Abstract
Since 1950, dramatic advances in human genetics have occurred, racial disparities in infant mortality have widened, and the United States’ international ranking in infant mortality has deteriorated. The quest for a “preterm birth gene” to explain racial differences is now under way.
Scores of papers linking polymorphisms to preterm birth have appeared in the past few years. Is this strategy likely to reduce racial disparities? We reviewed broad epidemiological patterns that call this approach into question.
Overall patterns of racial disparities in mortality and secular changes in rates of prematurity as well as birth-weight patterns in infants of African immigrant populations contradict the genetic theory of race and point toward social mechanisms. We postulate that a causal link to class disparities in health exists.
THE AVAILABILITY OF information on molecular genetics has exploded in recent decades. From the description of the double helix by Watson and Crick in 1953 to the sequencing of the human genome in 2003 and the beginnings of genomic medicine, scientific knowledge has accumulated at a breathtaking pace. Over the same decades, the United States, the world leader in newborn intensive care, fell from 6th to 27th in its international standing for infant mortality rate. At the same time, the racial gap for infant mortality in the United States has widened. The rate of death in the first year of life for Black infants increased from 1.6 times to 2.3 times the rate of White infants.1,2 The worsened national statistics for infant mortality are not just the result of including the poor outcomes for Blacks. In 2001 the infant mortality rate for White infants born in the United States was 5.7 per 1000 live births, which would give that subgroup a rank of 23rd in the world, not much better than 27th. The US rate for Whites was more than twice as high as the country with the best record in the world: Singapore, at 2.4 deaths per 1000 live births.2
Observation of these trends should give pause to those who are tempted to approach public health problems with strictly technological solutions, of which genomic medicine is the latest example. Despite having the world’s most advanced technology, the United States continues to fall farther behind other nations in health outcomes. The widening racial disparity in infant mortality during the era of molecular genetics should also prompt skepticism that genetic research holds the key to understanding and eliminating the disparities, a goal of the Healthy People 2010 objectives.3 Indeed, it has been argued by anthropologists for years that “race” has little or no meaning as a genetic category but rather derives all its usefulness from its very clear social, political, cultural, and historical meaning.4,5 These social meanings of race have clear public health implications.1,6–8 We evaluated the expected utility of 2 approaches to racial disparities: one based on race as a proxy for geographic ancestry and genetics, and the other based on race as a social construct.
“RACE,” GEOGRAPHIC ANCESTRY, AND HEALTH
“Race” in its traditional genetic conceptualization has been undermined by a wealth of information from molecular biology over the past 30 years. Most human genetic variation (90% to 95%) is found within the population of any continent, with only an additional 5% to 10% accounted for by differences in gene frequencies between continental populations.5,9 Patterns of human variation reflect our evolutionary history as a young species (anatomically modern Homo sapiens), originating in Africa roughly 200000 years ago. For more than half of the intervening time, all modern humans lived in Africa, with smaller founding populations arriving in Asia and Europe within the past 80000 to 50000 years.5,10
When hundreds of variable sites in the genome are sampled, geographical structures of diversity (statistical associations of DNA markers with populations native to different geographic locations) can be discerned, roughly corresponding to continental barriers to ancient migrations, such as oceans or mountain ranges. These statistical clusters do not fit into the traditional, essentialist concept of “races.” Attempts to conflate such constructs with traditional racial classification impede a more sophisticated understanding of genomic diversity.11,12 Geographical structuring is inferred from multiple neutrally varying sites. By contrast, medically relevant loci are often subject to strong natural selection or to genetic drift of rare alleles following population bottlenecks (evolutionary events in which a significant percentage of a population or species is killed or otherwise prevented from reproducing) and the populations of interest do not necessarily coincide with continental populations.12,13
Differences in allele frequencies between geographic populations have well-known effects on the incidence of uncommon diseases such as cystic fibrosis and sickle cell anemia.14 Whether they will turn out to have parallels in common complex diseases remains to be demonstrated.15–17 There is as yet no complex disease for which the genetic components are completely or even largely understood, but the popular genetic conception of “race” in medical research in the United States takes genetic differences between Whites and Blacks as a starting point.
Recently, researchers have suggested adding preterm birth to the list of such complex conditions as heart disease, hypertension, and diabetes.18,19 The hunt is on for “preterm birth genes” that can explain the disparity in prematurity and infant mortality between Blacks and Whites. The March of Dimes Research Agenda on Prematurity lists genetic factors as the second rubric under the category “racial/ethnic disparities.”20 A typical rationale for the genetic approach is that “African American women suffer twice the rate of preterm birth compared with Caucasians even when confounding social and economic variables are controlled for.”19(p57) Can all or even most of the multifaceted social, economic, political, and historical effects of racial discrimination be adequately “controlled for” with the variables commonly measured?1,21 Clearly some investigators believe they can.
Reports of polymorphisms associated with adverse birth outcomes appear to be growing exponentially at this time (Figure 1 ▶). Fiscella reviewed this literature extensively and published his findings in late 2005.22 He tabulated 70 reports describing 32 different genetic variants putatively implicated in the risk of preterm birth along with racial differences in gene frequency. A PubMed search in February 2006 identified 14 additional reports.23–36 Of the combined total of 84 articles, 59 have been published since 2002. Does existing population health evidence support this increasingly intense pursuit of a genetic basis for racial disparities in birth outcomes?
