Abstract
Bone metastasis is a common sequelae of breast cancer and the interaction of αvβ3-integrin with osteopontin (OPN) found in the extracellular matrix of mineralized tissues is implicated in this process. The integrin-dependent proadhesive and promigratory functions of OPN are particularly attributed to the 40-kD N-terminal fragment that derives upon matrix metalloproteinase (MMP) cleavage. Based on the broad repertoire of interactions between Staphylococcus aureus extracellular adherence protein (Eap) and host components, we here characterized Eap to specifically interact with recombinant full-length OPN and the 40-kD N-terminal MMP cleavage fragment, but not with the 32-kD or the 25-kD C-terminal fragments of OPN. Eap thereby prevented the OPN/αvβ3-integrin interaction, as well as the αvβ3-integrin-dependent adhesion of MDA-MB-231 breast cancer cells to full-length OPN or to the 40-kD fragment and the migration of these cells towards OPN. Furthermore, Eap treatment markedly impaired the development of osseous metastasis of human MDA-MB-231 cells in vivo. Taken together, Eap may represent an attractive novel treatment for the prevention of breast cancer bone metastasis.
INTRODUCTION
Metastasis is the major cause of death in women with breast cancer[1]. Organotropic metastasis to the bone occurs in >50% of patients with advanced (UICC stage IIIb and IV) breast cancer[2]. Bone metastasis renders breast cancer virtually incurable concomitant with significant and devastating morbidity including osteolytic fractures and poor life quality[2]. The bone-directed recruitment of breast cancer cells is governed by chemokines that attract the cells to the bone[3], as well as by their adhesion and migration on components of the extracellular matrix of the bone that is mediated by integrins and other adhesion receptors[4]. αvβ3-integrin expression confers on breast cancer cells a higher metastatic potential to the bone[5],[6]. αvβ3-integrin may facilitate the early steps of breast cancer cell bone colonization by mediating adhesion, migration and invasion through direct interactions with bone matrix–associated proteins such as osteopontin (OPN)[7],[8]. In addition, αvβ3 as well as its ligand OPN are important in osteoclast motility and osteoclast-mediated bone resorption[9],[10],[11]. Consistently, OPN-deficient tumours show reduced bone metastasis[12]. Consequently, interfering with αvβ3 integrin-dependent interactions of breast cancer cells represents a feasible therapeutic approach to block bone metastasis[13].
As a member of the SIBLING (small integrin-binding ligand N-linked glycoprotein) family, OPN is a secreted chemokine-like protein that interacts with CD44 and adhesion receptors of the integrin family[14], particularly due to its arginine-glycine-aspartate (RGD) integrin-binding motif[15]. OPN has a protease-hypersensitive site that is susceptible to cleavage by thrombin[16] or members of the matrix metalloproteinase (MMP) family, such as MMP-3 and MMP-7[17]. Upon limited proteolysis the proadhesive and promigratory functions of OPN are potentiated, as the formerly cryptic αvβ3 integrin binding site within the terminal fragment becomes exposed[18],[19].
Staphylococcus aureus secretes proteins with extracellular matrix binding properties that mediate bacterial adherence to host tissue[20]. Among them the 60–70 kD extracellular adherence protein (Eap) provides a broad repertoire of interactions with host extracellular matrix components, including fibrinogen, fibronectin, vitronectin, bone sialoprotein and OPN that enable S. aureus to colonize at various sites of infection[21],[22]. In addition, we could show that Eap may exert anti-inflammatory functions, in part due to blocking interactions of leukocyte integrins with their matrix ligands[23],[24]. These observations prompted us to investigate the interaction of Eap with OPN as well. Our findings indicate that Eap specifically interacts with the N-terminal MMP-cleavage fragment of OPN thereby interfering with the αvβ3-integrin-dependent OPN-mediated adhesion and migration of breast cancer cells in vitro. In addition, Eap blocked blood-borne breast cancer cell metastasis to the bone in vivo.
MATERIALS AND METHODS
Cell culture
The breast carcinoma MDA-MB-231 cells were obtained from ATCC (Manassas, VA) and cultivated as described by the supplier.
