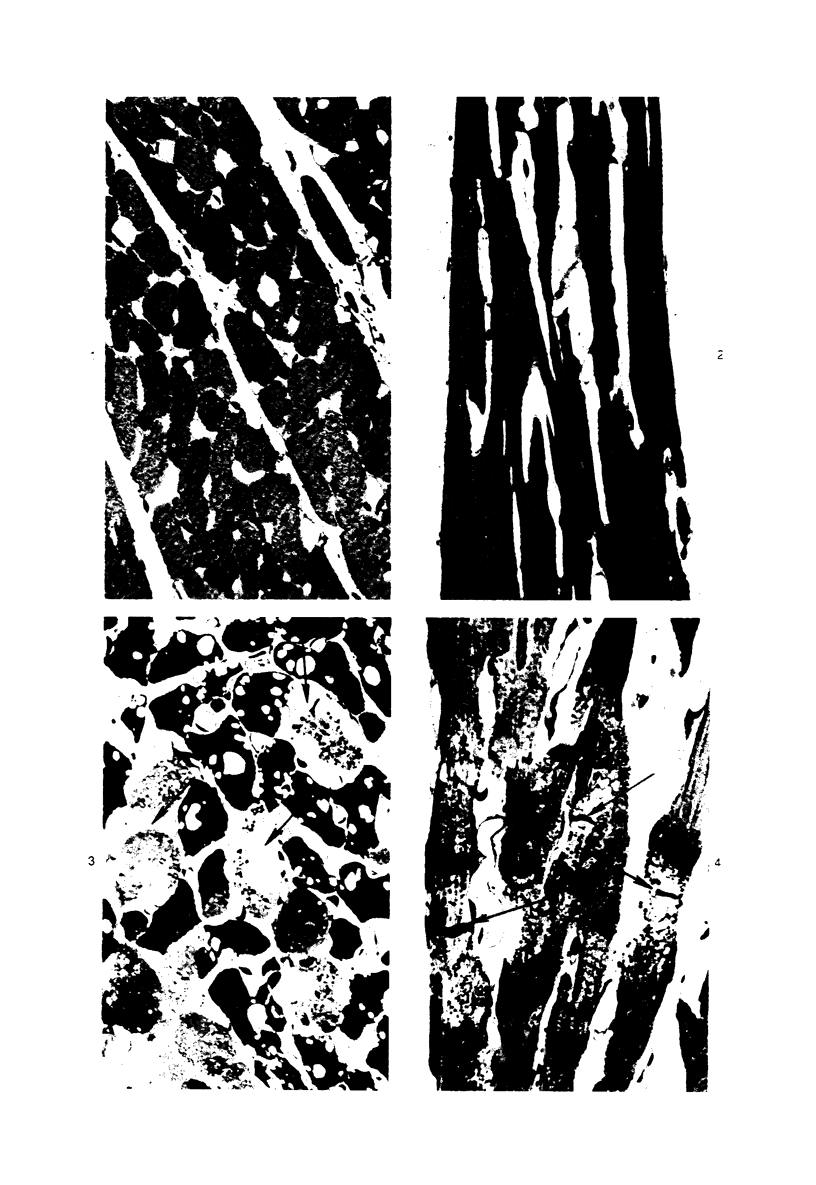
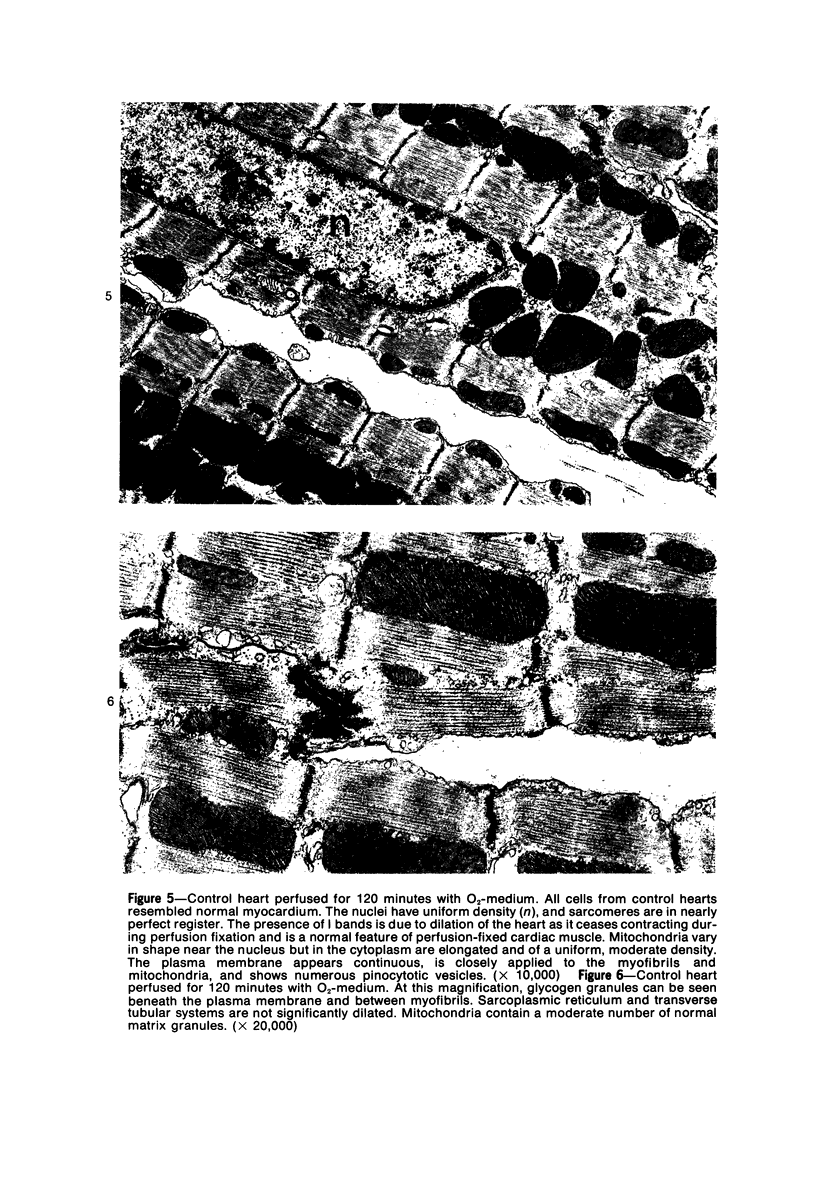
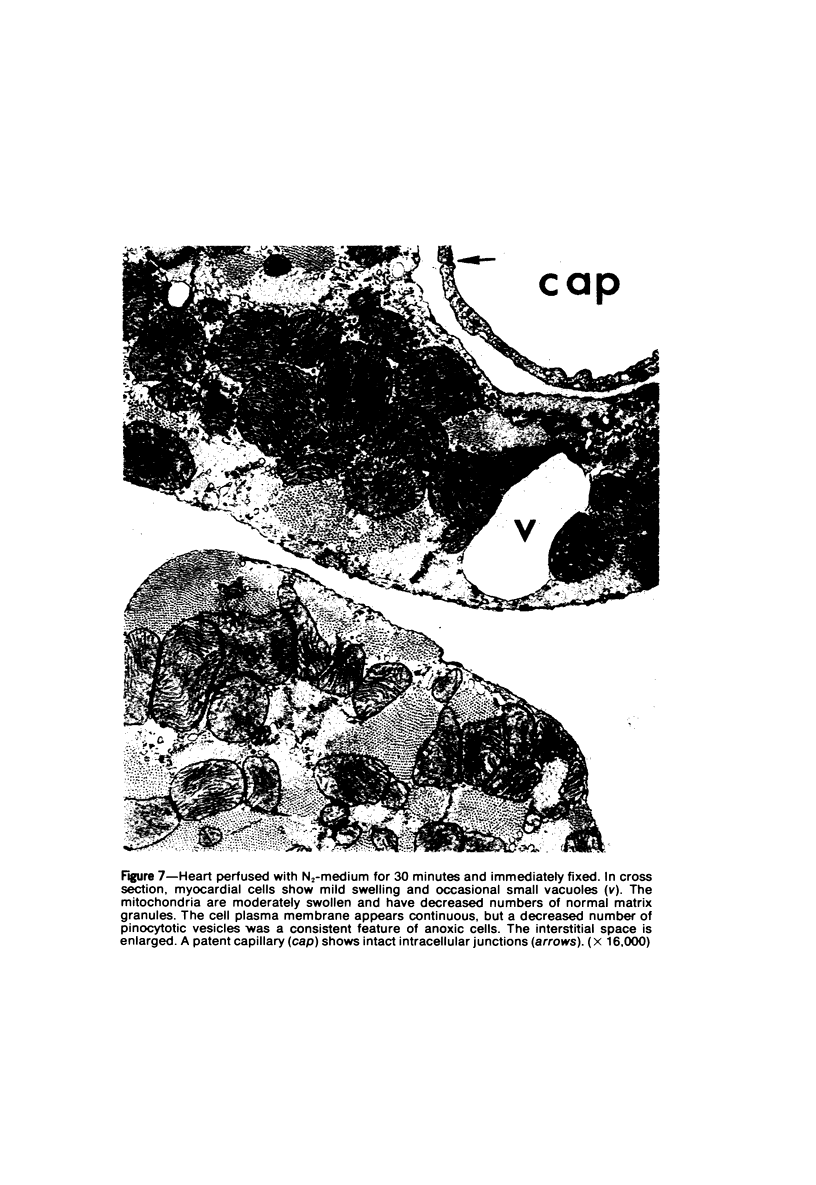
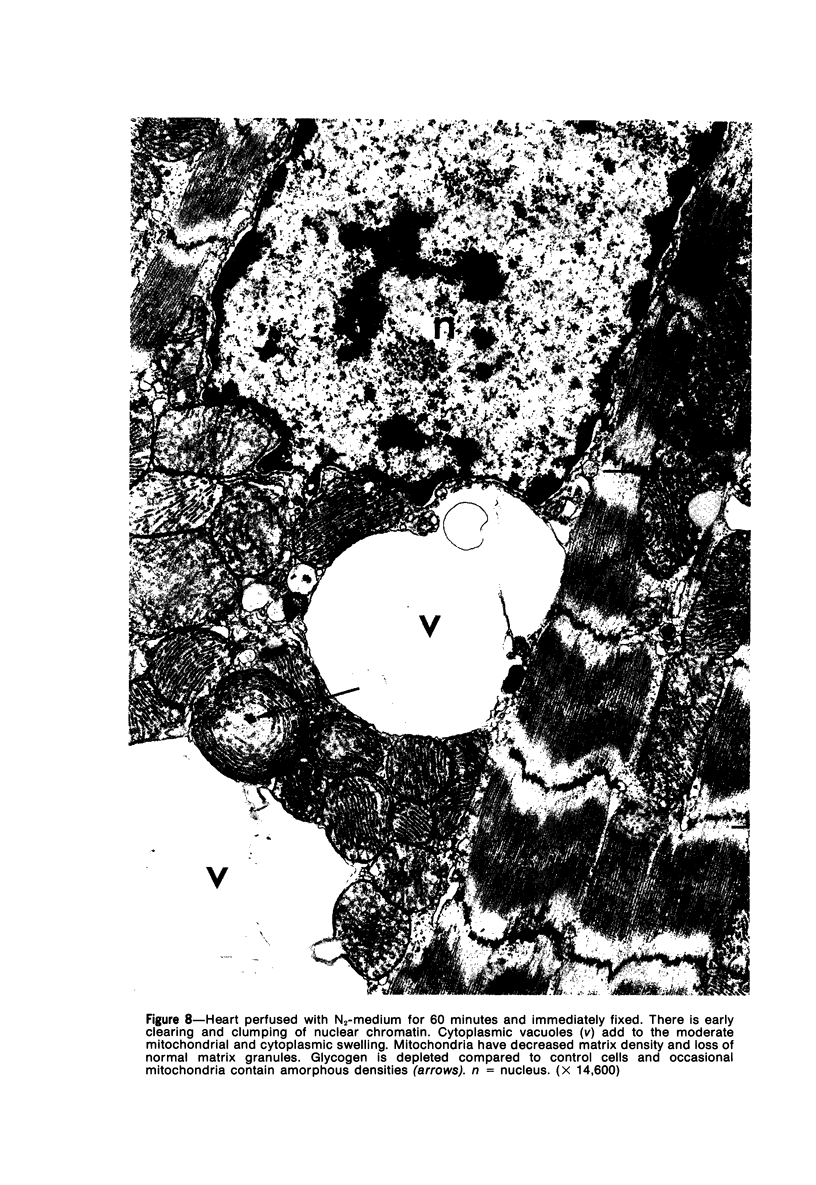
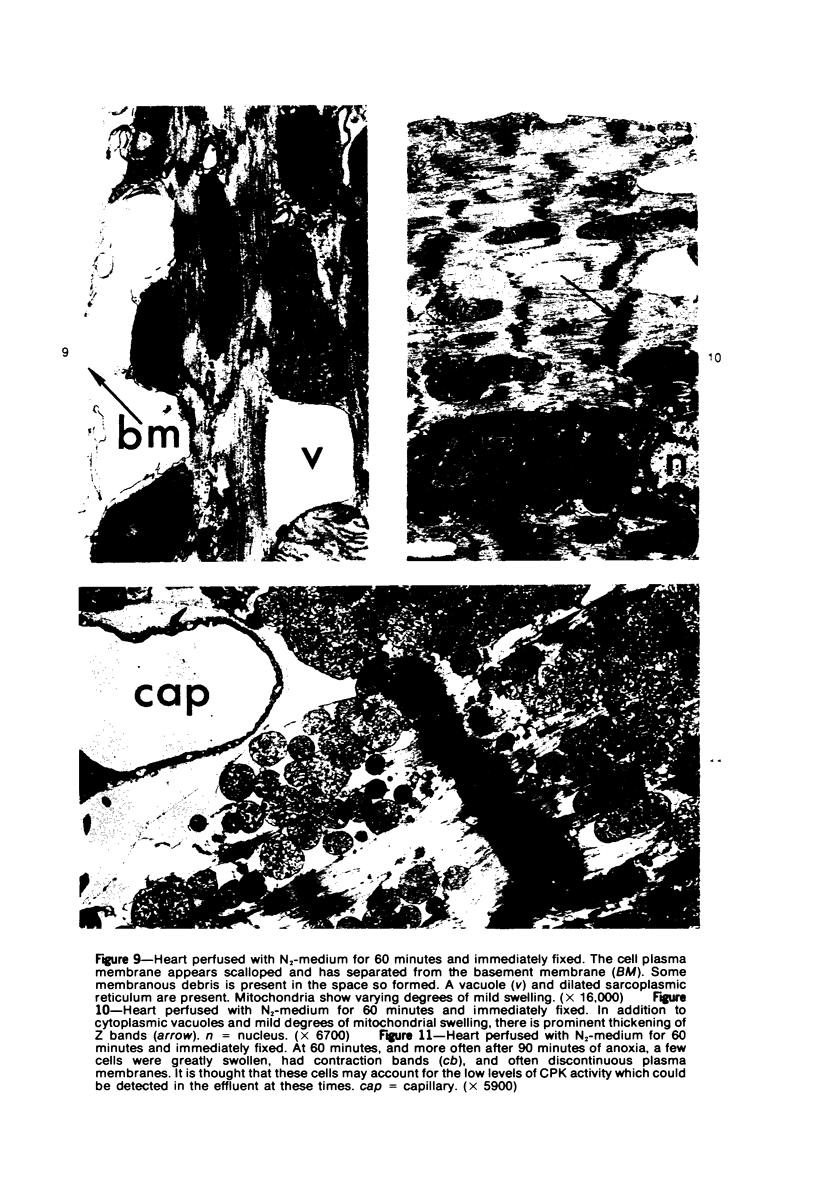
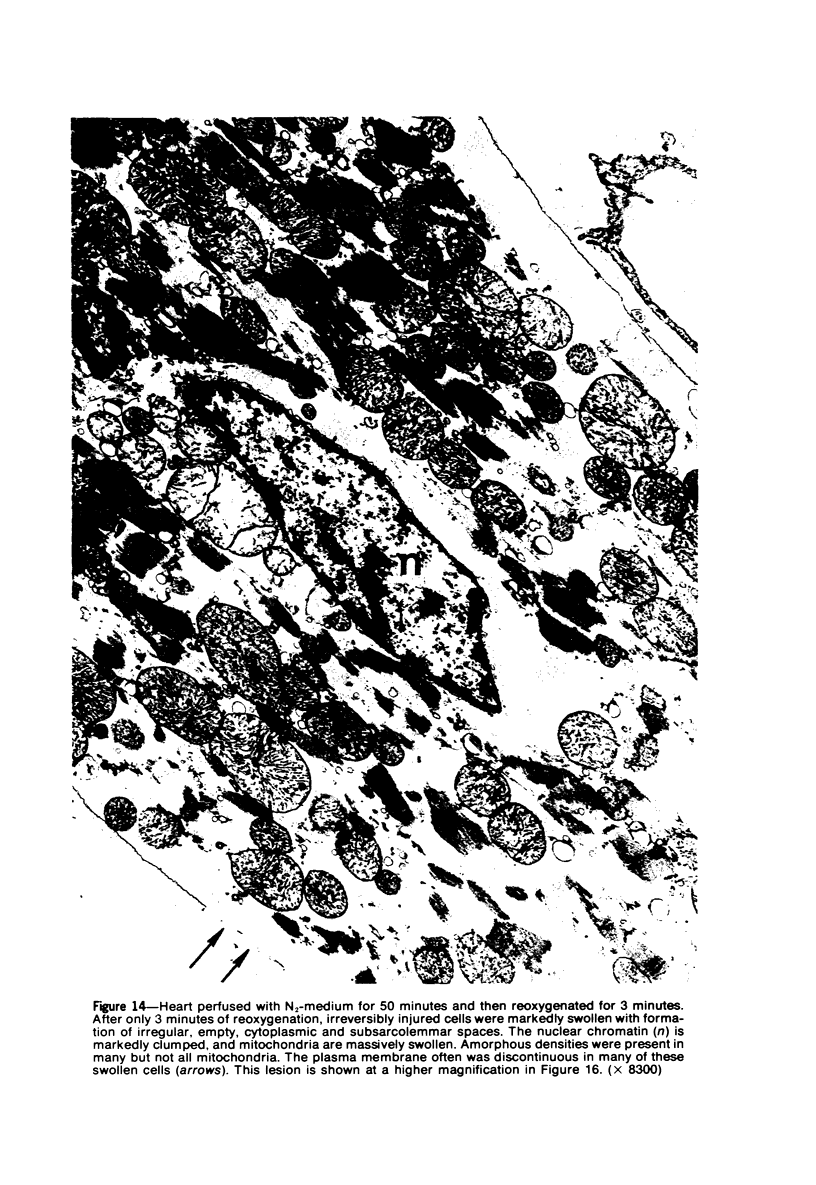
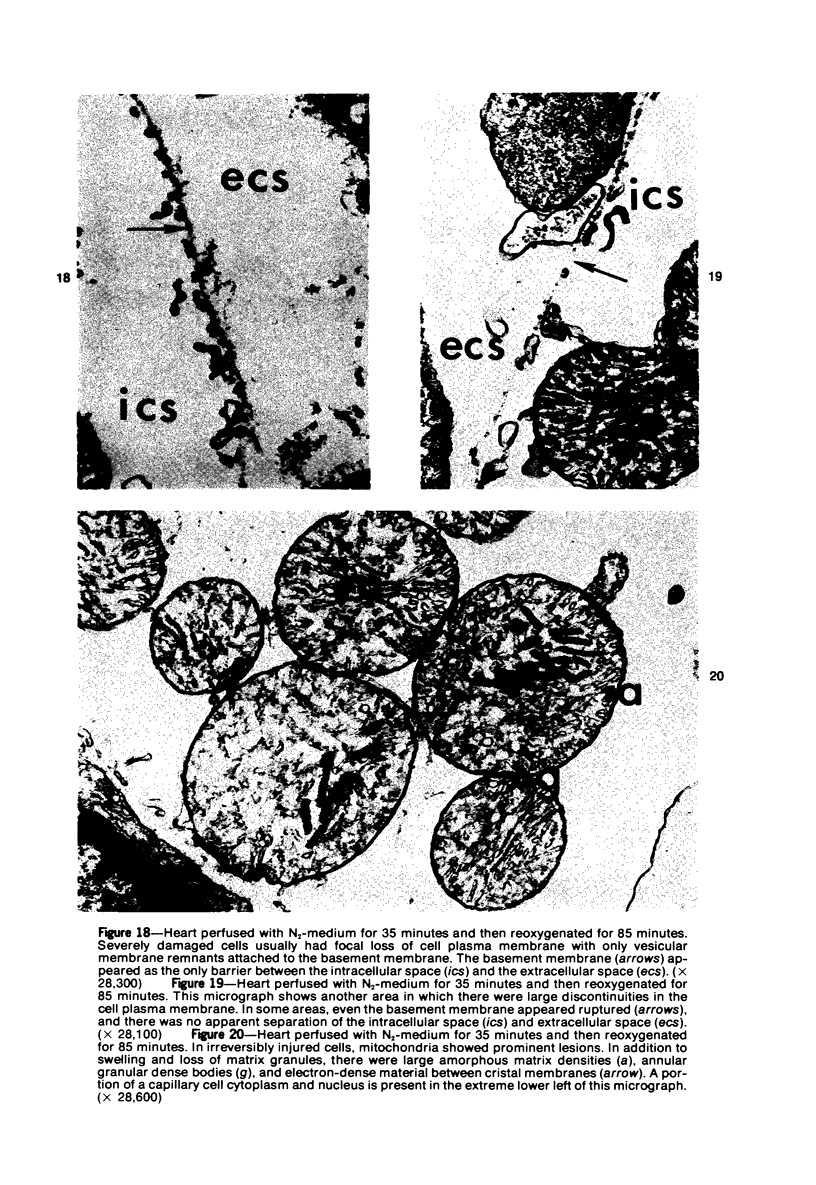
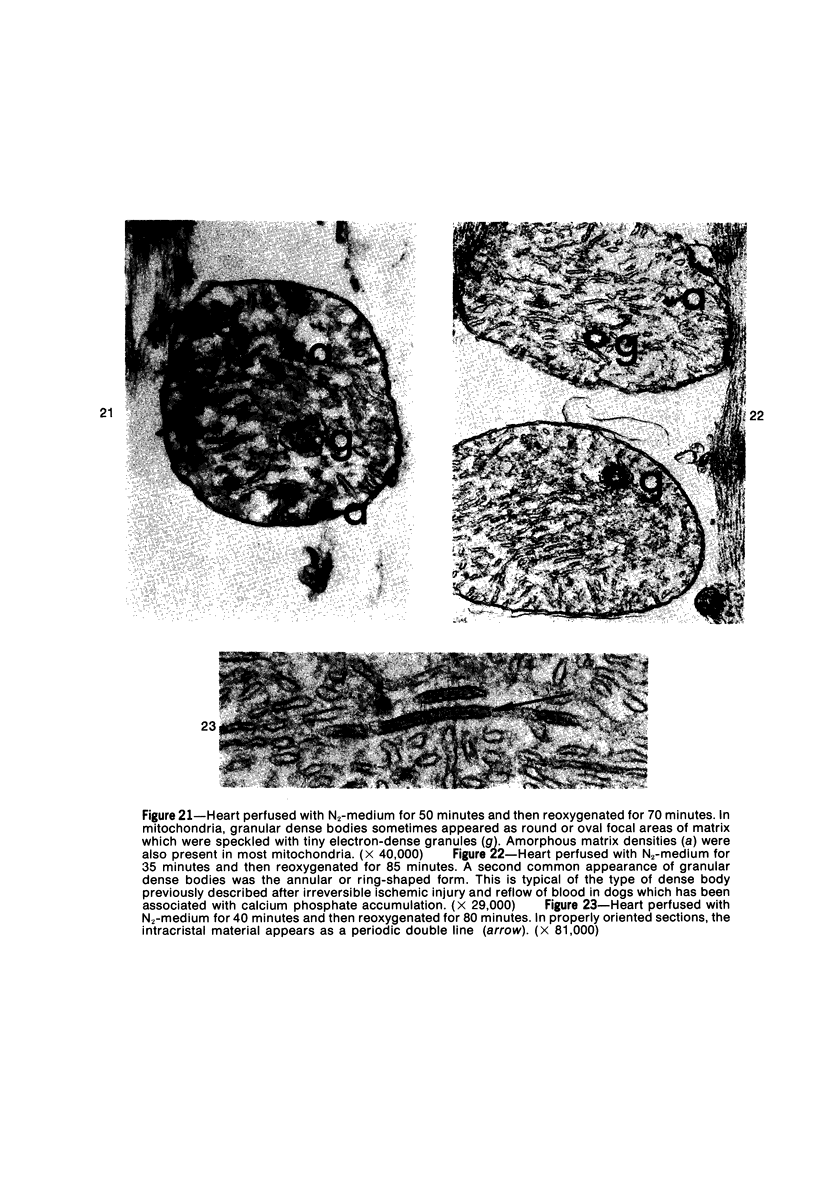
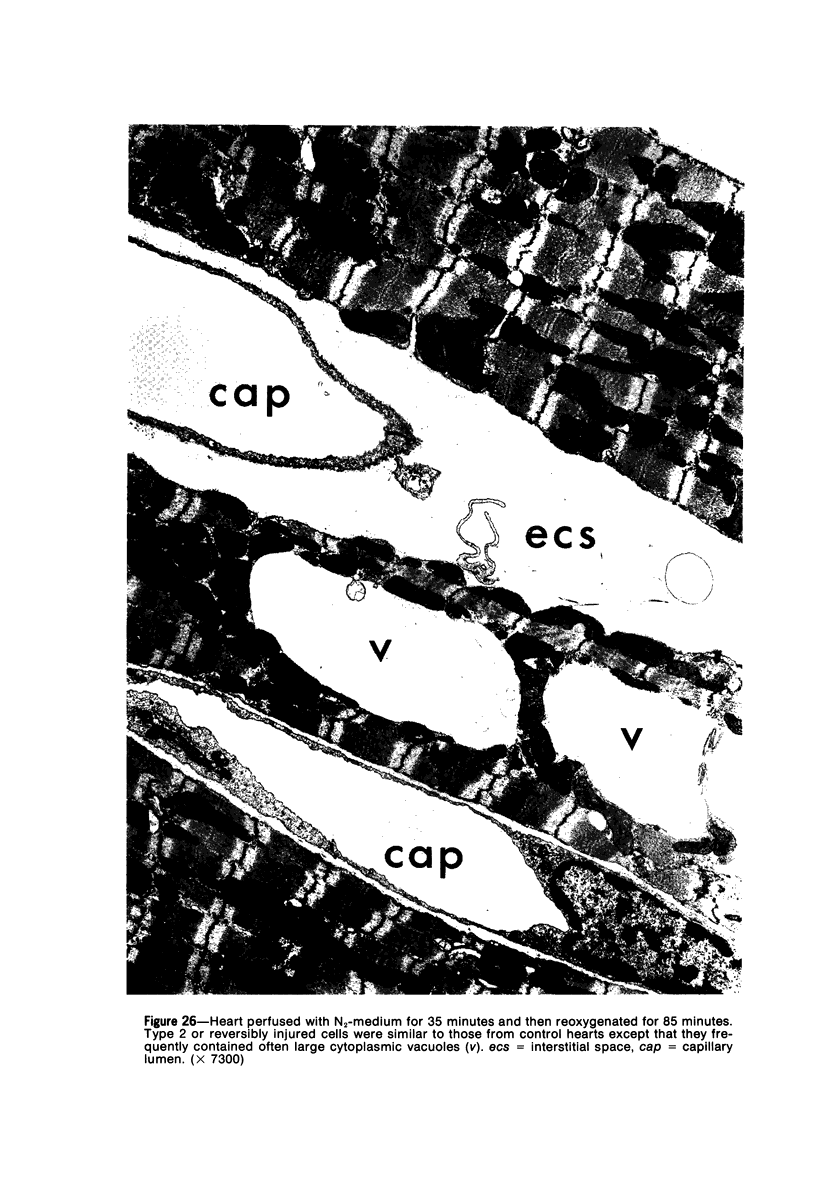
Isolated rat hearts were perfused at 37 C on a double reservoir, nonrecirculating Langendorff apparatus. For aerobic perfusion, Krebs-Henseleit medium containing 10 mM glucose was gassed with 95% O2-5% CO2; for anoxic perfusion, glucose was replaced with 10 mM mannitol, a nonmetabolizable substrate, and the medium was equilibrated with 95% N2-5% CO2. Heart effluent was serially collected during perfusion for creatine phosphokinase activity (CPK) analysis. Fixation was with 1% glutaraldehyde for morphologic studies. With aerobic control perfusion, hearts continued contracting, released no CPK, and were morphologically normal by light and electron microscopy examination after 120 minutes. With anoxic perfusion, contractions soon ceased, and by 60 minutes a sustained slow release of CPK was first observed. By electron microscopy, cells at 60 or 90 minutes were swollen and contained amorphous matrix densities in mitochondria; a few cells showed breaks in cell plasma membrane. When anoxic hearts were challenged with