FIGURE 1—
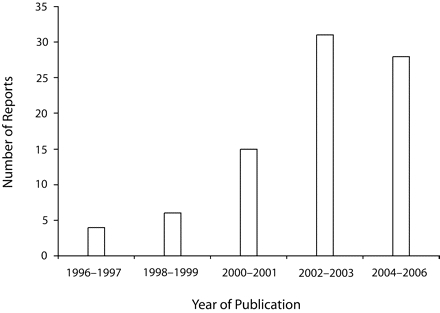
Quantity of reports describing polymorphisms putatively implicated in the risk of preterm birth or other adverse birth outcomes.
Source. All articles before 2003 and 45 of the 59 from 2003 onward were cited in Table 1 ▶ in Fiscella.22 The remaining 14 articles23–36 were obtained from a PubMed search in February 2006.
FROM THE NEW DEAL TO BIDIL
It can be argued that social and economic forces, rather than scientific evidence, underlie this proliferation of genetic research on racial disparities. Current social and political discourse in this country favors individual-level and technology-based solutions over extensions (or even maintenance) of the social contract implicit in US politics since the New Deal. More and more, a business model dominates medical research, with more than 20% of the genes in the human genome now under patent.37 In 2005, the first “ethnic drug,” BiDil, was approved by the US Food and Drug Administration for treatment of heart failure specifically in Blacks.38,39 The licensing of this product is seen by many as the first of many pharmaceuticals that will be tailored to different physiology in different people—genomic medicine. And of course, people belonging to different “races” will be assumed to differ at the genome level, providing distinct niche markets. Sociologist Troy Duster predicts that, because “race is such a dominant category,” such endeavors in biomedicine “can leave [their] own indelible mark once given the temporary imprimatur of scientific legitimacy by molecular genetics.”40(p1050) Given the risk of wasting large amounts of scientific effort and reinforcing popular misperceptions of “race,” do the existing population data justify this quest?
RACE AND MORTALITY
An overview of racial disparities in birth outcomes will help to put the current research agenda into perspective. The 5 leading causes of death in the first year of life in the United States for Black and White infants41 are shown in Table 1 ▶. Table 1 ▶ also shows the Black-to-White rate ratios of infant death for each of the major causes. These ratios range from a high of 3.9 for “disorders related to short gestation” to a low of 1.2 for “congenital malformations.” It is noteworthy that the mortality disadvantage of Black Americans is observed across all of the major categories of infant death. A similar pattern is seen in adults. Of the 10 leading causes of death, Blacks have lower death rates for only 2: chronic lung disease and Alzheimer disease.42 It is highly unlikely for any given population to have concentrated multiple deleterious mutations in such a way that they are at higher risk for almost all of the common complex disorders on a genetic basis. Social, economic, and cultural processes, on the other hand, could reasonably be hypothesized to cause adverse impacts on historically disadvantaged groups in a multifaceted and multilayered manner.1,6,8,21 Indeed, social-class gradients have been demonstrated for a variety of diseases in all age groups since the classic studies of the 19th century.43(p123–137),44(p109) A contemporary report from the United Kingdom reveals that the same pattern persists today: a significant social-class mortality gradient, as well as significant gradients for 15 of the 17 specific causes of child morbidity.45
TABLE 1—
Cause-Specific Infant Death Rates: United States, 2000
| Death Ratea | ||||
| Causes of Infant Death | Total | White | Black | RR |
| All causes | 688.9 | 571.2 | 1347.7 | 2.4 |
| Congenital malformations (Q00–Q99) | 141.8 | 138.5 | 167 | 1.2 |
| Disorders related to short gestation (P07) | 108.4 | 74.7 | 293.6 | 3.9 |
| Sudden infant death syndrome (R95) | 62.1 | 51.8 | 122.1 | 2.4 |
| Maternal pregnancy complications (P01) | 34.3 | 26.1 | 80.5 | 3.1 |
| Complications of placenta, cord, membranes (P02) | 25.7 | 22.3 | 45.6 | 2.0 |
Note. RR = rate ratio (Black to White). Data are from National Vital Statistics Reports.41 Codes (in parentheses) are from International Classification of Diseases, 10th Revision.
aPer 100 000 live births.
BIRTHWEIGHT AND ANCESTRY
Birthweight, a commonly used proxy for gestational maturity, is the most important determinant of infant mortality differences between Whites and Black Americans. Most of the Black–White gap in first-year mortality is attributable to the higher rate of Black infants born at very low birth-weight (less than 1500 g; 3 times that of White infants), essentially all of whom are preterm.46 Short gestation is tightly linked with low birthweight but is more difficult to measure, because it involves an estimate based on the recall of menstrual history as opposed to a straightforward measurement made by hospital staff. The superior reliability of birthweight as a proxy for gestational maturity is especially apparent at the population level.47,48 Researchers point out the persistence of a racial birthweight disparity after having controlled for various social or environmental risk factors.19,49 Do population patterns of birthweight support a genetic basis?
One feature of population patterns of preterm or low-birth-weight births that is at odds with genetic explanations of population differences is secular change. Average birth weights have risen in populations native to Japan, Pakistan, and Southeast Asia, among others, following either economic changes within the country of origin or immigration to more affluent societies.50–52 Similarly, birthweights in the state of Illinois—within both White and Black families—increased from 33 g to 74 g over the generation from the 1960s to the 1990s.53 More recently the National Center for Health Statistics reported that the rates of singleton preterm births changed significantly between 1989 and 1996 for both Whites and Blacks.54 Population changes in phenotype caused by genetic drift or natural selection occur over tens of thousands of years, not over decades. Clearly these changes over brief periods of time must have an environmental, not a genetic, basis.
