Abstract
Rathke's pouch contains progenitor cells that differentiate into the endocrine cells of the pituitary gland. It gives rise to gonadotrope, thyrotrope, somatotrope, corticotrope and lactotrope cells in the anterior lobe and the intermediate lobe melanotropes. Pituitary precursor cells express many members of the Notch signaling pathway including the downstream effecter gene Hes1. We hypothesized that Hes1 regulates the timing of precursor differentiation and cell fate determination. To test this idea, we expressed Hes1 in differentiating pituitary cells and found that it can inhibit gonadotrope and thyrotrope differentiation. Pituitaries of Hes1 deficient mice have anterior lobe hypoplasia. All cells in the anterior lobe are specified and differentiate, but an early period of increased cell death and reduced proliferation causes reduced growth, evident as early as e14.5. In addition, cells within the intermediate lobe differentiate into somatotropes instead of melanotropes. Thus, the Hes1 repressor is essential for melanotrope specification. These results demonstrate that Notch signaling plays multiple roles in pituitary development, influencing precursor number, organ size, cell differentiation and ultimately cell fate.
Keywords: Pituitary, Notch, Hes1, melanotrope, proliferation, transgenic
Introduction
The pituitary gland controls the release of hormones that direct growth, metabolism, fertility and the body's response to stress. The anterior and intermediate lobes of this gland arise from a structure known as Rathke's pouch that invaginates from the oral ectoderm at embryonic day 8.5 (e8.5) in the mouse (Burrows et al., 1999; Zhu and Rosenfeld, 2004). Before birth, a cascade of signaling events activates transcription factors that serve to specify the five cell types of the anterior pituitary in a temporally discrete manner. The order of appearance of the fully differentiated cell types in rodents is corticotropes, thyrotropes, somatotropes, lactotropes and gonadotropes that secrete adrenocorticotropic hormone (ACTH), thyroid stimulating hormone (TSH), growth hormone (GH), prolactin (PRL), and gonadotropins (FSH and LH). The intermediate lobe is composed of melanotropes that secrete melanocyte stimulating hormone (MSH).
We reported that many members of the Notch signaling pathway are expressed in the developing pituitary gland and hypothesized that Notch signaling regulates the transition from proliferation to differentiation (Raetzman et al., 2004). Notch is an evolutionarily conserved signaling system first identified in Drosophila. The transmembrane receptor Notch interacts with its ligand (Delta/Jagged) on a neighboring cell, resulting in the cleavage of the Notch intracellular domain (NICD), which then binds to Rbpsuh. This complex translocates to the nucleus and activates the expression of transcriptional repressor Hes and Hey genes. Hes genes classically function by inhibiting the transcription of bHLH genes that normally promote cellular differentiation. In the pituitary, there is a temporal and dorsal-ventral restriction of Notch receptors and ligands during development (Raetzman et al., 2004). The Notch2 and Notch3 receptors and Hes1 are present in Rathke's Pouch progenitor cells, but are excluded from differentiating progenitors.
Proper expression of Hes1 is essential for the development of many organs. In Hes1 null mice, the pancreas is hypoplastic due to premature precursor differentiation (Jensen et al., 2000) and the biliary epithelium undergoes cell fate conversion to pancreatic tissue (Apelqvist et al., 1999; Sumazaki et al., 2004). In the intestine, loss of Hes1 leads to increased NeuroD and Math1 expression concomitant with premature hormone expression (Jensen et al., 2000). Muscle precursor differentiation is blocked by Hes1 mediated repression of MyoD (Kuroda et al., 1999). Notch signaling through Hes1 can also have a specific influence on endocrine cell selection. In lung development, neuroendocrine cells express Notch ligands and inhibit Clara cells from becoming neuroendocrine cells by activating Notch receptors and Hes genes (Ito et al., 2000). Intestinal lineage specification from crypt cells is also reliant on Notch signaling. Inhibition of Notch signaling by blocking receptor cleavage or by deleting Hes1 causes Goblet and enteroendocrine cells to be formed preferentially, the other lineages are lost and there is overexpression of Ngn3 (Jensen et al., 2000; Wong et al., 2004). Ngn3 is necessary cell autonomously to promote enteroendocrine cell differentiation (Jenny et al., 2002). Taken together, these studies highlight a common theme in endocrine development, which is that Notch signaling represses endocrine cell development from undifferentiated precursors and/or from other differentiated cells.
To determine if Notch signaling mediates the transition from pituitary precursor proliferation to differentiation, or influences the specification hormone producing cell types, we undertook a gain and loss of function analysis of Hes1 in pituitary organogenesis. We demonstrate that persistent expression of Hes1 in pre-gonadotropes and pre-thyrotropes prevents their differentiation. Hes1 deficient mice have abnormally small pituitary glands, due in part to increased cell death and decreased proliferation. Additionally, the intermediate lobe melanotropes exhibit a cell fate switch to somatotropes in the absence of Hes1. These data suggest that Notch signaling through Hes1 both inhibits pituitary cell differentiation and promotes melanotrope cell fate.
Materials and Methods
Transgene Construction
To generate the Cga-Hes1 construct, the Hes1 open reading frame (Ishibashi et al., 1994) was inserted into a pBSK plasmid containing the mouse protamine intron, splice sites and polyadenylation sequences. A 4.6 kb fragment containing the mouse α subunit (Cga) promoter and enhancer (Brinkmeier et al., 1998; Kendall et al., 1994) was used to direct expression to the pre-gonadotropes and pre-thyrotropes of the anterior pituitary. Restriction enzyme mapping and partial sequencing were used to verify the identity of the construct. Prior to microinjection, the construct was released from the vector by a restriction enzyme digest with Kpn1 and Cla1.
Generation and Genotyping of Transgenic Mice
The purified insert was microinjected into F2 zygotes from F1 (C57BL/6J X SJL/J) parents (JAX labs) by the University of Michigan Transgenic Animal Model Core. Embryos at the two-cell stage were implanted in pseudopregnant CD-1 females (Charles River) at embryonic day 0.5 (e0.5). For transgenic founder analysis, 106 embryos were collected at e18.5. Genotyping to identify the presence of the transgene was conducted using genomic DNA isolated from tail biopsy samples. Oligonucleotides were designed to amplify a region spanning the Hes1 cDNA and the mouse protamine sequence: 5' TAACGCAGTGTCACCTTCCA 3' and 5' ATCTGCTCCTGCTTTTGCTG 3'. A standard reaction mixture was used containing the following concentrations of reagents per reaction; primers 12.5 pmol, BSA 5 μg, and Taq 5U. PCR amplification was conducted for 29 cycles of denaturing at 92°C for 30 seconds, annealing at 55°C for 30 seconds, and elongating at 72°C for 30 seconds with a final elongation step conducted at 72°C for 10 minutes.
Hes1 null mice
Hes1 null mice were previously generated by replacing the first 3 exons of Hes1, including the bHLH domain, with a neomycin-resistance cassette (Ishibashi et al., 1995). A breeding colony of Hes1 null heterozygotes was established at University of Michigan by re-derivation. Embryos resulting from matings between heterozygote males obtained from Dr. Ryoichiro Kageyama and C56BL6/J females (Jackson Laboratory) were transferred to specific pathogen free surrogate mothers. The resulting heterozygote progeny were intercrossed for the experiments. Genotyping was performed as previously described (Jensen et al., 2000). Embryos at specific ages were obtained from Hes1 heterozygote females mated with Hes1 heterozygote males. All procedures involving the use of mice were approved by the University of Michigan Committee on the Use and Care of Animals. All experiments were conducted in accordance with the principles and procedures outlined in the NIH guidelines for the Care and Use of Experimental Animals.
For bromo-deoxyuridine (BrdU) experiments, pregnant mice were injected intra-peritoneally with 0.1mg/g body weight BrdU two hours prior to collecting the fetuses (Nowakowski et al., 1989).