Reagents
Purified αvβ3-integrin, blocking monoclonal antibody (mAb) LM609 against αv-integrin and rat antibody against human osteopontin were from Chemicon (Hofheim, Germany). S. aureus protein A was from Sigma (Deisenhofen, Germany) and cRGDfV peptide was from Bachem (Heidelberg, Germany). Vitronectin (VN) was purified from human plasma and converted to the multimeric form as described[24]. Recombinant full-length OPN and the recombinant fragments corresponding to the fragments cleaved by MMP at residues 166 and 210, i.e. the 40-kD N-terminal fragment (residues 1–166), the 32-kD C-terminal fragment (residues 167–314), and the 25-kD C-terminal fragment (residues 211–314) were produced and purified as described[18]. The masses of recombinant full-length OPN, the 40-kD fragment, the 32-kD fragment, and the 25-kD fragment were 35,460, 18,350, 16,744, and 13,810, respectively, as assessed by mass spectrometry[18]. The designation of the recombinant fragments, i.e. 40-kD fragment, 32-kD fragment, and 25-kD fragment derives from the apparent molecular weight of the MMP-cleaved fragments as assessed by SDS-PAGE[18]. Eap from strain Newman was purified exactly as described[24]. Eap revealed a single protein band at 64 kDa upon SDS-PAGE and was devoid of detectable endotoxin. The polyclonal antibodies against Eap were previously described[23].
In vitro ligand-receptor interactions
Binding of Eap (2 μg/ml) to immobilized full-length OPN, to the 40-kD, 32-kD and 25-kD fragments, to VN, or to BSA as a control (10 μg/ml each), or the binding of full-length OPN, of the 40-kD, 32-kD and 25-kD fragments or of VN (2 μg/ml each) to immobilized Eap (10 μg/ml each) was performed as previously described[24],[25]. Briefly, plates precoated with the immobilized ligands were blocked with 3% BSA, followed by incubation of the ligands in the soluble phase in TBS containing 0.3% BSA, 0.05% Tween-20, 1mM Ca2+. After incubation for 2hr at 22°C in each case, the respective anti-ligand antibodies (rat polyclonal against OPN; rabbit polyclonal against Eap; rabbit polyclonal to VN) followed by addition of appropriate secondary peroxidase-conjugated antibodies were used. Alternatively, binding of full-length OPN, or the 40-kD, 32-kD and 25-kD fragments (2 μg/ml each) to immobilized αvβ3-integrin (10 μg/ml) was performed in TBS containing 0.3% BSA, 0.05% Tween-20, 1mM Ca2+ in the absence or presence of competitors. After incubation for 2hr at 22°C the rat polyclonal antibody against OPN was added followed by addition of appropriate secondary peroxidase-conjugated antibody. After extensive washing the substrate ABTS was added, and binding was quantitated at 405 nm. Nonspecific binding to BSA-coated wells was used as blank and was subtracted to calculate specific binding.
Adhesion assay
Cell adhesion to multiwell plates coated with OPN, fragments thereof, or BSA as a control, was examined as previously described[25]. Multiwell plates were coated with full-length OPN or its fragments (each 10 μg/ml) and blocked with 3% (wt/vol) BSA. MDA-MB-231 cells were detached with trypsin, which was subsequently neutralized with soybean trypsin inhibitor (Sigma), washed in serum-free Dulbecco’s modified Eagle’s medium (DMEM), and plated onto the precoated wells at 37°C in the absence or presence of competitors in serum-free DMEM. After an incubation period of 60min, the wells were washed and the number of adherent cells was quantified by staining with crystal violet and measuring the absorbance at 590 nm[25].
Migration assay
Chemotaxis of MDA-MB-231 towards OPN or fragments thereof was tested using gelatine-precoated Transwell membranes (8-μm pore size and 6.5-mm diameter; Corning Costar) as previously described[24]. After gentle trypsinization, the cells were resuspended in DMEM containing 0.2% FCS. Each factor was tested in triplicates using 100,000 cells in the upper well, with OPN or the 40-kD fragment in the lower wells. After 4hr at 37°C, the number of migrated cells was estimated[24].