Perhaps the most direct test of the hypothesized linkage between continent of ancestry (“race”) and birthweight was a comparison of birthweights among 3 groups of women delivering in Illinois over a 15-year period—US-born White women, US-born Black women, and African-born Black women.55 Earlier research showed that US Blacks have significant European genetic admixture. If birthweight differences between US Blacks and Whites in North America were determined by different frequencies of alleles responsible for low birthweight and these “low birthweight genes” were derived from African populations, then the birth weight difference should be most pronounced in African women, less so in US Blacks, and least in women with largely European ancestry (so-called “Whites.”). What we found was quite different. The overall birth-weight distributions for infants of US-born White women and African-born women were almost identical, with US-born Black women’s infants comprising a distinctly different population, weighing hundreds of grams less (Figure 2 ▶). Black women born in the United States also experienced higher rates of very low birthweight than either the White or African-born women once appropriate confounders were controlled.55
FIGURE 2—
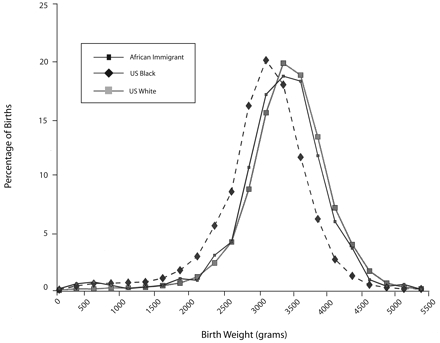
Birthweight distributions of 3 Illinois subpopulations.
Source. Reprinted with permission from David and Collins.55
We performed a similar analysis of births to Black Caribbean women immigrants to the United States and again found that those women gave birth to infants hundreds of grams heavier than the infants of US-born Black women.56 A recent report from Portugal57 showed similar findings, except that the birthweights of the infants of African-born women in Portugal were actually somewhat higher than the weights of both groups of Portuguese-born women—those of African and of European ancestry.
HEALTHY IMMIGRANTS OR UNHEALTHY SOCIETY?
The possibility exists that the phenomenon observed in these groups of women migrating from majority-Black countries in Africa and the Caribbean represents a “healthy immigrant” effect, similar to that described for other populations.58 We explored this hypothesis in a study of the birth-weight patterns in the generation after women migrated from African or Caribbean countries to the United States. Our findings again contradicted predictions based on genetic race. We analyzed the intergenerational birthweight patterns among the descendants of US-born and foreign-born White and Black women.59 Recent European immigrants to the United States gave birth to girls of similar birthweight to the girls born into established European American (White) families, and these girls grew up to have daughters whose average birthweight was higher than their own. This is the same pattern of rising birthweights over a generation that we had described previously in Illinois.52
Black African and Caribbean immigrants, on the other hand, gave birth to girls who were heavier than the girls born into established Black American families. Most striking, these first-generation Black girls grew up in the United States and went on to have daughters whose birth-weights were lower on average than their own weights had been at birth. This generational trend is opposite to that seen in the nonimmigrant population and opposite the trend in European immigrant families.59
It is possible that a “healthy immigrant” effect could exist for Africans and not for Europeans, given the more stringent visa requirements for African immigrants.60 However, if such immigration selection were in some way related to genetics, the pattern should persist into the next generation. That is not what we observed. Our findings were not readily explained by any genetic mechanism but rather suggested that negative effects of minority status are cumulative through the life course from fetus to childbearing woman.
An overview of the pattern of racial disparities in birth outcomes in the United States can be summarized as follows. Like health disparities in US adults, the disadvantage in cause-specific death rates for Black infants compared with White infants is distributed across nearly all causes. Racial disparity in infant deaths is highest for deaths related to prematurity and lowest for birth defects and chromosomal disorders. The pattern of low birthweight and prematurity in the population is not static but shows significant secular change over a generation or less. The low birthweights typical for Black infants in the United States or Portugal are not seen among infants born to recent immigrants from Africa or the Caribbean. After a generation of minority status, however, the birthweights in these families approximate those in the established Black minority population of the respective country.
DISPARITIES RESEARCH GROUNDED IN A SOCIAL CONCEPTION OF RACE
As suggested at the outset, the epidemiological evidence suggests that public health planners look to social and environmental rather than genetic differences between Black and White women in the campaign to eliminate health disparities. As Rudolph Virchow put it when considering mass diseases affecting German society in the 1860s, these conditions “indicate disturbances rooted in our social and governmental institutions, [and] hence [are] preventable.”61(p5) Even as biotechnology firms were applying for gene patents and molecular biologists were formulating their first studies in pursuit of a “preterm-birth gene” in Black women, another approach was being formulated. This approach to racial disparities research turned from “race” to “racism.” Stimulated by a series of conferences convened by the Centers for Disease Control and Prevention under the leadership of Diane Rowley and Carol Hogue in the early 1990s,62,63 a new picture of Black–White differences began to emerge: race as a social category is associated with a complex array of disparities in life experience in our highly racialized society.
