Abstract
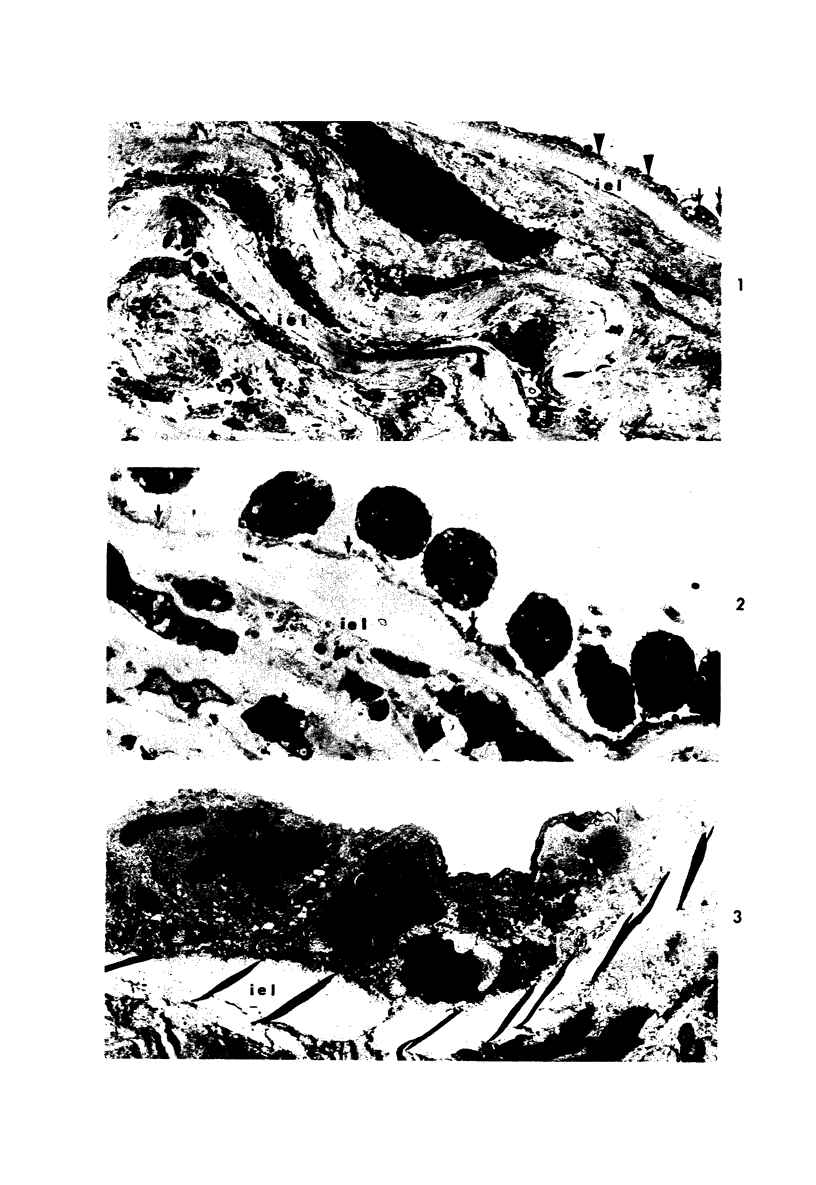
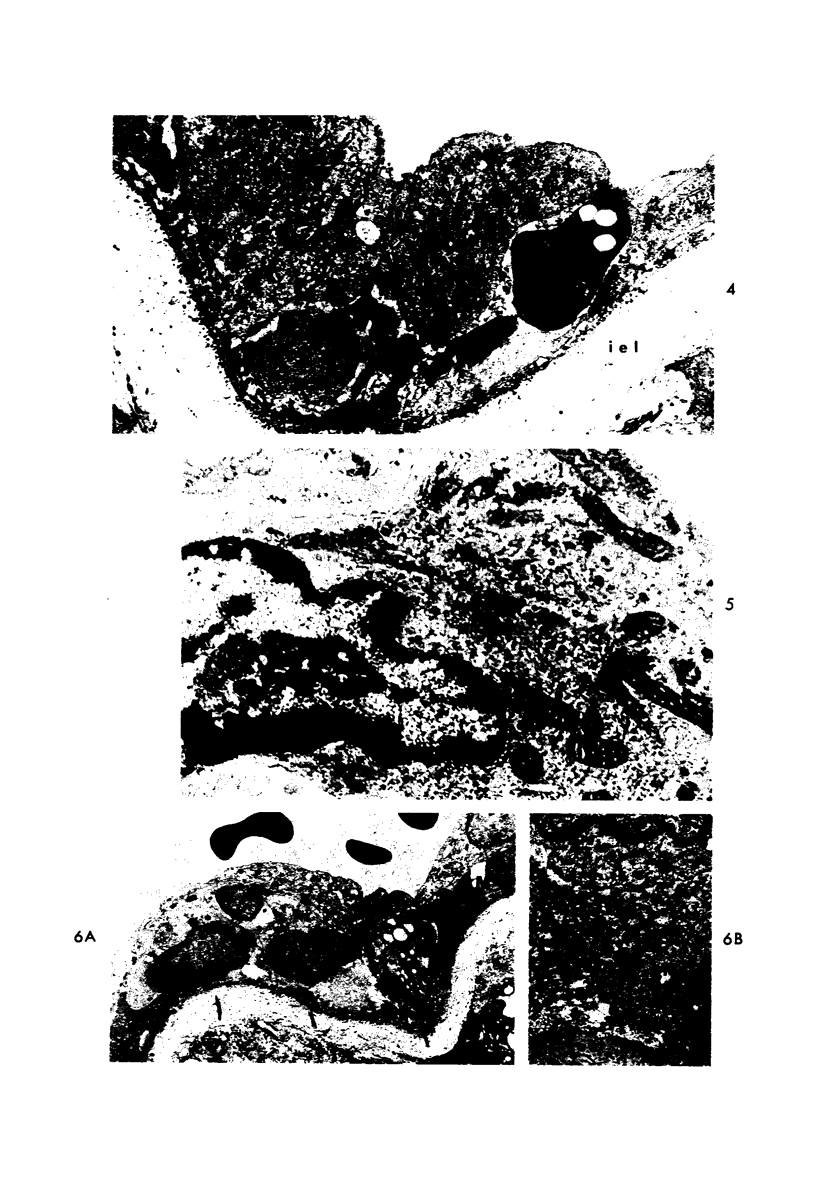
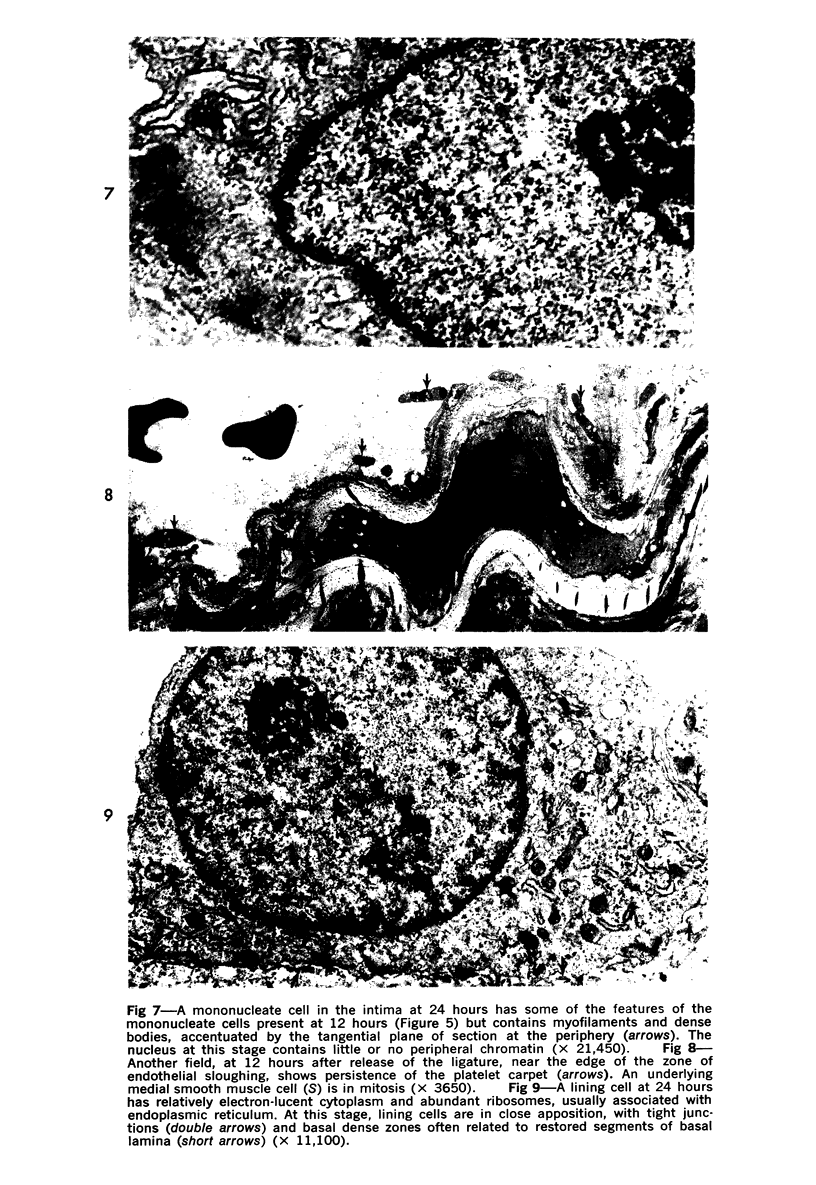
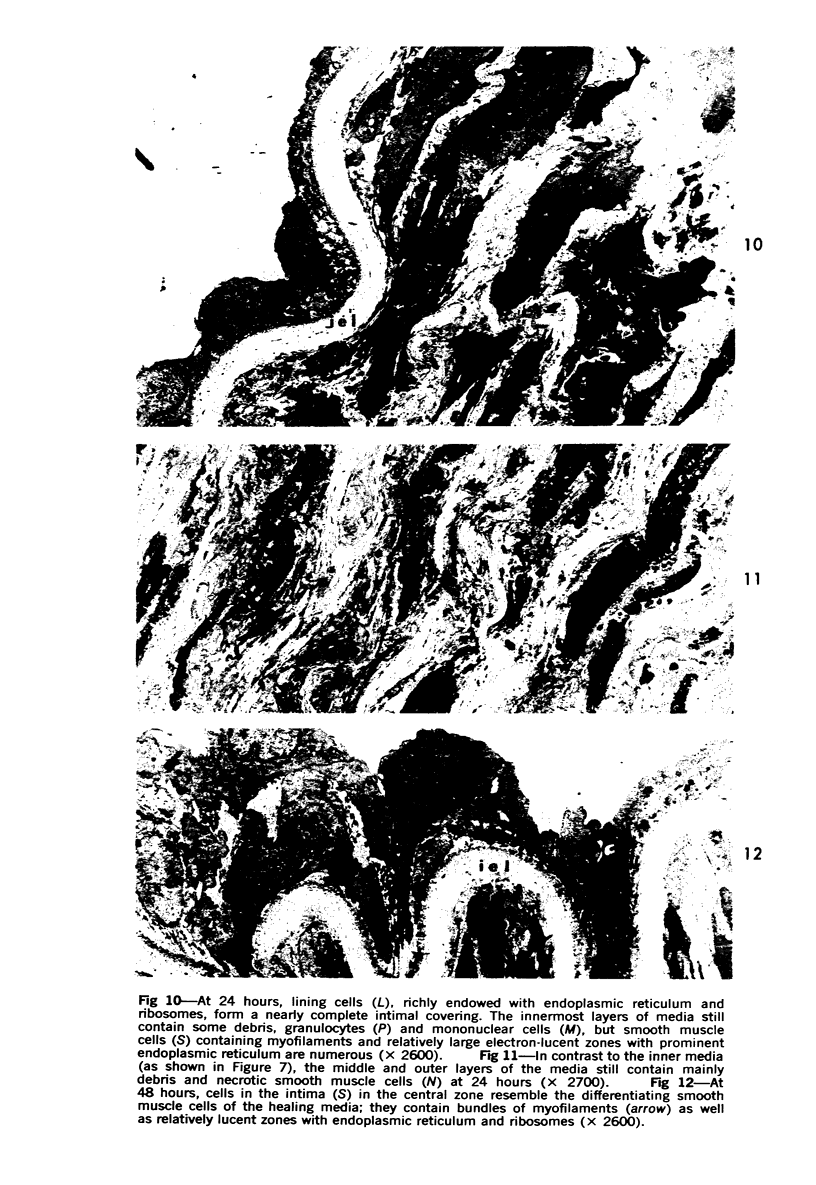
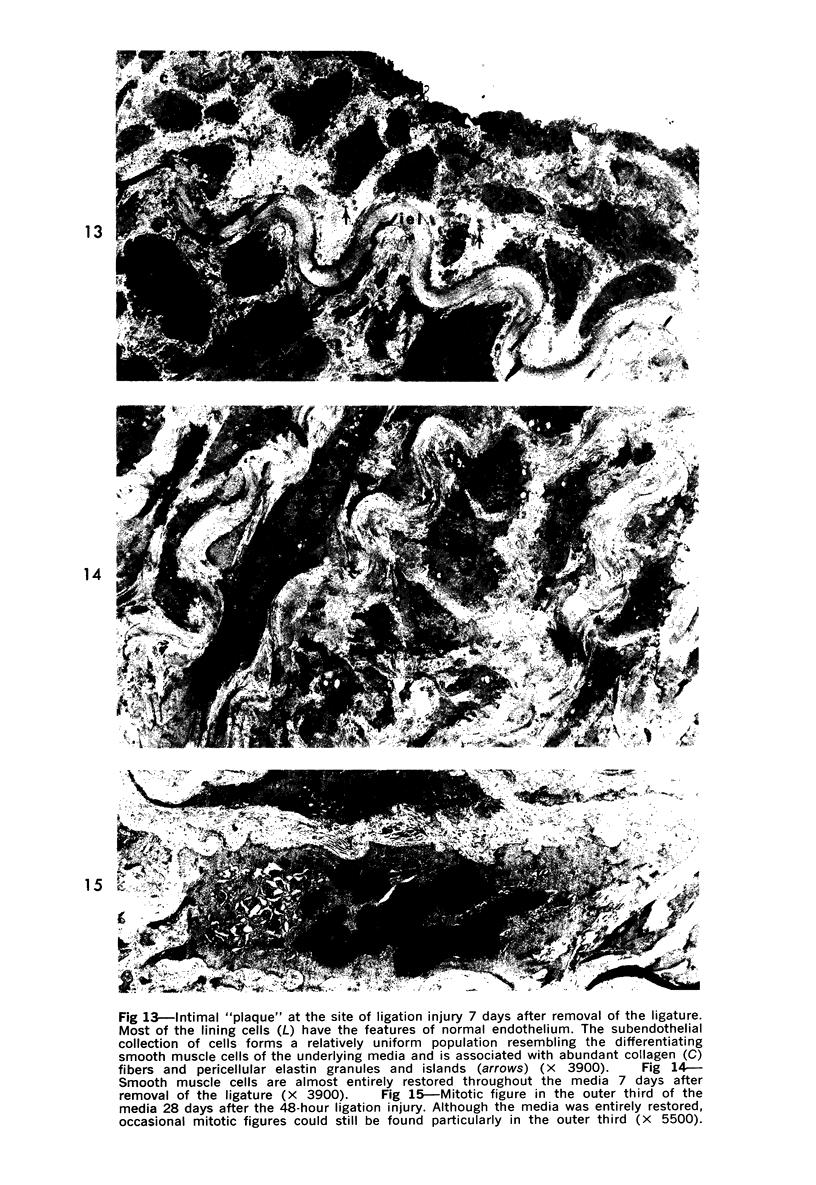
Partial ligation of the rabbit abdominal aorta with fine silk suture for 48 hours produced a circular band of transmural necrosis. On release of the ligature, blood cells from the lumen and from adventitial vasa vasorum, as well as cells derived by mitosis from the adjacent surviving endothelium and media, participated in the restitution of a continuous endothelial lining and an intact media containing well-differentiated smooth muscle cells within normal medial lamellar units. Initial deposition of a layer of blood platelets on the fibrillar material coating the denuded lumenal surface was followed by ingress from the lumen of polymorphonuclear granulocytes and mononuclear cells. These changes preceded the appearance of mitoses in surviving endothelial and medial smooth muscle cells at the margin of injury. By 24 hours, poorly differentiated cells had accumulated in the central portion of the intima and inner media. Similar cells formed a more extensive, nearly complete lumenal layer which was eventually continuous with and indistinguishable from the adjacent uninjured endothelium. By 7 days, smooth muscle cells repopulated the media, and a collection of less differentiated cells persisted between the restored endothelium and media. By 28 days, the only deviation from normal arterial structure was the