Histology, Immunohistochemistry and in situ hybridization
Wild type, Hes1 transgenic, and Hes1 null embryos and adult pituitaries were fixed for 2-24 h in 10% formalin in phosphate buffered saline pH 7.2, dehydrated, and embedded in paraffin. Sagittal or coronal sections of 6 micrometers were then prepared for immunostaining or in situ hybridization. For transcription factor immunostaining, slides were boiled in 10mM citric acid, pH6, for 10 minutes and then incubated with the mouse monoclonal LHX3 antibody (1:1000, C651.6DbHN developmental Studies Hybridoma Bank, University of Iowa, Iowa city IA), mouse monoclonal ISL1 (1:600; 40.2D6 DSHB) or rabbit polyclonal T-PIT (1:600; gift of J. Drouin, Montreal, Canada) which were diluted using the M.O.M antibody kit (Vector Laboratories) for LHX3 and ISL1 or PBS containing BSA (3%), Tween-20 (0.5%), and normal donkey serum (5% w/v) for T-PIT as previously described (Raetzman et al., 2004; Raetzman et al., 2002). The Perkin-Elmer TSA kit was used for antibody detection. For immunostaining with Cyclin D2 (1:250, Santa Cruz) Prohormone convertase 2 (1:300; Chemicon) or bromo-deoxyuridine (BrdU, 1:100; Harlan Sera), the slides were boiled in citrate (see above) for 5 minutes. Antibodies were detected with anti-rabbit-cy2 (1:200, Jackson ImmunoResearch), anti-rabbit-cy3 (1:200, Jackson ImmunoResearch) or anti-rat TRITC (1:200, Jackson ImmunoResearch), respectively. All fluorescently stained slides were mounted in an aqueous mounting media. Antibodies were also used to detect hormones: ACTH (also recognizes MSH; 1:1000; DAKO), MSH (1:500; Chemicon) LHβ (1:1500; National Hormone and Pituitary Program-NHPP), FSHβ (1:1800; NHPP), GH (1:1000; NHPP) and TSHβ (1:1000; NHPP). Vectastain kits (Vector Laboratories) were used for signal amplification and the Sigma Fast 3,3-Diaminobenzidine Tablet Sets (Sigma) were used for antibody detection. Before mounting in Permount (Fisher), slides were counterstained for 3 minutes with methyl green (Vector Laboratories).
Cell death was determined by the TUNEL (Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling) method using the in situ cell death detection kit (Roche, Indianapolis IN) according to the manufacturer's protocol.
For in situ hybridization (ISH), embryos were collected and embedded in paraffin as they were for immunohistochemistry. Gene expression was detected using digoxigenin-labeled riboprobes as previously described (Raetzman et al., 2004). The in situ probes used were Hes1 (Akazawa et al., 1992) and Fgf10 (Bellusci et al., 1997) (a gift from Brigid Hogan, Durham, NC). The Six6 ISH probe was derived from a sequence verified full length Six6 clone from a RIKEN pituitary cDNA library (Carninci et al., 2003). The Six6 clone in pFLCI was linearized with BamHI and transcribed with T7 polymerase to produce an antisense probe.
Results
Hes1 is expressed in the pituitary during development
Many members of the Notch signaling family are expressed in the pituitary at embryonic day 12.5 (e12.5) (Raetzman et al., 2004). To define the window of Hes1 expression, we performed in situ hybridization on sections of mouse embryos from e10.5 through e18.5. At e10.5, Hes1 expression is detected throughout most of Rathke's pouch and the adjacent ventral diencephalon (Fig. 1A). Hes1 transcripts are localized in the dorsal and medial aspects of Rathke's pouch and are absent in the ventral region of the pouch at e11.5 (Fig. 1B). This pattern of Hes1 expression corresponds to the area of the pouch that contains the proliferating, undifferentiated cells (Ikeda and Yoshimoto, 1991; Ward et al., 2006). Hes1 is excluded from the ventral aspect of the pituitary that contains differentiating cells expressing αGSU, the alpha subunit common to the glycoprotein hormones TSH, LH, and FSH (Japon et al., 1994). By e13.5, Hes1 expression is waning in Rathke's pouch and the ventral diencephalon (Fig. 1C) and is dramatically reduced by e14.5 (Fig. 1D). Hes1 is no longer detectable in the pituitary or ventral diencephalon at e18.5, at which time terminal differentiation markers are detectable for all the hormone producing cell types (Fig. 2A and data not shown). The expression pattern of Hes1 in the developing pituitary suggests that Hes1 could have a role in undifferentiated precursor cells, and its expression is extinguished as pituitary precursors initiate their differentiation into hormone producing cells (Japon et al., 1994).
Fig. 1.
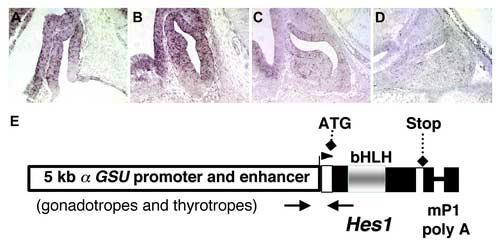
Hes1 is expressed in the developing pituitary in a temporally and spatially restricted pattern. Hes1 mRNA is detected by in situ hybridization in Rathke's Pouch, present in mid-sagittal sections of pituitaries, at e10.5 (A) and e11.5 (B). Hes1 expression wanes at e14.5 (C) and is undetectable by e16.5 (D). To increase the length of time Hes1 is expressed and to force its expression in differentiating cells, transgenic mice were generated with a construct (E) containing 4.6 kb of the mouse αGSU promoter and enhancer sequences, the Hes1 open reading frame cDNA, and mouse protamine 1 intron and polyadenylation sequences.
Fig. 2.
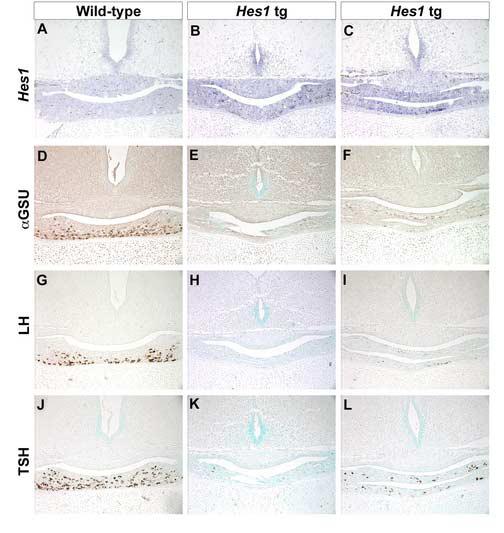
Mis-expression of Hes1 inhibits gonadotrope and thyrotrope differentiation. Coronal sections of pituitaries from e18.5 mice were probed for Hes1 expression by in situ hybridization. Hes1 in not detected in the anterior lobe of wild-type mice at this time (A), but transcripts are present in two independent, transient transgenic anterior pituitary lobes (B,C). The α subunit common to the dimeric hormones LH, FSH, and TSH is reduced in transgenic (E, F) pituitaries relative to wild-type (D). Both LHβ and TSHβ are also absent or substantially reduced in Hes1 transgenics (LH H, I; TSH K, L) compared to wild-type (LH G; TSH J). POMC, a hormone marker unrelated to α subunit, is expressed similarly in wild-type (M) and transgenic (N,O) pituitaries.
Hes1 expression must be silenced for pituitary cell differentiation to occur
Hes1 expression is spatially and temporally restricted during pituitary development. To test the hypothesis that Hes1 maintains Rathke's pouch precursor cells in an undifferentiated state and that Hes1 expression must be extinguished for differentiation to proceed, we created mice that express Hes1 constitutively under the control of the αGSU promoter and enhancer sequences, Cga (Fig. 1F). This well characterized promoter confers cell specific and developmentally regulated expression of a variety of transgenes in pre-gonadotrope and pre-thyrotrope cells which persists in the corresponding fully differentiated cells (Charles et al., 2005; Cushman et al., 2001). It also directs reporter gene expression in Rathke's pouch and the rostral tip thyrotropes, but this expression is extinguished rapidly.