reoxygenation, there was a sudden change in color to a pale opaque appearance, CPK was rapidly released, and there was massive cellular swelling. By electron microscopy, damaged cells showed contraction bands, clumping of nuclear chromatin, both amorphous densities and granular dense bodies in mitochondria, and prominent disruptions of cell plasma membranes. The number of damaged cells observed increased as a linear function of time between 30 and 55 minutes of anoxia. The results show that anoxic perfusion in vitro produces irreversible myocardial injury and that this injury is closely associated with loss of cell volume control, release of intracellular enzymes, and striking structural defects in the plasma membrane of the sarcolemma. Reoxygenation accelerates the development of lesions in irreversibly injured cells but protects reversibly injured cells.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuld R. A., Weiss A., Kruger F. A., Weissler A. M. Anaerobic performance and metabolism of the hyperthyroid heart. J Clin Invest. 1969 Oct;48(10):1905–1913. doi: 10.1172/JCI106157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachfeld N. Bioenergetics of the normal and anoxic myocardium. Adv Cardiopulm Dis. 1969;4:66–90. [PubMed] [Google Scholar]
- CAULFIELD J., KLIONSKY B. Myocardial ischemia and early infarction: an electron microscopic study. Am J Pathol. 1959 May-Jun;35(3):489–523. [PMC free article] [PubMed] [Google Scholar]
