Abstract
To study the influence of microwave induced thermo-chemotherapy on high-grade urothelial cell carcinomas. Five groups of each three patients were formed of whom initial biopsies and cystectomy samples were collected. Patients were treated 2 days prior to cystectomy with mitomycin-C (group 1), hyperthermia (group 2) or thermo-chemotherapy (group 3). Group 4 patients had been treated with a cycle of six thermo-chemotherapy treatments prior to cystectomy and group 5 patients served as control (no treatment). Tumour samples were stained with Haematoxylin and Eosin, monoclonal antibody Ki-67 and the monoclonal antibody p53. In six out of the nine patients treated with hyperthermia a decrease in proliferation activity in the tumour was found. Seven out of nine patients treated with hyperthermia showed a decrease in p53 activity. A decrease in proliferation activity and p53 activity illustrate the potential role of thermo-chemotherapy as a promising intravesical treatment.
Keywords: Superficial bladder cancer, Hyperthermia, Chemotherapy, Ki-67, p53
Introduction
Bladder cancer is the seventh most common cancer worldwide, accounting for 3.2% of all cancers. In 2000 an estimated 260,000 new cases in men and 76,000 in women were found worldwide [5]. The highest incidence rates of bladder cancer in both sexes are observed in Europe, North America and Australia [12]. Bladder cancer incidence is still rising moderately in most developed countries. Of all these malignant bladder tumours, more than 90% are transitional cell carcinomas of which two third is superficial and one third is muscle invasive. The management of these two types of transitional cell carcinoma differs enormously. The management of superficial bladder cancer consists of transurethral resection in the first place, whereas the primary treatment for muscle invasive bladder cancer consists of cystectomy, a complete different approach.
After transurethral resection of a superficial bladder tumour 30–85% of patients develop recurrences, despite the most accurate resection technique. This high risk of recurrences makes bladder cancer one of the most prevalent human tumours. To decrease the number of recurrences after transurethral resection, patients are treated subsequently with chemotherapeutic or immunotherapeutic agents. Immunotherapy, usually BCG treatment, is more effective than any intravesical chemotherapeutic agent, but has more serious and more frequent side effects. So, there is a need for more effective treatment options or treatment options with less frequent and less severe side effects. A new treatment option in patients with intermediate to high risk tumours is the combination of intravesical hyperthermia and intravesical chemotherapy [2, 6, 15].
The endocavitary location and ease of accessibility by the urethra makes thermo-chemotherapy a good therapeutic option for superficial bladder cancer. Local hyperthermia at temperatures of 40–44°C in combination with selected cytostatic agents in several tumours, including transitional cell carcinoma, results in a synergistic anti-tumour effect [10].
In this descriptive study the influence of different treatment modalities on bladder tumours (thermo-chemotherapy, chemotherapy and thermotherapy) was investigated by several immunohistochemical stainings.
Methods
Subjects
The study included 15 patients with high-grade transitional cell carcinoma of the bladder. All patients signed a patient informed consent and all these patients were facing cystectomy. The 15 patients were divided in five groups with each three patients. Group 1 patients were treated 2 days before cystectomy with an intravesical mitomycin-C (MMC) instillation. After the bladder was emptied, 20 mg MMC (Kyowa Hakko Kogyo Co., Tokyo, Japan) in 50 ml saline was instilled. In order to stabilize the MMC concentration in the bladder throughout the entire session, the bladder was emptied after 30 min and with urine diluted solution was replaced by a new solution containing 20 mg MMC. Group 2 patients were treated 2 days before cystectomy with sterile water and local hyperthermia. To standardize treatments the instillation was replaced after 30 min. The local microwave induced hyperthermia was delivered by the SB-TS 101 system as described by Colombo et al. [3]. This system consists of a 915 MHz intravesical microwave applicator that delivers hyperthermia of the bladder walls via direct irradiation. The applicator is part of the specially designed 20F transurethral catheter. The catheter also contains five thermocouples. Two thermocouples measure the temperature in the prostatic urethral tract; the other three are spread out and pushed tangentially against the posterior and lateral walls of the bladder. To avoid urethral overheating, the solution is continuously pumped out of the bladder and re-instilled after being cooled. Hyperthermia was delivered within a temperature range of 41–42°C.
Group 3 patients were treated 2 days before cystectomy with two times 30 min intravesical MMC (20 mg in 50 ml sterile water) combined with local microwave induced hyperthermia delivered by the SB-TS 101 system. Group 4 patients were treated with a cycle of six thermo-chemotherapy treatments during the last 3 months. Finally, group 5 patients served as control group. These patients did not receive any intravesical instillations within 3 months prior to cystectomy.