In vivo metastasis assay
6–8 week old female Balb/c nu/nu mice were from Charles River Laboratories (Sulzbach, Germany). Animal studies were approved by the Governmental Office Karlsruhe, Germany. After trypsinization, MDA-MB-231 cells were washed and resuspended at 106 cells/ml in sterile PBS. Mice were anesthetized by intraperitoneal injection of tribromoethanol (Sigma), and 105 cells were inoculated into the left heart ventricle as described previously[26]. Mouse health was monitored daily, and mice were sacrificed at 3 and 5 weeks after tumor cell inoculation. Mice received intraperitoneal injections of PBS or Eap diluted in PBS at a dose of 2.5 mg/kg at 0, 1, 3, 5, 7 and 10 days after tumor cell inoculation.
Three and 5 weeks following tumor cell inoculation, the mice were subjected to high resolution volumetric computed tomography (VCT) analysis to record skeletal defects. A research prototype VCT scanner (GE Global Research, Houston, TX) was used[27]. All scans were performed with 70kV/200mA (scan time: 8 s/rotation; 4.2 cm slab thickness/rotation; reconstructed voxel size 70×70×70 μm3, X-ray dose 136.6 mGy). Postprocessing of VCT data and quantitative assessment of metastasis count and volume were performed on an Advantage Workstation® 4.1 (GE Medical Systems Europe, Munich, Germany) using the Voxtool® Volume Viewer software.
Statistsical analysis
Data were analysed by the Student’s t Test or ANOVA as appropriate. P<0.05 was considered as significant.
RESULTS
Interaction of Eap with OPN
It was shown previously that Eap may interact with OPN[22]. To functionally characterize the interaction between Eap and OPN, we studied the binding of Eap to full-length OPN as well as to the OPN fragments derived from MMP-cleavage. Full-length OPN and the N-terminal 40-kD fragment bound specifically to immobilized Eap, and their binding was comparable to the binding of vitronectin to Eap that was previously demonstrated[23]. In contrast, only weak binding of the 32-kD fragment and the 25-kD fragment to immobilized Eap was detectable (Fig. 1A). In the reverse experiment, specific binding of Eap to both, immobilized full-length OPN and the N-terminal 40-kD fragment but not to the 32-kD fragment or the 25-kD fragment was noted. (Fig. 1B). Together, Eap specifically interacted with the N-terminal 40-kD portion of OPN.
Figure 1. Binding of Eap to osteopontin.
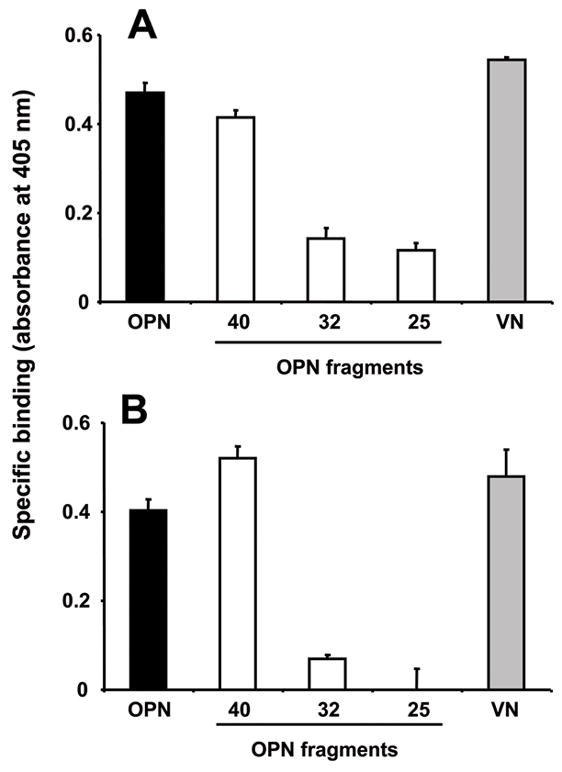
(A) Binding of full-length OPN (filled bar), the OPN fragments, (40-kD, 32-kD and 25-kD) (open bars) or vitronectin (gray bar) to immobilized Eap is shown. (B) Binding of Eap in solution to immobilized full-length OPN (filled bar), the OPN fragments, (40-kD, 32-kD and 25-kD) (open bars), or vitronectin (gray bar) is shown. Specific binding is expressed as absorbance at 405 nm. Data are mean±SD (n=3) of a typical experiment; similar results were observed in three separate experiments.