- Croker B. P., Jr, Saladino A. J., Trump B. F. Ion movements in cell injury: relationship between energy metabolism and the pathogenesis of lethal injury in the toad bladder. Am J Pathol. 1970 May;59(2):247–278. [PMC free article] [PubMed] [Google Scholar]
- Csapó Z., Dusek J., Rona G. Peculiar myofilament changes near the intercalated disc in isoproterenol-induced cardiac muscle cell injury. J Mol Cell Cardiol. 1974 Feb;6(1):79–83. doi: 10.1016/0022-2828(74)90009-1. [DOI] [PubMed] [Google Scholar]
- De la Iglesia F. A., Lumb G. Ultrastructural and circulatory alterations of the myocardium in experimental coronary artery narrowing. Lab Invest. 1972 Jul;27(1):17–31. [PubMed] [Google Scholar]
- Feuvray D., de Leiris J. Effect of short dimethylsulfoxide perfusions on ultrastructure of the isolated rat heart. J Mol Cell Cardiol. 1973 Feb;5(1):63–69. doi: 10.1016/0022-2828(73)90036-9. [DOI] [PubMed] [Google Scholar]
- Friedman I., Moravec J., Reichart E., Hatt P. Y. Subacute myocardial hypoxia in the rat. An electron microscopic study of the left ventricular myocardium. J Mol Cell Cardiol. 1973 Apr;5(2):125–132. doi: 10.1016/0022-2828(73)90045-x. [DOI] [PubMed] [Google Scholar]