Immunohistochemistry
All tumour tissue samples were fixed in a 10% buffered formaldehyde solution. The specimens were embedded in paraffin blocks and sections of 4 μm were cut. All specimens were deparaffinized and stained with Haematoxylin and Eosin. A microscopic examination of the samples was performed and the extend of inflammation (1+ to 3+) haemorrhage (1+ to 3+) were semi quantitatively scored.
Ki-67, a nuclear protein present during phases G1, S, G2 and M of cycling cells, is accepted as a good indicator of cell proliferation [11]. Sections were deparaffinized and immersed in a 10 mM sodium citrate buffer (pH 6.0) in a microwave oven for two times 5 min to enhance antigen retrieval. After washing, the slides were incubated with 0.3% H2O2 in methanol to quench endogenous peroxidase activity. After incubation at room temperature for 2 h with the anti-Ki67 monoclonal antibody clone MIB-1 (BioGenex) diluted 1:30, a biotinylated antibody that recognises murine IgG (BioGenex) was applied for 20 min, followed by incubation in streptavidin-peroxidase complex (BioGenex) for 20 min. The peroxidase reaction was developed using 0.5 mg/ml diaminobenzidine tetrahydrochloride (Sigma) in 0.01% H2O2. Haematoxylin was used as a light counter stain. The slides were dehydrated and mounted with a xylene-soluble mounting medium.
The mutated p53 tumour suppressor gene product can be detected immunohistochemically and is associated with a lower survival in patients with bladder cancer [7]. Sections were deparaffinized and immersed in a 10 mM sodium citrate buffer (pH 6.0) in a microwave oven for two times 5 min to enhance antigen retrieval. After cooling down, 0.6% H2O2 in a 40% methanol solution was used for 30 min to block endogenous peroxidase activity. Slides were pre-treated with 10% normal swine serum for 10 min to block non-specific staining followed by adding primary antibody DO-7 for 1 h at 20°C. After extensive rinsing, sections were incubated for 30 min with biotinylated swine anti mouse antibody (1:200 dilution) and then for 30 min with avidin-biotin complexes (1:50 dilution) at 20°C. Diaminobenzidine (DAB) staining were finally followed by haematoxylin nuclear counter staining. The slides were dehydrated and mounted with a xylene-soluble mounting medium.
Immunoreactivity scoring
The screening of tumour tissue samples was performed by two independent investigators (CH and AH). The sections were screened for positive cells, defined as cells with nuclear staining. The amount of Ki67 or p53 staining is scored in percentages. The threshold for p53 “positivity” is ≥20% positive staining. The areas with maximal immunohistochemical staining were used for scoring. In total, 300–500 tumour cells were scored. In the visual estimation only definitely brown nuclei were recorded as positive. The results were expressed as percentage of immunoreactive tumour cell nuclei.
Results
Fifteen patients (11 males, 4 females) signed a patient informed consent and participated in this study. Ages ranged from 42 to 75 years (mean 63.2 years). All patients underwent cystectomy for transitional cell carcinoma of the bladder. Eight patients had a de novo invasive bladder tumour, whereas seven patients had a history of high-risk superficial disease. Patient details are summarized in Table 1.
Table 1.