persistence at the point of ligature of intimal thickening, consisting of smooth muscle cells and collagen and elastin fibers. Though still present at 6 weeks, this zone became increasingly compact and layered. There was no evidence that fibrin thrombus formation was a consistent feature of the initial reaction or that it played a role in the healing process or in the formation of the intimal lesion. Despite complete circumferential necrosis at the site of ligature, there was no evidence of medial rupture or intramural hemorrhage.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUMGARTNER H. R. EINE NEUE METHODE ZUR ERZEUGUNG VON THROMBEN DURCH GEZIELTE UBERDEHNUNG DER GEFAESSWAND. Z Gesamte Exp Med. 1963 Sep 12;137:227–247. [PubMed] [Google Scholar]
- Barbolini G., Scilabra G. A., Botticelli A., Botticelli S. On the origin of foam cells in cholesterol-induced atherosclerosis of the rabbit. Virchows Arch B Cell Pathol. 1969;3(1):24–32. doi: 10.1007/BF02901924. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R., Stemerman M. B., Spaet T. H. Adhesion of blood platelets to subendothelial surface: distinct from adhesion to collagen. Experientia. 1971 Mar 15;27(3):283–285. doi: 10.1007/BF02138148. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkerud S., Bondjers G. Arterial repair and atherosclerosis after mechanical injury. 2. Tissue response after induction of a total local necrosis deep longitudinal injury. Atherosclerosis. 1971 Sep-Oct;14(2):259–276. doi: 10.1016/0021-9150(71)90055-4. [DOI] [PubMed] [Google Scholar]