- GREENAWALT J. W., ROSSI C. S., LEHNINGER A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol. 1964 Oct;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritzka T. L., Trump B. F. Renal tubular lesions caused by mercuric chloride. Electron microscopic observations: degeneration of the pars recta. Am J Pathol. 1968 Jun;52(6):1225–1277. [PMC free article] [PubMed] [Google Scholar]
- HERDSON P. B., SOMMERS H. M., JENNINGS R. B. A COMPARATIVE STUDY OF THE FINE STRUCTURE OF NORMAL AND ISCHEMIC DOG MYOCARDIUM WITH SPECIAL REFERENCE TO EARLY CHANGES FOLLOWING TEMPORARY OCCLUSION OF A CORONARY ARTERY. Am J Pathol. 1965 Mar;46:367–386. [PMC free article] [PubMed] [Google Scholar]
- Hatt P. Y., Moravec J. Acute hypoxia of the myocardium. Ultrastructural changes. Cardiology. 1971;56(1):73–84. doi: 10.1159/000169343. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Chain E. B. Effect of glucose on enzyme release from, and recovery of, the anoxic myocardium. Recent Adv Stud Cardiac Struct Metab. 1973;3:763–772. [PubMed] [Google Scholar]
- Hearse D. J., Chain E. B. The role of glucose in the survival and 'recovery' of the anoxic isolated perfused rat heart. Biochem J. 1972 Aug;128(5):1125–1133. doi: 10.1042/bj1281125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Chain E. B. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol. 1973 Aug;5(4):395–407. doi: 10.1016/0022-2828(73)90030-8. [DOI] [PubMed] [Google Scholar]