Patient details containing number of previous occurrences, interval period between initial diagnosis and cystectomy in months, pathology data of biopsy and cystectomy (grading according to WHO 2002)
| Groups | Patient no/age/sex | N previous occurrences | Interval biopsy–cystectomy (in months) | Histopathology initial biopsy | Histopathology cystectomy |
|---|---|---|---|---|---|
| 1 (MMC) | 1/75/M | 0 | 2,0 | ≥pT2aGIII | pT3aGIIIN2 |
| 2/46/F | 0 | 4,4 | pT2GII | pT3bGIIIN1 | |
| 3/67/F | 0 | 1,2 | ≥pT2GIII | ≥pT2GIII | |
| 2 (HT) | 4/64/F | 0 | 1,9 | pT1GIII | pT2aGIII |
| 5/71/M | 3 | 38,7 | pT1GIII + CIS | pT1GIII + CIS | |
| 6/42/M | 10 | 121,9 | pTaGII | pTaGII | |
| 3 (MMC + HT) | 7/55/M | 0 | 2,6 | pT1GIII | ≥pT2GIII |
| 8/67/M | 0 | 1,9 | pT2GIII | pT3aGIIIN1 | |
| 9/48/F | 1 | 3,3 | pT1GII | pT2bGIIIN1 | |
| 4 (History of MMC + HT) | 10/54/M | 18 | 107,5 | pTaGII | pTaGII |
| 11/73/M | 2 | 22,3 | CIS | pT4aGIIIN2 | |
| 12/75/M | 10 | 57,7 | pTaGII | pTaGII | |
| 5 (Control) | 13/71/M | 9 | 115,5 | CIS | pT2GIII |
| 14/71/M | 0 | 3,1 | pT2GIII | pT2GIII | |
| 15/71/M | 0 | 3,3 | pT2GIII | pT2GIIIN1 |
HT microwave induced hyperthermia
The initial biopsies and tumour tissues obtained with cystectomy were used for histopathological diagnosis and immunohistochemical analysis of Ki-67 and p53. The results from the different treatment groups are summarized in Table 2. In one patient of the MMC group no residual tumour could be retrieved in the cystectomy specimen. The proliferation activity and p53 activity could not be scored in that patient.
Table 2.
The p53 and Ki67 immunoreactivity scorings in percentages of all patients divided in five different treatment groups
| Groups | Material | Inflammation | Proliferation (%) | P53 (%) | Haemorrhage |
|---|---|---|---|---|---|
| 1 (MMC) | |||||
| a | Biopsy | +++ | 30 | 20 | + |
| Cystectomy | +++ | 75 | 20 | ++ | |
| b | Biopsy | ++ | 20 | 75 | − |
| Cystectomy | +++ | 15 | 75 | + | |
| c | Biopsy | +++ | 40 | 75 | +++ |
| Cystectomy | NA | NA | NA | NA | |
| 2 (HT) | |||||
| a | Biopsy | + | 60 | 80 | − |
| Cystectomy | +++ | 20 | 40 | ++ | |
| b | Biopsy | + | 30 | 90 | − |
| Cystectomy | ++ | 20 | 40 | − | |
| c | Biopsy | + | 10 | - | − |
| Cystectomy | ++ | 10 | - | + | |
| 3 (MMC + HT) | |||||
| a | Biopsy | +++ | 40 | >75 | − |
| Cystectomy | +++ | 25 | 75 | − | |
| b | Biopsy | +++ | >75 | >75 | + |
| Cystectomy | +++ | 60 | 15 | − | |
| c | Biopsy | +++ | 30 | >75 | + |
| Cystectomy | +++ | 15 | 75 | ++ | |
| 4 (History of MMC + HT) | |||||
| a | Biopsy | + | 10 | 75 | − |
| Cystectomy | + | 20 | − | − | |
| b | Biopsy | +++ | 30 | 25 | − |
| Cystectomy | +++ | 30 | 25 | − | |
| c | Biopsy | ++ | 50 | 20 | − |
| Cystectomy | ++ | 20 | 10 | + | |
| 5 (Control) | |||||
| a | Biopsy | + | 15 | >75 | − |
| Cystectomy | +++ | 40 | >75 | − | |
| b | Biopsy | ++ | 40 | 20 | − |
| Cystectomy | ++ | 40 | 20 | − | |
| c | Biopsy | ++ | 35 | 75 | + |
| Cystectomy | +++ | 35 | 75 | − | |
The extend of inflammation and haemorrhage is semi quantitatively scored (1+ to 3+)
NA not applicable due to absence of residual tumour
The intensity of inflammation increased in three out of nine patients treated with hyperthermia. In three out of five patients from the MMC group and control group an increase was seen. The intensity of haemorrhage increased in four out of nine patients treated with hyperthermia, one patient showed a decrease and four patients did not show any difference. In the MMC group and in the control group two patients showed an increase, one patient a decrease and two patients did not show any difference.
In six out of the nine patients treated with hyperthermia a decrease in proliferation activity in the tumour tissue sample was found (Fig. 1a). In the MMC group one patient showed a decrease and one showed an increase in proliferation activity. In the control group two out of three patients showed no changes, whereas in one patient an increase in proliferation activity was seen.
Fig. 1.