Geronimus, who was first to point out the deterioration of birth outcomes of Black women as they age from adolescence into their 20s, the so-called “weathering” phenomenon, noted the elevated and rising levels of lead in the blood of Black women living in polluted neighborhoods.64,66 Similarly, we described adverse birth outcomes for Black women exposed to neighborhood violence,66 other unsatisfactory aspects of their residential environments, and stressful life events.67 The subjective reports of increased levels of life stress described by Black women compared with White women fit with published statistics. To take 2 striking examples, Blacks—both women of childbearing age and their partners—are more than twice as likely to be in the US Army68,69 and 7 times as likely to be incarcerated69,70 as Whites. In our case–control study of Black women giving birth in 2 Chicago hospitals, a remarkably high 16% reported incarceration of their partner during the pregnancy.71 In addition to these examples of community- and institutional-level effects of racism, recent studies by our group72 and others73–75 demonstrated similar deleterious effects on the interpersonal level. These studies showed an adverse impact of perceived racial discrimination on the birth outcome for Black women.
ELIMINATING RACIAL DISPARITIES TO REDUCE WHITE INFANT MORTALITY
The importance of a socio-political approach to understanding and eliminating racial health disparities extends beyond its potential benefits for Blacks in America. As noted, Whites in America also fare poorly compared with people in other countries, despite the United States having the world’s largest per-capita expenditure on medical care.76 The US racial gap in infant mortality and the gap between the United States and the world’s leader in infant mortality reduction, Japan, have increased in tandem over the past half century.1 Is there a causal pathway that could explain this tight temporal correlation? The missing concept to formulate this pathway is social class. Health statistics in the United States record categories of ethnicity but not social class.15 However, an extensive literature documents the impact of social class on health in other wealthy, industrialized societies, including on infant mortality.77–80 In a recent cross-sectional study of 16 wealthy member countries of the Organisation for Economic Co-operation and Development, Muntaner et al. analyzed the impact of national-level politics on birth outcomes. The authors reported that “the rates of low birthweight and infant deaths from all causes were lower in those countries with more voter turnout, more left votes, more left members of parliament, more women in government, a stronger social pact and various aspects of the welfare state, and low income inequality, as measured in a variety of ways.”81(p651)
Understanding health outcomes for the majority population in the United States requires a model that incorporates race both as a social construct and as a social class. We propose a model that links the 2 by the mechanism of class power. That is, the political influence exercised by an economic class depends on its political unity in pursuing its class agenda. To the extent that racial identity inhibits class identity, it also reduces class unity and class political power. This process has been labeled “divide and conquer.” We speculate that the unique history of race in the United States has led to a situation in which political unity and influence of the working classes—ordinary wage earners—is relatively low, as indicated by the international comparison reported by Muntaner et al.81
IMPLICATIONS FOR HEALTH DISPARITIES
Our national history includes the decimation of one ethnic group and the enslavement of another. A different economic and social history was more common for European colonies. In India, for example, the English mercantile class exploited colonial laborers thousands of miles from the home country, unseen by the average English workingman. By contrast, Africans brought to North America labored alongside “White” servants, pressed into service from the streets of London or Bristol. This necessitated a set of ideological justifications and supporting institutions. The laws preventing intermarriage between “races,” forbidding voting or land ownership by Africans, and otherwise discriminating against them came into being in the late 1600s, a generation or more after the first Africans landed in the colonies. Bennett82 argues that these measures prevented unified rebellions by Black and White plantation laborers. The very terms “Black” and “White” came into use—replacing African and Englishman, (or Christian)—only in the late 1600s. By these mechanisms African Americans were placed in a special social category where they contributed disproportionately to the country’s wealth and acquired disproportionately little of it, a situation that began in the 17th century and continues today.82,83
No other modern nation shares our unique history. This past has led to the “peculiar institutions” of present-day US politics. As virtually the only industrialized country that has no labor party and no universal health care, our politics are indeed unusual. The speculation that our nation’s history of race relations has led to our lack of class-based political institutions derives from the fact that popular culture and consciousness revolve around racial identity in the United States. Although racial ideologies have had84,85—and continue to have86—their own ugly history in Europe and elsewhere, there is no other industrialized nation where racial politics have been so dominant and consistent over time as in the United States.87(p21ff)
To be understood, the renewed interest in “race” as a genetic concept must be viewed in this context: scientific discovery and technologic advancement proceed according to their own dynamic of discovery, but scientists are part of society and subject to its political and cultural influences. The yearning for simple solutions to irreconcilable contradictions within a class-stratified society such as the United States has led to the recurrent reinvention of the concept of genetic “race”88–90 despite abundant scientific evidence against it. It would almost appear that the idea is essential to the maintenance of class society as we know it.
In which direction does this point us in the ongoing effort to eliminate the glaring and shameful health disparities—racial and otherwise—that afflict our population? We can take encouragement from 2 observations. First, poor health outcomes for Blacks are inextricably connected to poor health in the US majority population relative to other affluent countries. This may seem like more bad news, but viewed from a different perspective it means that the objective basis exists for broad political unity to change the status quo. Second, despite the cultural influences that promote racial identity,91 popular attitudes have shown movement toward breaking down racial separation,92 with the number of mixed marriages increasing tenfold since 1960, now accounting for 4% of couples.93 Thus, social class unity—across racial lines—could develop over time, making possible deep political change.