We examined pituitary development e18.5 in six independent Hes1 transgenic founder mice. The transgenic mice with the highest levels of Hes1 expression expressers were identified by in situ hybridization with a Hes1-specific probe (Fig. 2 B, C). Out of the six founder mice, four expressed detectable levels of the Hes1 transgene, with the two shown being the highest expressers. Hes1 transcripts are present in the anterior lobe of the transgenics but not in non-transgenics at this time because endogenous Hes1 expression is already extinguished (Fig. 2A). The lumen of Rathke's pouch is dysmorphic in all of the transgenics, but there is no obvious difference in the overall size of these pituitary glands relative to those of non-transgenic mice.
The consequence of persistent Hes1 expression on cell differentiation was examined by immunostaining for hormone expression. We assessed the presence of gonadotropes and thyrotropes by expression of the common alpha subunit (αGSU) and the distinct beta subunits luteinizing hormone (LH) and thyroid-stimulating hormone (TSH). In wild-type pituitaries, αGSU (Fig. 2D) positive cells are readily detectable in the ventral aspect of the anterior lobe. In contrast, the anterior lobes of the Hes1 transgenics either lack αGSU positive cells (Fig. 2E) or only possess a few stained cells (Fig. 2F), indicating a failure to differentiate. The severity of the dysmorphology does not necessarily correlate with the degree to which differentiation is blocked. LHβ (Fig. 2G) and TSHβ (Fig. 2J) immunoreactive cells are abundant in the anterior lobe of wild-type pituitaries, however LHβ (Fig. 2H,I) and TSHβ (Fig. 2K,L) staining is severely reduced or absent in transgenic mice that express Hes1 persistently. In contrast, immunostaining for POMC-derived proteins is identical in the intermediate and anterior lobes of wild-type (Fig. 2M) and transgenic (Fig. 2N, O) pituitaries. Taken together, these data demonstrate that Hes1 can potently inhibit differentiation of gonadotropes and thyrotropes. A role of Hes1 in pituitary development, then, may be to prevent Rathke's pouch precursor cells from differentiating into hormone producing cells.
Loss of Hes1 prevents proper pituitary gland morphogenesis
To investigate the effect of Hes1 deficiency on pituitary development, we utilized Hes1 knockout mice (Ishibashi et al., 1995). This well-characterized null allele lacks the first 3 exons of the Hes1 gene, which encodes the bHLH domain of HES1. Hes1 null embryos die just before or at birth, allowing examination of the morphology of Rathke's Pouch and the anterior lobe up to that point in wild-type and Hes1 knockout embryos. At e11.5, the wild-type infundibulum has formed by evagination of the neural ectoderm and Rathke's pouch has fully separated from the oral ectoderm (Fig. 3A). The infundibulum is smaller than normal and the process of Rathke's pouch separation is delayed in the Hes1 mutants (arrow, Fig. 3E). By e14.5, the separation is complete in Hes1 mutants. The cartilage that underlies the pouch has not formed a unified structure in mutants (arrow, Fig. 3F), but it has in wild-type embryos of the same age (Fig. 3B).
Fig. 3.
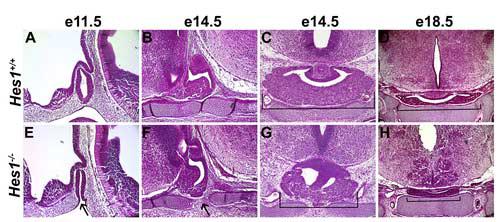
Pituitary size is reduced in Hes1 mutants. Hematoxylin and eosin (H&E) staining reveals the morphology of wild-type (A-D) and Hes1 null (E-H) pituitaries during development. In e11.5 sagittal sections, the pouch of the Hes1 mutant is formed, but has not completely separated from the underlying oral ectoderm (arrow, E) compared with the wild-type sagittal section (A). At e14.5, wild-type pituitaries have a nearly fused cartilage plate underneath Rathke's pouch (B), but Hes1 mutants exhibit a large gap between the rostral and caudal aspects of the cartilage (F). Coronal sections at e14.5 demonstrate that the size of the wild-type anterior pituitary (C, bracket) is substantially greater than the Hes1 mutant (G, bracket). This size difference between the wild-type (D, bracket) and Hes1 mutant (H, bracket) is even more pronounced in coronal sections of e18.5 pituitaries.
Normally, by e14.5 cells have migrated away from Rathke's pouch both ventrally and laterally to form the anterior lobe (Fig. 3B, C). The anterior lobe in Hes1 mutants is smaller due to reduced expansion laterally (Fig. 3G). At e18.5, the anterior pituitary is still undersized in Hes1 null embryos (Fig. 3H), and it appears to lack the posterior lobe that is prominent in wild-type pituitaries (Fig. 3E). These data demonstrate that the expression of Hes1 in and around Rathke's pouch is necessary for generating the correct size of the anterior and posterior lobe.
Hes1 is necessary for appropriate cell survival and cell proliferation in Rathke's pouch
To investigate the mechanism of pituitary hypoplasia in Hes1 mutant pituitaries, we compared proliferation and cell death during pituitary organogenesis in wild-type and Hes1 mutant pituitaries. Cells in the dorsal and medial aspects of Rathke's pouch are normally highly proliferative at e11.5, as demonstrated by BrdU labeling and Cyclin D2 expression (Fig. 4A and data not shown). Although dying cells are detectable in the adjacent mesenchyme, no cell death is occurring in Rathke's pouch at e11.5 in normal mice (Fig. 4B). In contrast, Hes1 mutants have fewer cells labeled with the proliferation marker BrdU (Fig. 4C, white bracket) and numerous dying cells within Rathke's pouch (Fig. 4D, white bracket). By e13.5, Cyclin D2 expression in proliferating Rathke's pouch cells is similar in wild-type (Fig. 4E) and Hes1 mutant (Fig. 4G) pituitaries. Additionally, cell death is no longer apparent in either wild-type (Fig. 4F) or Hes1 mutant (Fig. 4H) Rathke's pouches at this time. It is likely that both an early decrease in progenitor cell proliferation and elevated progenitor cell death contributes to the smaller pituitary glands of Hes1 mutants.
Fig. 4.
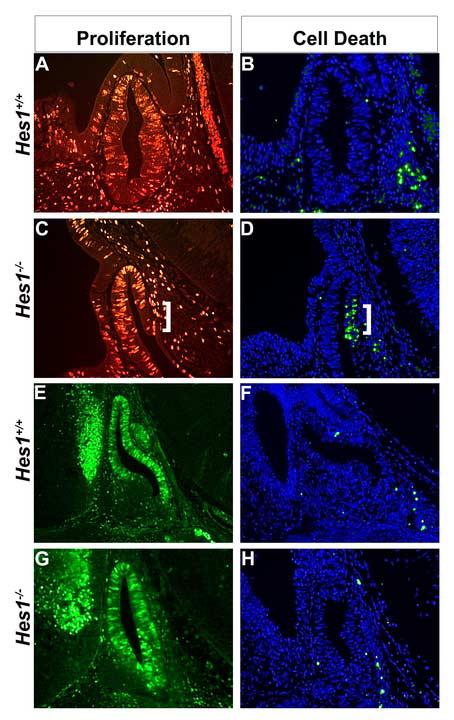
Decreased cell proliferation and increased cell death in Rathke's pouch of Hes1 mutants. Proliferation was assessed by BrdU immunohistochemistry at e11.5 (A, C) and Cyclin D2 immunohistochemistry at e12.5 (E,G). There is an area of decreased density of proliferating cells in the Hes1 mutants at e11.5 (C, white bracket), compared to the wild-type Rathke's pouch, where proliferating cells are evenly spacing throughout (A). At e12.5 proliferating cells are located throughout Rathke's pouch of both wild-type (E) and Hes1 mutants (G). Cell death was detected by the TUNEL method in sagittal sections of wild-type (B, F) and Hes1 mutant (D, H) pituitaries. Dying cells are labeled green and all nuclei are marked blue with DAPI. At e11.5, wild-type pituitaries have no dying cells within Rathke's pouch, but Hes1 mutants have many dying cells (D, white bracket). At e12.5, no cell death is detected in Rathke's pouch of wild-type (F) or Hes1 mutants (H).