Eap blocks the interaction between αvβ3-integrin and OPN
The N-terminal fragment of OPN mediates the adhesive functions of the molecule as it harbors the RGD-motif and thereby interacts with integrins, especially αvβ3-integrin. We therefore studied the effect of Eap on the interaction between OPN and αvβ3-integrin. A specific RGD-dependent interaction between αvβ3-integrin and the full-length OPN or the N-terminal 40-kD fragment was observed, whereas no binding of the 32-kD fragment or the 25-kD fragment to immobilized αvβ3-integrin was found (Fig. 2A). The interaction between αvβ3-integrin and the full-length OPN or the N-terminal 40 kD fragment was prevented by Eap, but not by another S. aureus protein, protein A (Fig. 2A). The inhibition of the interaction between αvβ3-integrin and full-length OPN or the N-terminal 40 kD fragment by Eap occurred in a dose-dependent manner (Fig. 2B; data with the N-terminal 40 kD fragment not shown). These data indicate that Eap blocks the OPN/αvβ3-integrin-interaction.
Figure 2. Eap blocks the interaction between αvβ3-integrin and osteopontin.
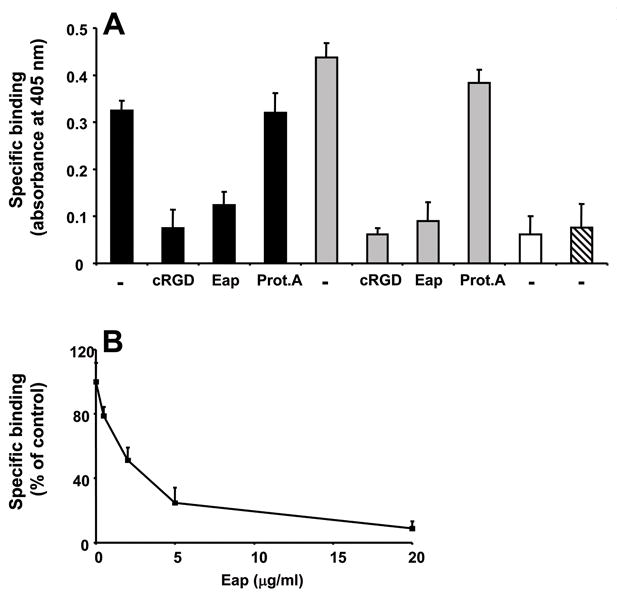
(A) The binding of full-length OPN (filled bars), the OPN 40-kD fragment (gray bars), the OPN 32-kD fragment (open bar) and the OPN 25-kD fragment (hatched bar) to immobilized αvβ3-integrin is shown in the absence (−) or presence of cyclic RGD, Eap or protein A (each 10 μg/ml). Specific binding is expressed as absorbance at 405 nm. (B) The binding of full-length OPN to immobilized αvβ3-integrin is shown in the absence or presence of increasing concentrations of Eap. Specific binding is expressed as % of control (the binding of OPN to αvβ3-integrin in the absence of competitor represents the 100% control). Data are mean±SD (n=3) of a typical experiment; similar results were observed in three separate experiments.
Inhibition of OPN-induced breast cancer cell adhesion and migration by Eap
The adhesion and migration of breast cancer cells to OPN is predominantly mediated by the αvβ3-integrin[5],[28]. We therefore investigated the effect of Eap on the adhesion of the invasive mammary carcinoma cells MDA-MB-231 to immobilized OPN. MDA-MB-231 cell adhesion to full-length OPN or the 40-kD N-terminal fragment was predominantly mediated by αvβ3-integrin, as demonstrated by inhibition with cyclic RGD peptide. Consistent with a previous report[18], the 40-kD N-terminal fragment promoted higher MDA-MB-231 cell adhesion than full-length OPN, whereas no adhesion to the 32-kD fragment or the 25-kD fragment was observed (Fig. 3A). Eap but not protein A prevented the adhesion of MDA-MB-231 cells to both full-length OPN or the 40-kD N-terminal fragment (Fig. 3A), and this effect of Eap was dose-dependent (IC50 about 2 μg/ml) (Fig. 3B).