Researchers, especially those in public health and epidemiology, can help guide reform efforts. In the attempt to reduce adverse infant outcomes, detailed comparisons of the United States and other countries will be required,94 as well as studies that link health outcomes to explicit social, economic, and political processes.95–97 Our evidence suggests that a redirection of disparities research will come as part of a more profound change, a change necessary to improve the health of the entire population.
Acknowledgments
The authors wish to thank Miriam Golomb, Richard Cooper, Jay Kaufman, Susan Echiveri, and Chris Stahl for suggestions on the manuscript.
Peer Reviewed
Contributors R. David originated and led the writing of the article. J. Collins Jr headed up many of the projects that led to the perspective presented here and assisted in the writing and editing of the article.
References
- 1.David RJ, Collins JW Jr. Bad outcomes in Black babies: race or racism? Ethn Dis.. 1991;1:236–244. [PubMed] [Google Scholar]
- 2.Martin JA, Kochanek KD, Strobino DM, Guyer B, MacDorman MF. Annual summary of vital statistics—2003. Pediatrics. 2005;115:619–634. [DOI] [PubMed] [Google Scholar]
- 3.Healthy People 2010. US Dept of Health and Human Services. Available at: http://www.healthypeople.gov/About/goals.htm. Accessed March 18, 2005.
- 4.Montagu A. Man’s Most Dangerous Myth: The Fallacy of Race. 5th ed. New York, NY: Oxford University Press; 1974.
- 5.Marks J. Human Biodiversity: Genes, Race, and History. New York, NY: Aldine De Gruyter; 1995.
- 6.Cooper R, Steinhauer M, Miller W, David R, Schatzkin A. Racism, society, and disease: an exploration of the social and biological mechanisms of differential mortality. Int J Health Serv. 1981;11: 389–414. [DOI] [PubMed] [Google Scholar]
- 7.Cooper R. A note on the biologic concept of race and its application in epidemiologic research. Am Heart J. 1984;108:715–722. [DOI] [PubMed] [Google Scholar]
- 8.Cooper R, David R. The biological concept of race and its application to public health and epidemiology. J Health Polit Policy Law. 1986;11:97–116. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg NA, Pritchard JK, Weber JL, et al. Genetic structure of human populations. Science. 2002;298: 2381–2385. [DOI] [PubMed] [Google Scholar]
- 10.Tishkoff SA, Kidd KK. Implications of biogeography of human populations for “race” and medicine. Nat Genet. 2004;36(suppl 11):S21–S27. [DOI] [PubMed] [Google Scholar]
- 11.Bamshad M, Wooding S, Salisbury BA, Stephens JC. Deconstructing the relationship between genetics and race. Nat Rev Genet. 2004;5:598–609. [DOI] [PubMed] [Google Scholar]
- 12.Foster MW, Sharp RR. Beyond race: towards a whole-genome perspective on human populations and genetic variation. Nat Rev Genet. 2004;5:790–796. [DOI] [PubMed] [Google Scholar]
- 13.Jorde LB, Wooding SP. Genetic variation, classification and “race.” Nat Genet.. 2004;36(suppl 11):S28–S33. [DOI] [PubMed] [Google Scholar]
- 14.Drayna D. Founder mutations. Sci Am. 2005;293:78–85. [DOI] [PubMed] [Google Scholar]
- 15.Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med. 2003;348:1166–1170. [DOI] [PubMed] [Google Scholar]
- 16.Colhoun HM, McKeigue PM, Davey Smith G. Problems of reporting genetic associations with complex outcomes. Lancet. 2003;361:865–872. [DOI] [PubMed] [Google Scholar]
- 17.Race ,, Ethnicity, and Genetics Working Group. The use of racial, ethnic, and ancestral categories in human genetics research. Am J Hum Genet. 2005;77:519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varner MW, Esplin MS. Current understanding of genetic factors in pre-term birth. BJOG. 2005;112(suppl 1): 28–31. [DOI] [PubMed] [Google Scholar]
- 19.Dizon-Townson DS. Preterm labour and delivery: a genetic predisposition. Paediatr Perinat Epidemiol. 2001; 15(suppl 2):57–62. [DOI] [PubMed] [Google Scholar]
- 20.Green NS, Damus K, Simpson JL, et al. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005; 193:626–635. [DOI] [PubMed] [Google Scholar]
- 21.Krieger N, Rowley DL, Herman AA, Avery B, Phillips MT. Racism, sexism, and social class: implications for studies of health, disease, and well-being. Am J Prev Med. 1993;9(suppl 6):82–122. [PubMed] [Google Scholar]
- 22.Fiscella K. Race, genes and pre-term delivery. J Natl Med Assoc. 2005; 97:1516–1526. [PMC free article] [PubMed] [Google Scholar]
- 23.Witkin SS, Vardhana S, Yih M, Doh K, Bongiovanni AM, Gerber S. Polymorphism in intron 2 of the fetal interleukin-1 receptor antagonist genotype influences midtrimester amniotic fluid concentrations of interleukin-1beta and interleukin-1 receptor antagonist and pregnancy outcome. Am J Obstet Gynecol. 2003;189:1413–1417. [DOI] [PubMed] [Google Scholar]