- Borgers M., Schaper J., Schaper W. The origin of subendothelial cells in developing coronary collaterals. A cytochemical approach. Virchows Arch A Pathol Pathol Anat. 1973 Feb 19;358(4):281–294. doi: 10.1007/BF00543269. [DOI] [PubMed] [Google Scholar]
- Esterly J. A., Glagov S., Ferguson D. J. Morphogenesis of intimal obliterative hyperplasia of small arteries in experimental pulmonary hypertension. An ultrastructural study of the role of smooth-muscle cells. Am J Pathol. 1968 Feb;52(2):325–347. [PMC free article] [PubMed] [Google Scholar]
- Friedman S. M., Scott G. H., Nakashima M. Vascular morphology in hypertensive states in the rat. Anat Rec. 1971 Dec;171(4):529–544. doi: 10.1002/ar.1091710409. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Geis W. P., Kaye M. P. Distribution of sympathetic fibers in the left ventricular epicardial plexus of the dog. Circ Res. 1968 Aug;23(2):165–170. doi: 10.1161/01.res.23.2.165. [DOI] [PubMed] [Google Scholar]
- HAUST M. D., WYLLIE J. C., MORE R. H. ELECTRON MICROSCOPY OF FIBRIN IN HUMAN ATHEROSCLEROTIC LESIONS; IMMUNOHISTOCHEMICAL AND MORPHOLOGIC IDENTIFICATION. Exp Mol Pathol. 1965 Apr;28:205–216. doi: 10.1016/0014-4800(65)90033-x. [DOI] [PubMed] [Google Scholar]
- Hassler O. The origin of the cells constituting arterial intima thickening. An experimental autoradiographic study with the use of H3-thymidine. Lab Invest. 1970 Apr;22(4):286–293. [PubMed] [Google Scholar]