Figure 3. Inhibition of MDA-MB-231 cell adhesion and migration to OPN by Eap.
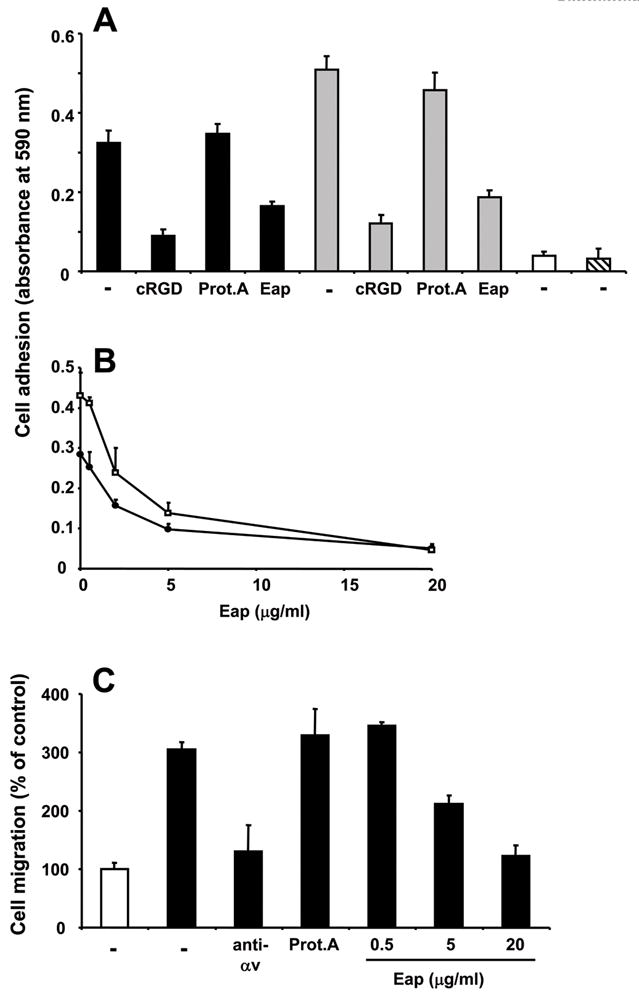
(A) The adhesion of MDA-MB-231 cells to immobilized full-length OPN (filled bars), the OPN 40-kD fragment (gray bars), the OPN 32-kD fragment (open bar) and the OPN 25-kD fragment (hatched bar) is shown in the absence (−) or presence of cyclic RGD, protein A or Eap (each 10 μg/ml). (B) The adhesion of MDA-MB-231 cells to immobilized full-length OPN (filled circles) or the OPN 40-kD fragment (open squares) is shown in the absence or presence of increasing concentrations of Eap. Specific adhesion (adhesion to BSA was subtracted) is expressed as absorbance at 590 nm. (C) The migration of MDA-MB-231 cells towards buffer (open bar) or OPN (filled bars) is shown in the absence (−) or presence of mAb to αvβ3-integrin (anti-αv), protein A (each 20 μg/ml) or increasing concentrations of Eap, as indicated. Migration is expressed as % of control (migration towards buffer in the absence of competitor represents the 100% control). Data are mean±SD (n=3) of a typical experiment; similar results were observed in three separate experiments.
OPN may also induce the migration of breast cancer cells in an integrin-dependent manner[28]. Accordingly, by using a transwell system, OPN induced a three-fold increase in the migration of MDA-MB-231 cells. In a dose-dependent manner, Eap prevented OPN-induced migration of MDA-MB-231 cells (Fig. 3C). In contrast, Eap did not interfere with adhesion to collagen or collagen-induced migration of MDA-MB-231 cells (data not shown). Together, Eap inhibits the OPN-mediated induction of αvβ3-integrin-dependent adhesion and migration of breast cancer cells.