Pituitary cell death in Hes1 mutants coincides with a loss of Lhx3 expression
Cell survival during pituitary gland ontogeny is supported by FGF signaling from the diencephalon and the expression of the several transcription factors in Rathke's pouch including the LIM homeodomain factor LHX3 (Charles et al., 2005; Ericson et al., 1998; Norlin et al., 2000; Sheng et al., 1996; Takuma et al., 1998; Treier et al., 1998). It is possible that the cell death observed in Hes1 mutant pituitaries at e11.5 is due to a decrease in one of these survival cues. At e10.5, the wild-type pituitary has detectable cell death along the oral ectoderm (Fig. 5A). This zone of death is expanded in the Hes1 null mutant (Fig. 5B, white bracket) and extends into Rathke's pouch. Lhx3 is normally expressed throughout Rathke's pouch at e10.5 (Fig. 5C), but expression is absent in Hes1 mutant pouches in the area where increased cell death is observed (Fig. 5D, white bracket). The LIM homeodomain transcription factor, ISL1, exhibits indistinguishable expression patterns in wild-type (Fig. 5E) and Hes1 mutant (Fig. 5F) Rathke's pouches. Likewise, the transcription factor SIX6, which promotes pituitary precursor proliferation (Li et al., 2002), is expressed similarly in Hes1 mutants (Fig. 5H) and wild-type pouches (Fig. 5G) and the ventral diencephalon (Fig. 5 G, H, arrow). This indicates that Hes1 deficiency alters LHX3 expression specifically.
Fig. 5.
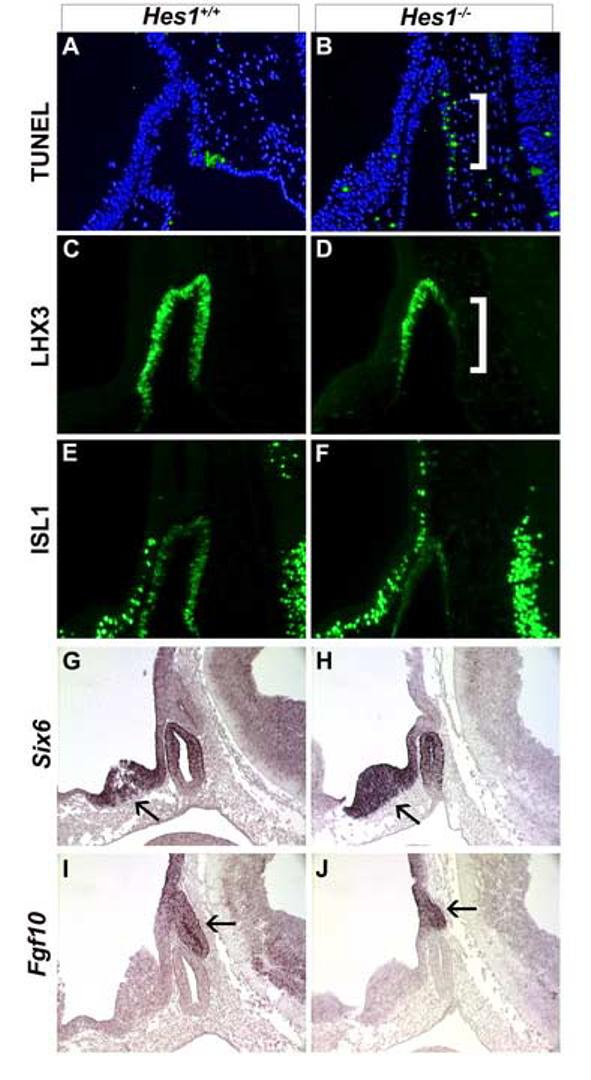
Hes1 is necessary for cell survival and LHX3 expression. Cell death (A, B), LHX3 (C, D) and ISL1 (E, F) expression were examined in wild-type and Hes1 mutant embryos collected at e10.5. Six6 (G, H) and Fgf10 (I, J) mRNA expression was examined by in situ hybridization on sagittal sections of e11.5 embryos. At e10.5, wild-type embryos exhibit cell death at the junction between Rathke's pouch and the attached oral ectoderm (A, green stained cells). Hes1 mutants have ectopic dying cells in the caudal aspect of Rathke's pouch (B, white bracket) that corresponds with lack of LHX3 expression (D, white bracket). Six6 expression in the diencephalon (arrow) and Rathke's pouch is equivalent in wild-type (G) and Hes1 mutants (H). Fgf10 expression in the infundibulum (arrow) is similar in wild-type (I) and Hes1 mutants (J).
FGFs released from the infundibulum are important for proliferation and survival of cells within Rathke's pouch (De Moerlooze et al., 2000; Ericson et al., 1998; Norlin et al., 2000; Ohuchi et al., 2000; Takuma et al., 1998). We examined the expression of Fgf8 and Fgf10 to determine whether the reduced cell proliferation and increased cell death in Hes1 mutants resulted from deficiencies in these growth factors. Fgf10 mRNA is detected in the infundibulum in wild-type mice at e11.5 (Fig. 5I, arrow). The levels and boundaries of Fgf10 expression appear unchanged in Hes1 mutants relative to wild-type (Fig. 5J, arrow). Similar results were obtained with Fgf8 expression (data not shown). Because Hes1 expression is not necessary for proper FGF expression in the infundibulum, but it is required for normal LHX3 expression in Rathke's pouch, the hypoplasia characteristic of Hes1 mutants may be intrinsic to Rathke's pouch.
Intermediate lobe melanotropes are not specified in the absence of Hes1
Hes1 is necessary to specify enteroendocrine cells during intestinal organogenesis (Jensen et al., 2000). To determine if Hes1 has a similar role the specification of endocrine cell types in the developing pituitary gland, we examined pituitary cell differentiation markers at e18.5. Wild-type and Hes1 null embryos were immunostained with an antibody that recognizes the proteins produced from differential cleavage of pro-opiomelanocortin (POMC): MSH in the intermediate lobe (IL) and ACTH in the anterior lobe (AL). Wild-type pituitaries have POMC immunoreactive cells in the IL and AL (Fig. 6A), but Hes1 null pituitaries only have immunoreactive cells in the anterior lobe, (Fig. 6B). The mutant intermediate lobe is completely devoid of POMC immunoreactive cells and POMC mRNA (data not shown), suggesting that Hes1 is required for melanotropes but not corticotropes. The lack of melanotropes in Hes1 null mice is confirmed in two ways. An antibody that recognizes αMSH reveals many stained cells in the IL of wild-type mice (Fig. 6C) but none in Hes1 null mice (Fig. 6D). Pro-hormone convertase 2 (PC2), the enzyme that processes POMC to produce αMSH, is clearly expressed in wild-type (Fig. 6E) but not mutant IL (Fig. 6F).
Fig. 6.
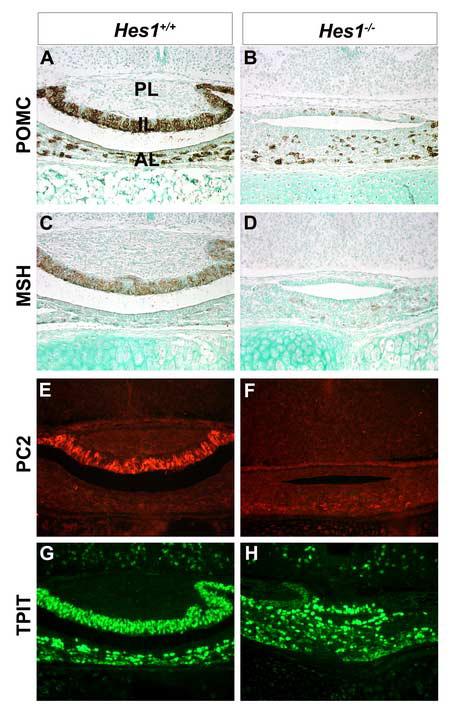
Intermediate lobe melanotropes are lost in the absence of Hes1. Coronal sections of wild-type (A, C, E, G) and Hes1 null (B, D, F, H) pituitaries were examined at e18.5. An antibody that recognizes POMC derivatives in the intermediate lobe (IL) and anterior lobe (AL) shows that in the wild-type pituitary, the posterior lobe (PL) is devoid of staining whereas the IL and AL contain many stained cells. In Hes1 mutants, the AL contains POMC stained cells, but the IL has a dramatic reduction in staining. MSH and PC2 immunoreactivity is apparent in the intermediate lobe of wild-type pituitaries (C, E, respectively) but not in Hes1 mutants (D, F, respectively). T-PIT immunoreactivity is similar in wild-type (G) and Hes1 mutant anterior and intermediate lobes (H).