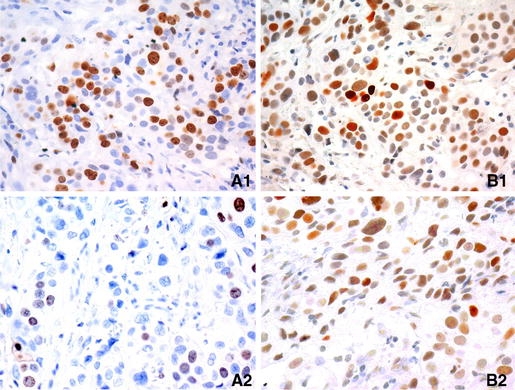
The p53 and Ki67 positive staining in patient no. 4 (hyperthermia group). a1 shows 60% Ki67 positivity before treatment; a2 shows 20% Ki67 positivity after treatment; b1 shows 80% p53 positivity before treatment and b2 shows 40% p53 positivity after treatment
As regards p53, seven out of nine patients treated with hyperthermia showed a decrease in p53 activity (Fig. 1b). In one patient no change was found. The control group and the group treated with MMC did not show any differences concerning p53.
Discussion
Thermo-chemotherapy has shown to be a promising method for treating several kinds of malignant tumours including superficial bladder cancer [15]. While hyperthermia is important in cancer therapy, it can also damage normal tissues adjacent to the tumour. Fajardo [4] reviewed the effects of hyperthermia from several studies on various tissues of mammals and humans. Hyperthermia with a maximum applied temperature of 44.5°C showed no gross or microscopic alterations of the bladder of dogs and rabbits. Subsequently, Rath-Wolfson et al. [13] studied the effect of hyperthermia, with a maximum applied temperature of 46°C, and simultaneous MMC treatment in sheep. In this study thermo-chemotherapy showed no significant macroscopic or microscopic differences in the bladder wall as compared to a control group with untreated sheep.
In the current study the effect of the combination thermo-chemotherapy is compared with solely hyperthermia, solely chemotherapy and no treatment (control group) in human patients with high-grade urothelial cell carcinoma of the bladder. The system SB-TS 101 used to deliver local microwave induced hyperthermia makes it possible to dose the hyperthermia very precisely [1]. In this study hyperthermia was delivered within a temperature range of 41–42°C, the clinical situation. This makes comparison between the different groups more accurate.
The sample size of the group studied is small due to the fact that recruitment of patients was difficult. Patients had to agree on an extra treatment session only two days before cystectomy, which did not give them any benefit at all.
The degree of inflammation increased in the group treated with solely hyperthermia (N = 3), solely MMC (N = 1) and the control group (N = 2). In the group treated with thermo-chemotherapy 2 days before surgery the degree of inflammation was initially already maximal. In the group treated with a cycle of six thermo-chemotherapy treatments during the last 3 months the degree of inflammation apparently had returned to baseline again.
At least one patient per group (except the control group) showed an increase in the degree of haemorrhage. Patients from group 1 (solely MMC) showed the highest degree of haemorrhage.
Group 3 patients (treated with thermo-chemotherapy prior to cystectomy) show in one patient an increase, in one patient a decrease and in one patient a stable extend of haemorrhage. This is in line with the results from Rath-Wolfson et al. [13]. Nevertheless, since solely MMC and solely hyperthermia gives an increase in haemorrhage, it would have been logical to find a higher extend of haemorrhage after the combination of both treatments. Possibly, this is not found due to the small sample size.
The inhibition of proliferation activity is one of the most important goals in cancer treatment. All groups treated with hyperthermia showed to a different extend a decrease in proliferation activity. Most interesting are the three patients treated with thermo-chemotherapy preceding transurethral resection. All three patients showed a decrease in proliferation activity. The control group on the other hand shows in two patients no difference and in one patient an increase in proliferation activity. In earlier in vitro studies using hyperthermia and bladder cancer cell lines, this decrease in cell proliferation due to hyperthermia combined with chemotherapeutic agents was already shown [14, 16]. However, in these in vitro studies solely hyperthermia did not cause a significant decrease in cell proliferation. Nevertheless, there is a difference between in vitro and in vivo studies. This in vivo study is the first one showing a decreased proliferation activity in humans treated with microwave-induced hyperthermia with or without MMC. The effect of hyperthermia was seen in six out of nine patients treated with hyperthermia preceding cystectomy.
Furthermore, the group treated with solely MMC did not show a significant decrease in proliferation activity. This is probably due to the limited penetration properties of this intravesical used drug, especially after one single treatment.
p53 is known to be responsible for repair or apoptosis in response to DNA damage. The p53 activity, in other words the expression of mutant p53, decreased exclusively in patients treated with hyperthermia with or without MMC.