- Haudenschild C., Studer A. Early interactions between blood cells and severely damaged rabbit aorta. Eur J Clin Invest. 1971 Nov;2(1):1–7. doi: 10.1111/j.1365-2362.1971.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Helin P., Lorenzen I., Garbarsch C., Matthiessen M. E. Repair in arterial tissue. Morphological and biochemical changes in rabbit aorta after a single dilatation injury. Circ Res. 1971 Nov;29(5):542–554. doi: 10.1161/01.res.29.5.542. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., Gottlob R. Ultrastructural changes of large rabbit blood vessels following mild mechanical trauma. Virchows Arch A Pathol Pathol Anat. 1968;345(2):93–106. doi: 10.1007/BF00548644. [DOI] [PubMed] [Google Scholar]
- Honour A. J., Pickering G. W., Sheppard B. L. Ultrastructure and behaviour of platelet thrombi in injured arteries. Br J Exp Pathol. 1971 Oct;52(5):482–494. [PMC free article] [PubMed] [Google Scholar]
- KJAERHEIM A., HOVIG T. The ultrastructure of haemostatic blood platelet plugs in rabbit mesenterium. Thromb Diath Haemorrh. 1962 Mar 15;7:1–15. [PubMed] [Google Scholar]
- Kennedy L. J., Jr, Weissman I. L. Dual origin of intimal cells in cardiac-allograft arteriosclerosis. N Engl J Med. 1971 Oct 14;285(16):884–887. doi: 10.1056/NEJM197110142851603. [DOI] [PubMed] [Google Scholar]
- Poole J. C., Cromwell S. B., Benditt E. P. Behavior of smooth muscle cells and formation of extracellular structures in the reaction of arterial walls to injury. Am J Pathol. 1971 Mar;62(3):391–414. [PMC free article] [PubMed] [Google Scholar]