To explore the molecular signature of the cells in the intermediate lobe of Hes1 mutants further, we examined expression of the T box transcription factor T-PIT (Tbx19) and the bHLH transcription factor NeuroD1. Both transcription factors are normally expressed prior to Pomc. TPIT is necessary for IL cell specification (Pulichino et al., 2003), and NeuroD1 deficient mice exhibit delayed corticotrope development (Lamolet et al., 2001). T-PIT expression is similar in the IL and AL of wild-type (Fig 6G) and Hes1 mutant (Fig. 6H) pituitaries at e18.5. Both T-PIT and NeuroD1 expression are unchanged in Hes1 mutants at e14.5 (data not shown). This indicates that the mechanism whereby Hes1 controls melanotrope fate does not involve either T-PIT or NEUROD1.
Intermediate lobe cells adopt a somatotrope cell fate in Hes1 mutants
The existing IL cells in Hes1 mutants may be committed to the melanotrope fate, but not express the terminal differentiation markers MSH and PC2. Alternatively, the cells located in the Hes1 null IL may have taken on another cell fate. To distinguish these possibilities, wild-type and Hes1 mutant pituitaries were immunostained with an antibody to the homeodomain transcription factor PIT1. PIT1 is normally expressed in the anterior lobe and is necessary for TSH, GH, and PRL expression and lineage specific proliferation of thyrotropes, somatotropes, lactotropes (Lin et al., 1994; Ward et al., 2006). PIT1 expression is observed in the AL of wild-type pituitaries at e16.5, (Fig. 7A) and e18.5 (Fig. 7C). The Hes1 null AL contains ample numbers of PIT1 positive cells, but PIT1 is aberrantly expressed in the IL at e16.5 (Fig. 7B, bracket) and at e18.5 (Fig. 7D, bracket). These data indicate that Hes1 expression in Rathke's pouch is necessary to repress PIT1 expression in the IL.
Fig. 7.
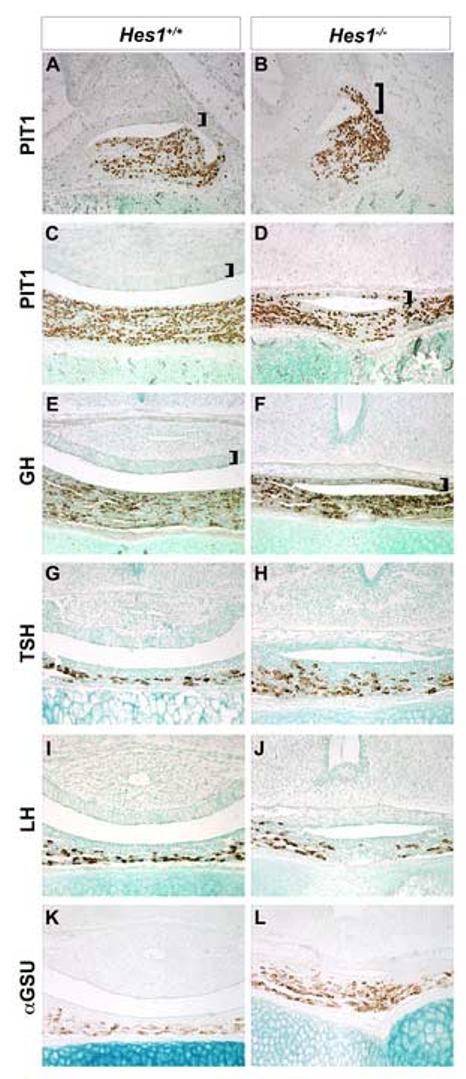
Hes1 deficiency causes intermediate lobe cell fate switch to somatotrope. PIT1 immunoreactive cells are normally detected only in the anterior lobe of wild-type mice at e16.5 (A) and e18.5 (C). The PIT1 negative intermediate lobe is denoted with a bracket. Hes1mutant mice express PIT1 becomes expressed in the caudal part of the intermediate lobe at e16.5 (B, bracket), and PIT1 is expressed throughout by e18.5 (D, bracket). Additionally, GH is expressed in the intermediate and anterior lobes of Hes1 mutants (F, bracket), but only in the anterior lobe of wild-type mice (E, bracket). There is no change in TSHβ, LHβ or αGSU immunostaining between wild-type (G, I, K, respectively) and Hes1 mutant (H, J, L, respectively) pituitaries at e18.5.
PIT1 is thought to act in combination with other cell type restricted transcription factors to generate thyrotropes and lactotropes (Bradford et al., 1997; Charles et al., 2005; Gordon et al., 1997). Misexpression of PIT1 can be sufficient for GH expression, however (Dasen et al., 1999). Ectopic PIT1 expression in the IL of the Hes1 null pituitaries, also leads to GH expression (Fig. F, bracket), while GH is only expressed in the anterior lobe of wild-type mice (Fig. E, bracket). No other ectopic hormone expression was detected in the IL of Hes1 mutants (Fig. 7F, H, J, L). The absence of Hes1, therefore, results in a cell fate change from melanotropes to somatotropes.
Anterior lobe cell specification does not require Hes1
The anterior lobe is substantially smaller in Hes1 mutants, but it contains all hormone producing cell types. POMC (Fig. 6B) and GH (Fig. 7F) are expressed in a normal pattern in the AL of Hes1 mutants when compared to wild-type pituitaries at e18.5 (Fig. 6A, Fig. 7E), in spite of their altered expression within the IL. In addition, similar levels of TSHβ, LHβ and αGSU immunoreactivity are seen in wild-type (Fig. 7G, I, K, respectively) and Hes1 mutant (Fig. 7H, J, L, respectively) pituitaries. To determine whether Hes1 deficiency promotes premature differentiation of pituitary cells like it does in many other organ systems, we examined the appearance of hormones that mark corticotropes, somatotropes, thyrotropes, and gonadotropes during development. There was no evidence for consistent, precocious expression of hormone genes in Hes1 mutants (Supp. Fig. 1 and 2).
Discussion
Notch signaling through Hes1 is critically important in the normal development of many organs. A combination of gain of function and loss of function approaches has proven useful in deciphering the distinct role Hes1 plays in cell fate selection. Hes1 may participate in 2 distinct processes: directing a precursor to remain uncommitted while a daughter cell differentiates or biasing a precursor pool that usually generates diverse types of cells towards one particular differentiated cell fate. We have uncovered a key role for Hes1 in pituitary cell fate specification (Fig. 8). Hes1 is normally expressed in the progenitor cells of Rathke's pouch and its levels decrease as differentiation proceeds. Constitutive, ectopic expression of Hes1 in developing gonadotropes and thyrotropes, results in dramatic reduction of LH and TSH production, indicating that Hes1 expression is sufficient to inhibit differentiation. Besides its role in maintaining pituitary cells in an undifferentiated state, Hes1 is also required for adoption of the melanotrope cell fate. The absence of Hes1 results in ectopic somatotrope specification, at the expense of melanotropes. There are several possible mechanisms for this cell fate switch. Hes1 may actively promote melanotrope differentiation and/or suppress Pit1 expression. Alternatively, somatotropes may form in the intermediate lobe because Hes1 deficiency permits cells to respond signaling molecules that stimulate differentiation at a time when Hes1 should be inhibiting differentiation. By the time the cues are present to stimulate melanotrope differentiation, the precursor cells may have already assumed the somatotrope fate. The latter hypothesis is consistent with the requirement for Hes1 to stimulate Rathke's pouch precursor cell proliferation. The undersized pituitary gland in Hes1 mutants caused by a combination of reduced cell proliferation and increased cell death. These studies are the first to demonstrate a definitive role for Notch signaling in pituitary cell fate choices and organ growth.