- 24.Kalish RB, Vardhana S, Gupta M, Perni SC, Witkin SS. Interleukin-4 and -10 gene polymorphisms and spontaneous preterm birth in multifetal gestations. Am J Obstet Gynecol. 2004;190: 702–706. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Parry S, Macones G, et al. Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes (PPROM). Hum Mol Genet. 2004;13:2659–2669. [DOI] [PubMed] [Google Scholar]
- 26.Valdez LL, Quintero A, Garcia E, et al. Thrombophilic polymorphisms in preterm delivery. Blood Cells Mol Dis. 2004;33:51–56. [DOI] [PubMed] [Google Scholar]
- 27.Nukui T, Day RD, Sims CS, Ness RB, Romkes M. Maternal/newborn GSTT1 null genotype contributes to risk of pre-term, low birthweight infants. Pharmacogenetics. 2004;14:569–576. [DOI] [PubMed] [Google Scholar]
- 28.Doh K, Sziller I, Vardhana S, Kovacs E, Papp Z, Witkin SS. Beta2-adrenergic receptor gene polymorphisms and pregnancy outcome. J Perinat Med. 2004;32:413–417. [DOI] [PubMed] [Google Scholar]
- 29.Perni SC, Vardhana S, Tuttle SL, Kalish RB, Chasen ST, Witkin SS. Fetal interleukin-1 receptor antagonist gene polymorphism, intra-amniotic inter-leukin-1beta levels, and history of spontaneous abortion. Am J Obstet Gynecol. 2004;191:1318–1323. [DOI] [PubMed] [Google Scholar]
- 30.Amory JH, Adams KM, Lin MT, Hansen JA, Eschenbach DA, Hitti J. Adverse outcomes after preterm labor are associated with tumor necrosis factor-alpha polymorphism −863, but not −308, in mother–infant pairs. Am J Obstet Gynecol. 2004;191:1362–1367. [DOI] [PubMed] [Google Scholar]
- 31.Resch B, Gallistl S, Kutschera J, Mannhalter C, Muntean W, Mueller WD. Thrombophilic polymorphisms–factor V Leiden, prothrombin G20210A, and methylenetetrahydrofo-late reductase C677T mutations–and preterm birth. Wien Klin Wochenschr. 2004;116:622–626. [DOI] [PubMed] [Google Scholar]
- 32.Kalish RB, Nguyen DP, Vardhana S, Gupta M, Perni SC, Witkin SS. A single nucleotide A>G polymorphism at position −670 in the Fas gene promoter: relationship to preterm premature rupture of fetal membranes in multifetal pregnancies. Am J Obstet Gynecol. 2005; 192:208–212. [DOI] [PubMed] [Google Scholar]
- 33.Engel SA, Erichsen HC, Savitz DA, Thorp J, Chanock SJ, Olshan AF. Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology. 2005;16: 469–477. [DOI] [PubMed] [Google Scholar]
- 34.Hartel C, von Otte S, Koch J, et al. Polymorphisms of haemostasis genes as risk factors for preterm delivery. Thromb Haemost. 2005;94:88–92. [DOI] [PubMed] [Google Scholar]
- 35.Erichsen HC, Engel SA, Eck PK, et al. Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. Am J Epidemiol. 2006;163:245–254. [DOI] [PubMed] [Google Scholar]
- 36.Kalish RB, Vardhana S, Normand NJ, Gupta M, Witkin SS. Association of a maternal CD14–159 gene polymorphism with preterm premature rupture of membranes and spontaneous preterm birth in multifetal pregnancies. J Reprod Immunol. 2006;70:109–117. [DOI] [PubMed] [Google Scholar]
- 37.Jensen K, Murray F. Intellectual property. Enhanced: intellectual property landscape of the human genome. Science. 2005;310:239–240. [DOI] [PubMed] [Google Scholar]
- 38.Kahn J. Perspective: ethnic drugs. In: The Hastings Center Report. Available at http://www.thehastingscenter.org/pf/news/ethnicdrugspf.htm. Accessed March 16, 2005. [PubMed]
- 39.Kahn J. Getting the numbers right: statistical mischief and racial profiling in heart failure research. Perspect Biol Med. 2003;46:473–483. [DOI] [PubMed] [Google Scholar]
- 40.Duster T. Medicine. Race and reification in science. Science. 2005;307: 1050–1051. [DOI] [PubMed] [Google Scholar]
- 41.Table 7. Infant deaths and mortality rates for the five leading causes of infant death by race and Hispanic origin of mother: United States, 2000 linked file. In: National Vital Statistics Reports. 2002;50:21. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr50/50_12t7.pdf. Accessed March 18, 2005. [Google Scholar]
- 42.Centers for Disease Control and Prevention. Table 2. Percentage of total deaths, death rates, age-adjusted death rates for 2003, percentage change in age-adjusted death rates from 2002 to 2003 and ratio of age-adjusted death rates by race and sex for the 15 leading causes of death for the total population in 2003: United States. Available at: http://www.cdc.gov/nchs/data/hestat/finaldeaths03_tables.pdf#2. Accessed February 19, 2006.
- 43.Ackerknecht EH. Rudolph Virchow, Doctor, Statesman, Anthropologist. Madison: University of Wisconsin Press; 1953.
- 44.Engels F. The Condition of the Working Class in England. Stanford, Calif: Stanford University Press; 1968. (Originally published as Die Lage der arbeitenden Klasse in England. Leipzig; 1845.)