- RODBARD S. Physical forces and the vascular lining. Ann Intern Med. 1959 Jun;50(6):1339–1351. doi: 10.7326/0003-4819-50-6-1339. [DOI] [PubMed] [Google Scholar]
- Rodbard S. Physical factors in arterial sclerosis and stenosis. Adv Cardiol. 1970;4:72–93. doi: 10.1159/000387606. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- SPIRO D., LATTES R. G., WIENER J. THE CELLULAR PATHOLOGY OF EXPERIMENTAL HYPERTENSION. I. HYPERPLASTIC ARTERIOLARSCLEROSIS. Am J Pathol. 1965 Jul;47:19–49. [PMC free article] [PubMed] [Google Scholar]
- STUMP M. M., JORDAN G. L., Jr, DEBAKEY M. E., HALPERT B. ENDOTHELIUM GROWN FROM CIRCULATING BLOOD ON ISOLATED INTRAVASCULAR DACRON HUB. Am J Pathol. 1963 Sep;43:361–367. [PMC free article] [PubMed] [Google Scholar]
- Sheppard B. L., French J. E. Platelet adhesion in the rabbit abdominal aorta following the removal of the endothelium: a scanning and transmission electron microscopical study. Proc R Soc Lond B Biol Sci. 1971 Jan 12;176(1045):427–432. doi: 10.1098/rspb.1971.0006. [DOI] [PubMed] [Google Scholar]
- Sheppard B. L. Platelet adhesion in the rabbit abdominal aorta following the removal of endothelium with EDTA. Proc R Soc Lond B Biol Sci. 1972 Jul 25;182(1066):103–108. doi: 10.1098/rspb.1972.0069. [DOI] [PubMed] [Google Scholar]
- Stehbens W. E., Ludatscher R. M. Ultrastructure of the renal arterial bifurcation of rabbits. Exp Mol Pathol. 1973 Feb;18(1):50–67. doi: 10.1016/0014-4800(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh A. D., Stemerman M. B., Spaet T. H. Rabbit heart valve basement membrane: low platelet reactivity. Blood. 1973 Mar;41(3):359–367. [PubMed] [Google Scholar]