Figure 8.
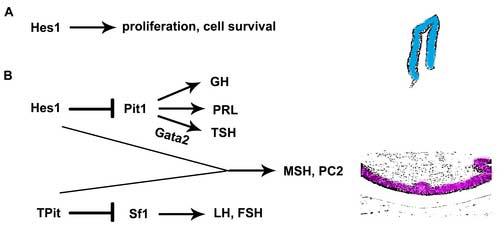
Genetic model of pituitary development and dependence on Hes1. During initial organogenesis, Hes1 is necessary for survival and proliferation of Rathke's pouch precursors (A). Hes1 is also necessary for intermediate lobe cells to become melanotropes and not PIT1-containing somatotropes (B).
Actively dividing progenitor cells line Rathke's pouch during the time Hes1 is expressed (Ikeda and Yoshimoto, 1991). These cells cease dividing and migrate to the anterior lobe before expressing terminal markers of differentiation. The expression of Hes1 in the same cells that express Notch2 and Notch3 suggests that Hes1 is a major effecter of Notch signaling in Rathke's pouch. Hey1 is expressed in the same cell population as Hes1 (Raetzman et al., 2006), while Hes6 appears to be in differentiating anterior lobe cells (Raetzman et al., 2004). It is possible that Hes1 and Hey1 have similar roles such that a significant amount of Notch signaling could occur in Hes1 mutant pituitaries. Consistent with the idea that Hes1 is only a part of Notch readout is that Notch2 knockouts are embryonic lethal by e10.5 (Hamada et al., 1999), but Hes1 knockouts can live until birth. In addition, loss of the pituitary specific transcription factor Prop1 results in a profound diminution of Notch2 expression, while Hes1 levels are not appreciably altered. The consequence of Prop1 loss in both humans and mice is failure of the Pit1 lineage, which is composed of somatotropes, lactotropes and thyrotropes (Gage et al., 1996a; Gage et al., 1996b; Wu et al., 1998). The fact that these cell types are specified in the Hes1 mutant, but melanotropes are absent, further indicates that the Prop1 mutant phenotype is unlikely to involve Hes1, even though Notch2 expression is dramatically reduced. Taken together, these data indicate that Hes1 performs an important role in pituitary development, but that other downstream Notch targets also regulate cell proliferation and specification.
The function of Hes1 in pituitary development is similar to its role during neurogenesis. In vertebrates, retinal progenitors rely on Notch signaling at two stages, first to block neuronal differentiation and next to direct precursors to produce glia. Hes1 null retinas contain fewer neurons due to premature depletion of the precursors (Tomita et al., 1996). Reciprocally, misexpression of the Notch target Hes1 in the retina inhibits neural differentiation and promotes production of Müller glial precursor cells (Bae et al., 2000; Furukawa et al., 2000). The Notch signal allows retinal progenitors to instructively generate glia despite the presence of neurogenic signals at the same time in development. In the pituitary it is likely that Notch signaling interacts with other signaling pathways present during the early stages of development as well.
There are several other signaling pathways that are critical for early pituitary cell proliferation and specification. Many members of the Wnt signaling pathway are detected in embryonic Rathke's pouches (Douglas et al., 2001). Wnt appears to act at several different stages of pituitary development, influencing proliferation (Kioussi et al., 2002), growth (Brinkmeier et al., 2003) and shape (Cha et al., 2004). Additionally, the Wnt target β-catenin interacts with Prop1 to specify the PIT1 lineage: somatotropes, lactotropes and thyrotropes (Olson et al., 2006). Wnts or LEF1 can increase the levels of Notch ligands (Ayyanan et al., 2006; Galceran et al., 2004; Hofmann et al., 2004). Additionally, Hes1 interacts with the groucho related genes known as transducin-like enhancer of split (Tle) to function as a co-repressor (Grbavec and Stifani, 1996; Ju et al., 2004). Notch and Wnt signals are also integrated to maintain hematopoetic stem cells. As in the pituitary, Notch is down-regulated as differentiation proceeds (Duncan et al., 2005). Notch signaling together with Wnt signaling maintains the cells in an undifferentiated state, while Wnt signaling alone promotes proliferation and survival. In the pituitary, it is likely that Wnt and Notch have overlapping roles in precursor maintenance while also performing distinct functions.
BMPs and FGFs provide important survival and differentiation cues during early pituitary organogenesis. We propose that these signaling pathways interact with the Notch pathway in the pituitary in a manner similar to that described for other organs. FGF8 and FGF10 are secreted from the diencephalon and are essential for proliferation and cell survival in Rathke's pouch (Ericson et al., 1998; Norlin et al., 2000; Ohuchi et al., 2000; Takuma et al., 1998). FGFs may enhance Notch signaling in the pituitary like they do in developing nervous system cells and pancreatic progenitor cells (Akai et al., 2005; Miralles et al., 2006; Norgaard et al., 2003; Yoon et al., 2004). BMP signaling appears to oppose FGF signaling and regulate the choice of Rathke's pouch precursors to differentiate into rostral tip thyrotropes or corticotropes (Ericson et al., 1998). BMPs may suppress corticotrope differentiation, in part, by augmenting the inhibitory effects of Notch signaling. This idea is supported by the observation that BMPs synergize with Notch signaling to increase the levels of Hes gene expression in developing osteoblasts (Nobta et al., 2005), myocytes (Dahlqvist et al., 2003) and endothelial cells (Itoh et al., 2004). BMP4 is expressed in the diencephalon dorsal to Rathke's pouch near the progenitors where it may suppress differentiation, and BMP2 is expressed in the ventral mesenchyme near the differentiating cells, where it may promote differentiation. Both of these processes may be linked with Notch signaling in the pituitary. This idea is supported by the observation that BMP4 acts with Notch to inhibit myogenic differentiation while BMP2 induced osteoblastic differentiation is promoted by Notch signaling.
A common role of Hes1 is to prevent premature differentiation of precursor cells. This is evident in both neurogenesis and intestinal endocrine cell development (Jensen et al., 2000; Nakamura et al., 2000). Hes1 functions to repress cell-patterning genes and when Hes1 is absent, premature cell specification occurs. We predicted that we would also observe precocious hormone expression in the pituitary of Hes1 deficient mice. Surprisingly, there were no consistent changes in the timing of pituitary hormone gene expression during development. It is difficult to eliminate the possibility that premature differentiation is occurring, however. The onset of hormone gene expression varied by one day among individual mice that were either wild type or Hes1 mutants, possibly due to the mixed genetic background of this colony. Genetic background effects have been observed in analysis of Pax6 and Prop1 alleles, and this variation could obscure subtle changes in the timing of differentiation (Bentley et al., 1999; Cushman et al., 2001; Kioussi, 1999; Vesper et al., 2006). Premature differentiation could also be missed due to a paucity of markers for stages leading to terminal differentiation and hormone production.
Hes1 is necessary for the survival of cells in the caudal aspect of Rathke's pouch. The reason why cell death only occurs in the caudal region is not clear, but it could be due to interaction of Notch and other signaling pathways. Chordin and Noggin, potent inhibitors of BMP signaling, are expressed near the caudal and dorsal aspects of Rathke's pouch, respectively, and affect FGF expression and pituitary cell survival (Treier et al., 1998)(Davis and Camper, unpublished observation). Reduced FGF signaling causes increased cell death in Rathke's Pouch (De Moerlooze et al., 2000). There may be a common mechanism whereby a combination of extracellular FGF signals and intrinsic Notch signaling establishes a milieu of transcription factors, all of which are essential for pituitary cell survival. This idea is supported by the observation that deficiencies in the transcription factors Lhx3 or Lhx4, or the combination of both Pitx1 and Pitx2 causes increased cell death in the pituitary primordium (Charles et al., 2005; Raetzman et al., 2002)(Ellsworth, Butts, Camper, unpublished observation). Like Hes1, lack of Lhx4 or the combination of Pitx1 and Pitx2 also decreases Lhx3 expression levels. Hes1 appears to play a role in cell survival in other organ systems. For example, Hes1 null duodenal crypt cells exhibit similar increases in cell death (Jensen et al., 2000), and Hes1 can repress the initiation of apoptosis in melanoblasts (Moriyama et al., 2006).