Previous experiments showed that the p53 pathway is heat sensitive and that the p53 protein is inactivated at temperatures above 41°C [8, 9]. When cells were heated to 42.5°C and returned to normal temperatures, a strong p53 response with an increase in protein levels was observed. In our study we found the opposite, but the time interval between treatment and cystectomy was with 2 days (group 3) and several months (group 4) significantly longer than the time interval used by Guan et al. [8]. Furthermore the results of this study are based on a small number of patients, which makes statistic analysis unreliable. On the other hand, the current results suggest that thermo-chemotherapy could be an effective treatment in patients with a p53 tumour suppressor gene mutated tumour. In all, the results show several trends and encourage carrying out larger experimental studies.
Conclusion
The degree of inflammation and haemorrhage in bladder tumours did not increase in patients treated with hyperthermia. This, in combination with a decrease in proliferation activity and a decrease in p53 activity, implies that thermo-chemotherapy is a safe and promising treatment.
References
- 1.Colombo R, Da Pozzo LF, Lev A et al. (1998) Local microwave hyperthermia and intravesical chemotherapy as bladder sparing treatment for select multifocal and unresectable superficial bladder tumors. J Urol 159:783–787 [DOI] [PubMed]
- 2.Colombo R, Da Pozzo LF, Salonia A et al. (2003) Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol 21:4270–4276 [DOI] [PubMed]
- 3.Colombo R, Lev A, Da Pozzo LF et al. (1995) A new approach using local combined microwave hyperthermia and chemotherapy in superficial transitional bladder carcinoma treatment. J Urol 153:959–963 [DOI] [PubMed]
- 4.Fajardo LF (1984) Pathological effects of hyperthermia in normal tissues. Cancer Res 44:4826s–4835s [PubMed]
- 5.Ferlay J, Bray F, Pisani P et al. (2001) Cancer Incidence, mortality and prevalence worldwide, version 1.0. IARC Press, Lyon
- 6.Gofrit ON, Shapiro A, Pode D et al. (2004) Combined local bladder hyperthermia and intravesical chemotherapy for the treatment of high-grade superficial bladder cancer. Urology 63:466–471 [DOI] [PubMed]
- 7.Grossman HB, Liebert M, Antelo M et al. (1998) p53 and RB expression predict progression in T1 bladder cancer. Clin Cancer Res 4:829–834 [PubMed]
- 8.Guan J, Stavridi E, Leeper DB et al. (2002) Effects of hyperthermia on p53 protein expression and activity. J Cell Physiol 190:365–374 [DOI] [PubMed]
- 9.Hainaut P, Butcher S, Milner J (1995) Temperature sensitivity for conformation is an intrinsic property of wild-type p53. Br J Cancer 71:227–231 [DOI] [PMC free article] [PubMed]
- 10.Kowal CD, Bertino JR (1979) Possible benefits of hyperthermia to chemotherapy. Cancer Res 39:2285–2289 [PubMed]
- 11.Nakopoulou L, Vourlakou C, Zervas A, Tzonou A, Gakiopoulou H, Dimopoulos MA (1998) The prevalence of blc-2, p53, and Ki-67 immunoreactivity in transitional cell bladder carcinomas and their clinicopathologic correlates. Hum Pathol 29(2):146–154 [DOI] [PubMed]
- 12.Parkin DM, Whelan SL, Ferlay J et al. (2003) Cancer incidence in five continents. IARC Press, Lyon
- 13.Rath-Wolfson L, Moskovitz B, Dekel Y, Kugel V, Koren R (2003) Combined intravesical hyperthermia and mitomycin chemotherapy: a preliminary in vivo study. Int J Exp Pathology 84:145–152 [DOI] [PMC free article] [PubMed]
- 14.van der Heijden AG, Jansen CFJ, Verhaegh G, O’Donnell MA, Schalken JA, Witjes JA (2004) The effect of hyperthermia on mitomycin-C induced cytotoxicity in four human bladder cancer cell lines. Eur Urol 46(1):670–674 [DOI] [PubMed]
- 15.van der Heijden AG, Kiemeney LA, Gofrit ON et al. (2004) Preliminary European results of local microwave hyperthermia and chemotherapy treatment in intermediate or high risk superficial transitional cell carcinoma of the bladder. Eur Urol 46:65–72 [DOI] [PubMed]
- 16.van der Heijden AG, Verhaegh G, Jansen CF, Schalken JA, Witjes JA (2005) Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: an in vitro study. J Urol 173(4):1375–1380 [DOI] [PubMed]