- Herdson P. B., Kaltenbach J. P., Jennings R. B. Fine structural and biochemical changes in dog myocardium during autolysis. Am J Pathol. 1969 Dec;57(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- JENNINGS R. B., BAUM J. H., HERDSON P. B. FINE STRUCTURAL CHANGES IN MYOCARDIAL ISCHEMIC INJURY. Arch Pathol. 1965 Feb;79:135–143. [PubMed] [Google Scholar]
- JENNINGS R. B., KALTENBACH J. P., SMETTERS G. W. Ensymatic changes in acute myocardial ischemic injury; glutamic oxaloacetic transaminase, lactic dehydrogenase, and succinic dehydrogenase. AMA Arch Pathol. 1957 Jul;64(1):10–16. [PubMed] [Google Scholar]
- JENNINGS R. B., SOMMERS H. M., SMYTH G. A., FLACK H. A., LINN H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960 Jul;70:68–78. [PubMed] [Google Scholar]
- Kjekshus J. K., Sobel B. E. Depressed myocardial creatine phosphokinase activity following experimental myocardial infarction in rabbit. Circ Res. 1970 Sep;27(3):403–414. doi: 10.1161/01.res.27.3.403. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Kübler W., Spieckermann P. G. Regulation of glycolysis in the ischemic and the anoxic myocardium. J Mol Cell Cardiol. 1970 Dec;1(4):351–377. doi: 10.1016/0022-2828(70)90034-9. [DOI] [PubMed] [Google Scholar]
- LADUE J. S., WROBLEWSKI F. The significance of the serum glutamic oxalacetic transaminase activity following acute myocardial infarction. Circulation. 1955 Jun;11(6):871–877. doi: 10.1161/01.cir.11.6.871. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., LA GATTUTA M., THOMPSON T. E. Cellular death and necrosis: chemical, physical and morphologic changes in rat liver. Virchows Arch Pathol Anat Physiol Klin Med. 1960;333:421–465. doi: 10.1007/BF00955327. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res. 1966 Jun;15(3):242–282. doi: 10.1016/s0022-5320(66)80109-0. [DOI] [PubMed] [Google Scholar]
- Moravec J., Corsin A., Guillemot H., Hatt P. Absence of the relationship between ultrastructure and metabolic state of heart mitochondria in situ. J Mol Cell Cardiol. 1971 Jun;2(2):161–171. doi: 10.1016/0022-2828(71)90068-x. [DOI] [PubMed] [Google Scholar]
- Myocardial ischemia--observations, definitions and speculations. J Mol Cell Cardiol. 1970 Dec;1(4):345–349. [PubMed] [Google Scholar]
- Neely J. R., Liebermeister H., Battersby E. J., Morgan H. E. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 1967 Apr;212(4):804–814. doi: 10.1152/ajplegacy.1967.212.4.804. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J., Whitmer J. T., Morgan H. E. Effects of ischemia on function and metabolism of the isolated working rat heart. Am J Physiol. 1973 Sep;225(3):651–658. doi: 10.1152/ajplegacy.1973.225.3.651. [DOI] [PubMed] [Google Scholar]
- OLIVER I. T. A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955 Sep;61(1):116–122. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poche R. Ultrastructure of heart muscle under pathological conditions. Ann N Y Acad Sci. 1969 Jan 31;156(1):34–47. doi: 10.1111/j.1749-6632.1969.tb16716.x. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Ganote C. E., Jennings R. B. Alterations in renal cortex following ischemic injury. 3. Ultrastructure of proximal tubules after ischemia or autolysis. Lab Invest. 1972 Apr;26(4):347–363. [PubMed] [Google Scholar]
- Sapsford R. N., Blackstone E. H., Kirklin J. W., Karp R. B., Kouchoukos N. T., Pacifico A. D., Roe C. R., Bradley E. L. Coronary perfusion versus cold ischemic arrest during aortic valve surgery. A randomized study. Circulation. 1974 Jun;49(6):1190–1199. doi: 10.1161/01.cir.49.6.1190. [DOI] [PubMed] [Google Scholar]
- Scheuer J., Stezoski S. W. Protective role of increased myocardial glycogen stores in cardiac anoxia in the rat. Circ Res. 1970 Nov;27(5):835–849. doi: 10.1161/01.res.27.5.835. [DOI] [PubMed] [Google Scholar]
- Scheuer J., Stezoski S. W. Protective role of increased myocardial glycogen stores in cardiac anoxia in the rat. Circ Res. 1970 Nov;27(5):835–849. doi: 10.1161/01.res.27.5.835. [DOI] [PubMed] [Google Scholar]
- Shell W. E., Kjekshus J. K., Sobel B. E. Quantitative assessment of the extent of myocardial infarction in the conscious dog by means of analysis of serial changes in serum creatine phosphokinase activity. J Clin Invest. 