One of the most significant changes in observed in the Hes1 mutant pituitaries is a cell fate change from melanotropes to somatotropes. The scenario is similar in foregut-derived cells in Hes1 mutants in that biliary epithelial cells are absent, and pancreatic cells form in their place ((Fukuda et al., 2006; Sumazaki et al., 2004). Thus, Hes1 either actively represses one cell fate or directs a multi-potential precursor to a specific fate in multiple organ systems. The cell fate change in Hes1 deficient mice is another example in support of the idea that the cells in Rathke's pouch have the potential to differentiate into hormone producing cells of either the anterior or the intermediate lobe. Mice lacking the T box transcription factor T-PIT (TBX19) exhibit ectopic differentiation of gonadotropes in the intermediate lobe at the expense of POMC expression, presumably because T-PIT suppresses expression of the gonadotrope lineage-specific orphan nuclear receptor transcription factor SF1 (Nur5a1) (Pulichino et al., 2003). Hes1 may be required to suppress Pit1 expression in the cells normally fated to become melanotropes. This suggests that suppression of lineage specific transcription factor gene expression may be equally important as activation of gene expression to produce multiple types of hormone-producing cells from Rathke's pouch precursors. Cell fate choices in the pituitary may be more complicated than a series of binary choices (Fig. 8).
Supplementary Material
Acknowledgements
We thank Wanda Filipiak, Galina Gavrilina, and Maggie Van Keuren for preparation of transgenic mice and acknowledge funding for the Transgenic Animal Model Core: NIH grants (CA46592, AR20557, DK07367), the University of Michigan Center for Organogenesis, the Michigan Economic Development Corporation, and the Michigan Technology Tri-Corridor (Michigan Animal Models Consortium grant 085P1000815). Additionally, we are grateful to Dr. Ryoichiro Kageyama (Kyoto University, Kyoto, Japan) for providing the Hes1 null mice. We also thank the National Hormone Pituitary Program for the pituitary hormone antibodies, Developmental Studies Hybridoma Bank at the University of Iowa for the LHX3 and ISL1 antibodies, Jacques Drouin (IRCM, Montreal, Canada) for the TPIT antibody and Simon Rhodes (IUPUI, Indianapolis, IL) for the PIT1 antibody. We thank Amanda Vesper for her expert assistance with mice and experiments. This research was funded by the National Institutes of Health (grants F32DK60306 and P30DK34933 to L.T.R. and R37HD30428 and R01HD34283 to S.A.C.).
Footnotes
This research was funded by the National Institutes of Health (grants F32DK60306 and P30DK34933 to L.T.R. and R37HD30428 and R01HD34283 to S.A.C.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akai J, Halley PA, Storey KG. FGF-dependent Notch signaling maintains the spinal cord stem zone. Genes Dev. 2005;19:2877–87. doi: 10.1101/gad.357705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa C, Sasai Y, Nakanishi S, Kageyama R. Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J Biol Chem. 1992;267:21879–85. [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–81. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, Mandinova A, Raffoul W, Fiche M, Dotto GP, Brisken C. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103:3799–804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S, Bessho Y, Hojo M, Kageyama R. The bHLH gene Hes6, an inhibitor of Hes1, promotes neuronal differentiation. Development. 2000;127:2933–43. doi: 10.1242/dev.127.13.2933. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–78. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Bentley CA, Zidehsarai MP, Grindley JC, Parlow AF, Barth-Hall S, Roberts VJ. Pax6 is implicated in murine pituitary endocrine function. Endocrine. 1999;10:171–177. doi: 10.1385/ENDO:10:2:171. [DOI] [PubMed] [Google Scholar]
- Bradford AP, Wasylyk C, Wasylyk B, Gutierrez-Hartmann A. Interaction of Ets-1 and the POU-homeodomain protein GHF-1/Pit-1 reconstitutes pituitary-specific gene expression. Mol Cell Biol. 1997;17:1065–74. doi: 10.1128/mcb.17.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeier ML, Gordon DF, Dowding JM, Saunders TL, Kendall SK, Sarapura VD, Wood WM, Ridgway EC, Camper SA. Cell-specific expression of the mouse glycoprotein hormone alpha-subunit gene requires multiple interacting DNA elements in transgenic mice and cultured cells. Mol Endocrinol. 1998;12:622–33. doi: 10.1210/mend.12.5.0103. [DOI] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Cha KB, Gridley T, Stifani S, Meeldijk J, Clevers H, Camper SA. TCF and Groucho-related genes influence pituitary growth and development. Mol Endocrinol. 2003;17:2152–61. doi: 10.1210/me.2003-0225. [DOI] [PubMed] [Google Scholar]
- Burrows HL, Douglas KR, Seasholtz AF, Camper SA. Genealogy of the anterior pituitary gland: Tracing a family tree. Trends in Endocrinology & Metabolism. 1999;10:343–352. doi: 10.1016/s1043-2760(99)00189-7. [DOI] [PubMed] [Google Scholar]
- Carninci P, Waki K, Shiraki T, Konno H, Shibata K, Itoh M, Aizawa K, Arakawa T, Ishii Y, Sasaki D, Bono H, Kondo S, Sugahara Y, Saito R, Osato N, Fukuda S, Sato K, Watahiki A, Hirozane-Kishikawa T, Nakamura M, Shibata Y, Yasunishi A, Kikuchi N, Yoshiki A, Kusakabe M, Gustincich S, Beisel K, Pavan W, Aidinis V, Nakagawara A, Held WA, Iwata H, Kono T, Nakauchi H, Lyons P, Wells C, Hume DA, Fagiolini M, Hensch TK, Brinkmeier M, Camper S, Hirota J, Mombaerts P, Muramatsu M, Okazaki Y, Kawai J, Hayashizaki Y. Targeting a complex transcriptome: the construction of the mouse full-length cDNA encyclopedia. Genome Res. 2003;13:1273–89. doi: 10.1101/gr.1119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. WNT5A signaling affects pituitary gland shape. Mech Dev. 2004;121:183–94. doi: 10.1016/j.mod.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol. 2005;19:1893–903. doi: 10.1210/me.2005-0052. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Watkins-Chow DE, Brinkmeier ML, Raetzman LT, Radak AL, Lloyd RV, Camper SA. Persistent Prop1 expression delays gonadotrope differentiation and enhances pituitary tumor susceptibility. Hum Mol Genet. 2001;10:1141–53. doi: 10.1093/hmg/10.11.1141. [DOI] [PubMed] [Google Scholar]
- Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, Ibanez CF, Lendahl U. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130:6089–99. doi: 10.1242/dev.00834. [DOI] [PubMed] [Google Scholar]
- Dasen JS, O'Connell SM, Flynn SE, Treier M, Gleiberman AS, Szeto DP, Hooshmand F, Aggarwal AK, Rosenfeld MG. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell. 1999;97:587–98. doi: 10.1016/s0092-8674(00)80770-9. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Douglas KR, Kennell JA, Eswara P, Harrison TA, Patrianakos AI, Sprecher BS, Potok MA, Lyons RH, MacDougald OA, Camper SA. Identification of members of the Wnt signaling pathway in the embryonic pitutiary gland. Mammalian Genome. 2001 doi: 10.1007/s00335-001-2076-0. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–22. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–15. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Koizumi M, Boyer DF, Fujimoto K, Doi R, Kageyama R, Wright CV, Chiba T. Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest. 2006;116:1484–93. doi: 10.1172/JCI27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–94. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA. The Ames dwarf gene, df, is required early in pituitary ontogeny for the extinction of Rpx transcription and initiation of lineage specific cell proliferation. Molecular Endocrinology. 1996a;10:1570–1581. doi: 10.1210/mend.10.12.8961267. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Roller ML, Saunders TL, Scarlett LM, Camper SA. Anterior pituitary cells defective in the cell-autonomous factor, df, undergo cell lineage specification but not expansion. Development. 1996b;122:151–60. doi: 10.1242/dev.122.1.151. [DOI] [PubMed] [Google Scholar]