- 45.Petrou S, Kupek E, Hockley C, Goldacre M. Social class inequalities in childhood mortality and morbidity in an English population. Paediatr Perinat Epidemiol. 2006;20:14–23. [DOI] [PubMed] [Google Scholar]
- 46.Iyasu S, Becerra JE, Rowley DL, Hogue CJ. Impact of very low birthweight on the Black–White infant mortality gap. Am J Prev Med. 1992;8:271–277. [PubMed] [Google Scholar]
- 47.David RJ. The quality and completeness of birthweight and gestational age data in computerized birth files. Am J Public Health. 1980;70:964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David R. Commentary: birth-weights and bell curves. Int J Epidemiol. 2001;30:1241–1243. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman JD, Ward K. Genetic factors in preterm delivery. Obstet Gynecol Surv. 1999;54:203–210. [DOI] [PubMed] [Google Scholar]
- 50.Gruenwald P. Fetal growth as an indicator of socioeconomic change. Public Health Rep. 1968;83:867–872. [PMC free article] [PubMed] [Google Scholar]
- 51.Clarson CL, Barker MJ, Marshall T, Wharton BA. Secular change in birth-weight of Asian babies born in Birmingham. Arch Dis Child. 1982;57:867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li DK, Ni HY, Schwartz SM, Daling JR. Secular change in birth-weight among southeast Asian immigrants to the United States. Am J Public Health. 1990;80:685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chike-Obi U, David RJ, Coutinho R, Wu SY. Birth weight has increased over a generation. Am J Epidemiol. 1996; 144:563–569. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. Preterm singleton births—United States, 1989–1996. MMWR Morbid Mortal Wkly Rep. 1999;48: 185–189. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00056645.htm. Accessed March 15, 2005. [Google Scholar]
- 55.David RD, Collins JW. Differing birthweight among infants of US-born Blacks, African-born Blacks, and US-born Whites. N Engl J Med. 1997;337: 1209–1214. [DOI] [PubMed] [Google Scholar]
- 56.Pallotto EK, Collins JW Jr, David RJ. Enigma of maternal race and infant birth weight: a population-based study of US-born Black and Caribbean-born Black women. Am J Epidemiol. 2000; 151:1080–1085. [DOI] [PubMed] [Google Scholar]
- 57.Harding S, Santana P, Cruickshank JK, Boroujerdi M. Birth weights of Black African babies of migrant and nonmi-grant mothers compared with those of babies of European mothers in Portugal. Ann Epidemiol. 2006;16:572–579. [DOI] [PubMed] [Google Scholar]
- 58.Marmot MG, Adelstein AM, Bulusu L. Lessons from the study of immigrant mortality. Lancet. 1984;1:1455–1457. [DOI] [PubMed] [Google Scholar]
- 59.Collins JW Jr, Wu SY, David RJ. Differing intergenerational birth weights among the descendants of US-born and foreign-born Whites and African Americans in Illinois. Am J Epidemiol. 2002; 155:210–216. [DOI] [PubMed] [Google Scholar]
- 60.Brandon G. Yoruba. In: American Immigrant Cultures: Builders of a Nation. Levinson D, Ember M, eds. Vol. 2. New York, NY: MacMillan Reference; 1997.
- 61.Virchow R. Collected Essays on Public Health and Epidemiology. Sagamore Beach, Mass: Science History Publications, USA. 1985;2:5. (Originally published as Gesammelte Abhandlungen aus dem Gebiete der öffentlichen Medicin und der Seuchenlehre. Berlin, Germany: August Hirschwald Verlag; 1879.)
- 62.Blackmore CA, Ferre CD, Rowley DL, Hogue CJ, Gaiter J, Atrash H. Is race a risk factor or a risk marker for preterm delivery? Ethn Dis.. 1993;3: 372–377. [PubMed] [Google Scholar]
- 63.Rowley DL, Hogue CJ, Blackmore CA, et al. Preterm delivery among African-American women: a research strategy. Am J Prev Med. 1993;9(6 suppl): 1–6. [PubMed] [Google Scholar]
- 64.Geronimus AT. The effects of race, residence, and prenatal care on the relationship of maternal age to neonatal mortality. Am J Public Health. 1986;76: 1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2: 207–221. [PubMed] [Google Scholar]
- 66.Collins JW Jr, David RJ. Urban violence and African-American pregnancy outcome: an ecologic study. Ethn Dis. 1997;7:184–190. [PubMed] [Google Scholar]
- 67.Collins JW Jr, David RJ, Symons R, Handler A, Wall S, Andes S. African-American mothers’ perception of their residential environment, stressful life events, and very low birth weight. Epidemiology. 1998;9:286–289. [PubMed] [Google Scholar]
- 68.US Bureau of the Census. Statistical Abstract of the United States: 2001. Section 10: Annual National Defense and Veteran Affairs, Table No. 500: Department of Defense Manpower: 1950 to 2000. Available at http://www.census.gov/prod/2002pubs/01statab/defense.pdf. Accessed March 19, 2005.
- 69.Grieco EM, Cassidy RC. Overview of Race and Hispanic Origin, Census 2000 Brief, US Census Bureau. Available at: http://www.census.gov/prod/2001pubs/c2kbr01-1.pdf. Accessed March 19, 2005.
- 70.US Department of Justice, Bureau of Justice Statistics. Correctional Populations in the United States, 1998. Available at: http://www.ojp.usdoj.gov/bjs/pub/pdf/cpus98.pdf. Accessed March 19, 2005.