- Ts'ao C. H., Glagov S. Platelet adhesion to subendothelial components in experimental aortic injury. Role of fine fibrils and basement membrane. Br J Exp Pathol. 1970 Aug;51(4):423–427. [PMC free article] [PubMed] [Google Scholar]
- Ts'ao C. H. Graded endothelial injury of the rabbit aorta. With special reference to platelet deposition. Arch Pathol. 1970 Sep;90(3):222–229. [PubMed] [Google Scholar]
- Ts'ao C. H., Spaet T. H. Ultramicroscopic changes in the rabbit inferior vena cava following partial constriction. Am J Pathol. 1967 Nov;51(5):789–813. [PMC free article] [PubMed] [Google Scholar]
- Ts'ao C. In vitro platelet reaction with isolated glomerular basement membrane. Ultrastructural comparison with platelet-collagen reaction. Thromb Diath Haemorrh. 1971 Jun 30;25(3):507–516. [PubMed] [Google Scholar]
- Veress B., Kádár A., Jellinek H. Ultrastructural elements in experimental intimal thickening. I. Electron microscopic study of the development and cellular elements of intimal proliferation. Exp Mol Pathol. 1969 Oct;11(2):200–211. doi: 10.1016/0014-4800(69)90008-2. [DOI] [PubMed] [Google Scholar]
- WOLINSKY H., GLAGOV S. STRUCTURAL BASIS FOR THE STATIC MECHANICAL PROPERTIES OF THE AORTIC MEDIA. Circ Res. 1964 May;14:400–413. doi: 10.1161/01.res.14.5.400. [DOI] [PubMed] [Google Scholar]
- Weiss P., Kranz D., Marx I., Fuhrmann I. Elektronenmikroskopische Befunde bei dopelter Stenosierung der Bauchaorta von Ratten und Katzen. Exp Pathol (Jena) 1971;5(1):70–77. [PubMed] [Google Scholar]