1971 Dec;50(12):2614–2625. doi: 10.1172/JCI106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Kinetics of calcium accumulation in acute myocardial ischemic injury. Am J Pathol. 1972 Jun;67(3):441–452. [PMC free article] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972 Jun;67(3):417–440. [PMC free article] [PubMed] [Google Scholar]
- Sobel B. E., Shell W. E. Serum enzyme determinations in the diagnosis and assessment of myocardial infarction. Circulation. 1972 Feb;45(2):471–482. doi: 10.1161/01.cir.45.2.471. [DOI] [PubMed] [Google Scholar]
- TRUMP B. F., GOLDBLATT P. J., STOWELL R. E. STUDIES ON NECROSIS OF MOUSE LIVER IN VITRO. ULTRASTRUCTURAL ALTERATIONS IN THE MITOCHONDRIA OF HEPATIC PARENCHYMAL CELLS. Lab Invest. 1965 Apr;14:343–371. [PubMed] [Google Scholar]
- Tyers G. F., Hughes H. C., Jr, Todd G. J., Williams D. R., Andrews E. J., Prophet G. A., Waldhausen J. A. Protection from ischemic cardiac arrest by coronary perfusion with cold Ringer's lactate solution. J Thorac Cardiovasc Surg. 1974 Mar;67(3):411–418. [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966 Nov 10;241(21):5026–5036. [PubMed] [Google Scholar]
- YOKOYAMA H. O., JENNINGS R. B., WARTMAN W. B. Intercalated disks of dog myocardium. Exp Cell Res. 1961 Feb;23:29–44. doi: 10.1016/0014-4827(61)90061-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. N., Hülsmann W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966 Aug 6;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]