- 71.Lespinasse AA, David RJ, Collins JW, Handler AS, Wall SN. Maternal support in the delivery room and birth-weight among African American women. J Natl Med Assoc. 2004;96:187–195. [PMC free article] [PubMed] [Google Scholar]
- 72.Collins JW Jr, David RJ, Handler A, Wall S, Andes S. Very low birth weight in African American infants: the role of maternal exposure to interpersonal racial discrimination. Am J Public Health. 2004;94:2132–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, Kiefe CI. Self-reported experiences of racial discrimination and Black–White differences in preterm and low-birthweight deliveries: the CARDIA study. Am J Public Health. 2004;94:2125–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stancil TR, Hertz-Picciotto I, Schramm M, Watt-Morse M. Stress and pregnancy among African American women. Paediatr Perinat Epidemiol. 2000;14:127–135. [DOI] [PubMed] [Google Scholar]
- 75.Dole N, Savitz DA, Siega-Riz AM, Hertz-Picciotto I, McMahon MJ, Buekens P. Psychosocial factors and preterm birth among African American and white women in central North Carolina. Am J Public Health. 2004;94:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reinhardt UE, Hussey PS, Anderson GF. US health care spending in an international context. Health Aff (Mill-wood). 2004;23:10–25. [DOI] [PubMed] [Google Scholar]
- 77.Dyer O. Disparities in health widen between rich and poor in England. BMJ. 2005;331:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaw C, Blakely T, Atkinson J, Crampton P. Do social and economic reforms change socioeconomic inequalities in child mortality? A case study: New Zealand 1981–1999. J Epidemiol Community Health. 2005;59:638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Devlieger H, Martens G, Bekaert A. Social inequalities in perinatal and infant mortality in the northern region of Belgium (the Flanders). Eur J Public Health. 2005;15:15–19. [DOI] [PubMed] [Google Scholar]
- 80.Arntzen A, Nybo Andersen AM. Social determinants for infant mortality in the Nordic countries, 1980–2001. Scand J Public Health. 2004;32:381–389. [DOI] [PubMed] [Google Scholar]
- 81.Muntaner C, Lynch JW, Hillemeier M, et al. Economic inequality, working-class power, social capital, and cause-specific mortality in wealthy countries. Int J Health Serv. 2002;32:629–656. [DOI] [PubMed] [Google Scholar]
- 82.Bennett L. Before the Mayflower: A History of Black America. Chicago, Ill: Johnson Publishing Co; 1982.
- 83.Farley R. Blacks and Whites: Narrowing the Gap? Cambridge, Mass: Harvard University Press; 1984.
- 84.Peukert DJK. Inside Nazi Germany: Conformity, Opposition, and Racism in Everyday Life. New Haven, Conn: Yale University Press; 1987.
- 85.Müller-Hill B. Tödlishe Wissenschaft: Die Aussonderung von Juden, Zigeunern und Geisteskranken, 1933–1945 [Murderous Science: Elimination by Scientific Selection of Jews, Gypsies, and Others in Germany, 1933–1945]. Hamburg, Germany: Rowolt Taschenbuch Verlag GmbH; 1984.
- 86.Dickey C. Racism’s rising tide. Newsweek International Edition. August 22, 2004. Available at: http://www.msnbc.msn.com/id/5709026/site/newsweek. Accessed March 25, 2005.
- 87.Black E. War Against the Weak: Eugenics and America’s Campaign to Create a Master Race. New York, NY: Four Walls Eight Windows; 2003.
- 88.Chase A. The Legacy of Malthus: The Social Costs of the New Scientific Racism. Chicago: University of Illinois Press; 1980.
- 89.Muntaner C, Nieto FJ, O’Campo P. The Bell Curve: on race, social class, and epidemiologic research. Am J Epidemiol. 1996;144:531–536. [DOI] [PubMed] [Google Scholar]
- 90.Leroi AL. A family tree in every gene. New York Times, March 14, 2005:A3.
- 91.Kleinfield NR. Guarding the borders of the hip-hop nation. In: How Race Is Lived in America: Pulling Together, Pulling Apart. New York, NY: Henry Holt and Company; 2001.
- 92.Sack K, Elder J. The New York Times Poll on race: Optimistic outlook but enduring racial division. In: How Race Is Lived in America: Pulling Together, Pulling Apart. New York, NY: Henry Holt and Company; 2001.
- 93.Fletcher MA. America’s racial and ethnic divides: interracial marriages eroding barriers. Washington Post. December 28, 1998:A1. Available at: http://www.washingtonpost.com/wp-srv/national/daily/dec98/melt29.htm. Accessed March 26, 2005.
- 94.Thompson LA, Goodman DC, Little GA. Is more neonatal intensive care always better? Insights from a cross-national comparison of reproductive care. Pediatrics. 2002;109: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 95.Daniels N, Kennedy B, Kawachi I. Is Inequality Bad for Our Health? Boston, Mass: Beacon Press; 2000.
- 96.Cooper RS, Kennelly JF, Durazo-Arvizu R, Oh HJ, Kaplan G, Lynch J. Relationship between premature mortality and socioeconomic factors in black and white populations of US metropolitan areas. Public Health Rep. 2001;116: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003; 7:13–30. [DOI] [PubMed] [Google Scholar]