- Galceran J, Sustmann C, Hsu SC, Folberth S, Grosschedl R. LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis. Genes Dev. 2004;18:2718–23. doi: 10.1101/gad.1249504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Lewis S, Haugen B, James R, McDermott M, Wood W, Ridgeway E. Pit-1 and GATA-2 interact and functionally cooperate to activate the thyrotropin β-subunit promoter. Journal of Biological Chemistry. 1997;272:24339–24347. doi: 10.1074/jbc.272.39.24339. [DOI] [PubMed] [Google Scholar]
- Grbavec D, Stifani S. Molecular interaction between TLE1 and the carboxyl-terminal domain of HES-1 containing the WRPW motif. Biochem Biophys Res Commun. 1996;223:701–5. doi: 10.1006/bbrc.1996.0959. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Kadokawa Y, Okabe M, Ikawa M, Coleman JR, Tsujimoto Y. Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development. 1999;126:3415–24. doi: 10.1242/dev.126.15.3415. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Schuster-Gossler K, Watabe-Rudolph M, Aulehla A, Herrmann BG, Gossler A. WNT signaling, in synergy with T/TBX6, controls Notch signaling by regulating Dll1 expression in the presomitic mesoderm of mouse embryos. Genes Dev. 2004;18:2712–7. doi: 10.1101/gad.1248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Yoshimoto T. Developmental changes in proliferative activity of cells of the murine Rathke's pouch. Cell and Tissue Research. 1991;263:41–47. doi: 10.1007/BF00318398. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–48. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Moriyoshi K, Sasai Y, Shiota K, Nakanishi S, Kageyama R. Persistent expression of helix-loop-helix factor HES-1 prevents mammalian neural differentiation in the central nervous system. Embo J. 1994;13:1799–805. doi: 10.1002/j.1460-2075.1994.tb06448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127:3913–21. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- Itoh F, Itoh S, Goumans MJ, Valdimarsdottir G, Iso T, Dotto GP, Hamamori Y, Kedes L, Kato M, ten Dijke Pt P. Synergy and antagonism between Notch and BMP receptor signaling pathways in endothelial cells. Embo J. 2004;23:541–51. doi: 10.1038/sj.emboj.7600065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. Journal of Histochemistry and Cytochemistry. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Ju BG, Solum D, Song EJ, Lee KJ, Rose DW, Glass CK, Rosenfeld MG. Activating the PARP-1 sensor component of the groucho/ TLE1 corepressor complex mediates a CaMKinase IIdelta-dependent neurogenic gene activation pathway. Cell. 2004;119:815–29. doi: 10.1016/j.cell.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Kendall SK, Gordon DF, Birkmeier TS, Petrey D, Sarapura VD, O'Shea KS, Wood WM, Lloyd RV, Ridgway EC, Camper SA. Enhancer-mediated high level expression of mouse pituitary glycoprotein hormone α-subunit transgene in thyrotropes, gonadotropes, and developing pituitary gland. Molecular Endocrinology. 1994;8:1420–1433. doi: 10.1210/mend.8.10.7531821. [DOI] [PubMed] [Google Scholar]
- Kioussi C. Pax6 is essential for establishing ventral-dorsal cell boundaries in pituitary gland development. Proceedings of National Academy of Sciences. 1999;96:14378–14382. doi: 10.1073/pnas.96.25.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–85. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Tani S, Tamura K, Minoguchi S, Kurooka H, Honjo T. Delta-induced Notch signaling mediated by RBP-J inhibits MyoD expression and myogenesis. J Biol Chem. 1999;274:7238–44. doi: 10.1074/jbc.274.11.7238. [DOI] [PubMed] [Google Scholar]
- Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;23:849–859. doi: 10.1016/s0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Li X, Perissi V, Liu F, Rose DW, Rosenfeld MG. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297:1180–3. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- Lin SC, Li S, Drolet DW, Rosenfeld MG. Pituitary ontogeny of the Snell dwarf mouse reveals Pit-1-independent and Pit-1-dependent origins of the thyrotrope. Development. 1994;120:515–22. doi: 10.1242/dev.120.3.515. [DOI] [PubMed] [Google Scholar]
- Miralles F, Lamotte L, Couton D, Joshi RL. Interplay between FGF10 and Notch signalling is required for the self-renewal of pancreatic progenitors. Int J Dev Biol. 2006;50:17–26. doi: 10.1387/ijdb.052080fm. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Osawa M, Mak SS, Ohtsuka T, Yamamoto N, Han H, Delmas V, Kageyama R, Beermann F, Larue L, Nishikawa S. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol. 2006;173:333–9. doi: 10.1083/jcb.200509084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Sakakibara S, Miyata T, Ogawa M, Shimazaki T, Weiss S, Kageyama R, Okano H. The bHLH gene hes1 as a repressor of the neuronal commitment of CNS stem cells. J Neurosci. 2000;20:283–93. doi: 10.1523/JNEUROSCI.20-01-00283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobta M, Tsukazaki T, Shibata Y, Xin C, Moriishi T, Sakano S, Shindo H, Yamaguchi A. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem. 2005;280:15842–8. doi: 10.1074/jbc.M412891200. [DOI] [PubMed] [Google Scholar]
- Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev Biol. 2003;264:323–38. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Norlin S, Nordstrom U, Edlund T. Fibroblast growth factor signaling is required for the proliferation and patterning of progenitor cells in the developing anterior pituitary. Mech Dev. 2000;96:175–82. doi: 10.1016/s0925-4773(00)00393-2. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–8. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–9. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev. 2003;17:738–47. doi: 10.1101/gad.1065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol. 2004;265:329–40. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129:4229–39. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol. 2006 doi: 10.1210/me.2005-0394. in press. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Zhadanov AB, Mosinger B, Jr., Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science. 1996;272:1004–7. doi: 10.1126/science.272.5264.1004. [DOI] [PubMed] [Google Scholar]
- Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–7. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke's pouch requires dual induction from the diencephalon. Development. 1998;125:4835–40. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Tomita K, Ishibashi M, Nakahara K, Ang SL, Nakanishi S, Guillemot F, Kageyama R. Mammalian hairy and Enhancer of split homolog 1 regulates differentiation of retinal neurons and is essential for eye morphogenesis. Neuron. 1996;16:723–34. doi: 10.1016/s0896-6273(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes & Development. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper AH, Raetzman LT, Camper SA. Role of prophet of Pit1 (PROP1) in gonadotrope differentiation and puberty. Endocrinology. 2006;147:1654–63. doi: 10.1210/en.2005-1080. [DOI] [PubMed] [Google Scholar]
- Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20:1378–90. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, Engstrom L, Pinzon-Ortiz M, Fine JS, Lee H-JJ, Zhang L, Higgins GA, Parker EM. Chronic Treatment with the {gamma}-Secretase Inhibitor LY-411,575 Inhibits {beta}-Amyloid Peptide Production and Alters Lymphopoiesis and Intestinal Cell Differentiation. J. Biol. Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- Wu W, Cogan JD, Pfaffle RW, Dasen JS, Frisch H, O'Connell SM, Flynn SE, Brown MR, Mullis PE, Parks JS, Phillips JA, 3rd, Rosenfeld MG. Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat Genet. 1998;18:147–9. doi: 10.1038/ng0298-147. [DOI] [PubMed] [Google Scholar]
- Yoon K, Nery S, Rutlin ML, Radtke F, Fishell G, Gaiano N. Fibroblast growth factor receptor signaling promotes radial glial identity and interacts with Notch1 signaling in telencephalic progenitors. J Neurosci. 2004;24:9497–506. doi: 10.1523/JNEUROSCI.0993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Rosenfeld MG. Transcriptional control of precursor proliferation in the early phases of pituitary development. Curr Opin Genet Dev. 2004;14:567–74. doi: 10.1016/j.gde.2004.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