Eap inhibits breast cancer metastasis to the bone
As the interaction between αvβ3-integrin and OPN has been implicated in breast cancer cell metastasis to the bone, we next tested whether Eap could be used as a therapeutic anti-metastatic modality. We engaged an arterial seeding model that involves the intraventricular inoculation of the human MDA-MB-231 mammary carcinoma cells into nude mice. Interestingly, Eap treatment reduced the metastasis count in both lower extremities (femur, tibia) and in vertebrae (Table 1). Concomitant with the decrease in metastasis count, Eap administration significantly diminished the volume of metastases (Fig. 4A and B). Thus, an overall lower degree of metastasis-associated bone damage was detected in Eap-treated mice (Fig. 4C). Together, Eap administration significantly ameliorated blood-borne breast cancer metastasis to the bone.
TABLE 1.
Eap blocks breast cancer metastasis in vivo.
| Metastasis Count | ||
|---|---|---|
| Lower Extremities | Vertebral Spine | |
| 21 days | ||
| Buffer | 8.28±1.42 | 3.85±1.09 |
| Eap | 0.38±0.26 | 1.0±0.50 |
| 35 days | ||
| Buffer | 9.0±1.69 | 5.00±1.27 |
| Eap | 2.88±1.31 | 1.63±0.78 |
The metastasis count in lower extremities (femur+tibia) and the vertebral spine in buffer- or Eap-treated mice 21 days 35 days following intracardial inoculation of MDA-MB-231 breast cancer cells is shown. Data are mean±SD (n=5 mice per group).
Figure 4. Reduction of MDA-MB-231 metastasis to the bone by Eap.
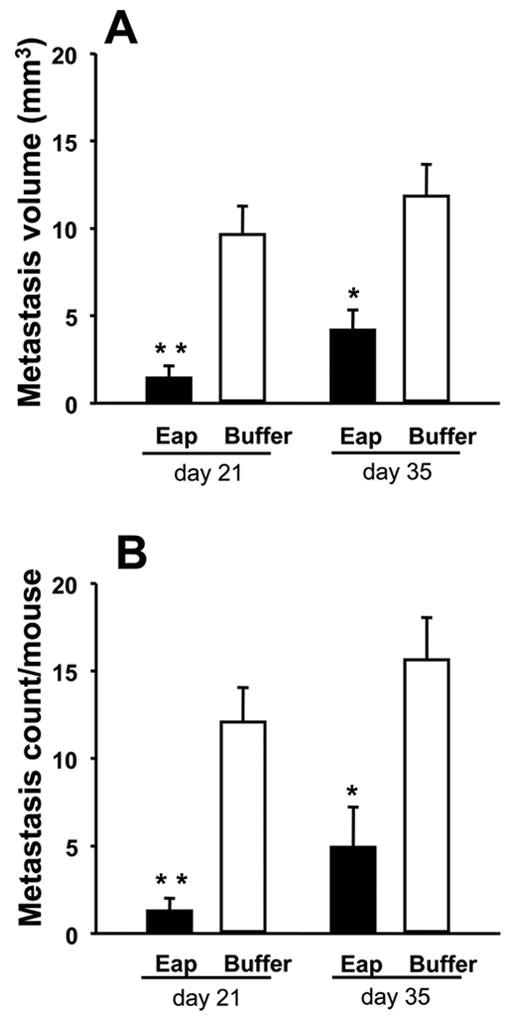
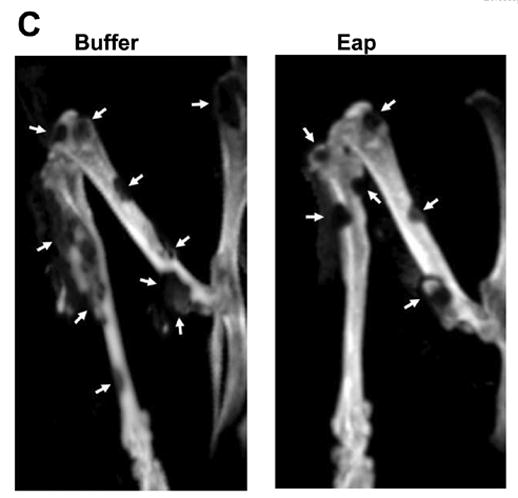
The metastasis of MDA-MB-231 cells to the bone was studied in buffer- or Eap-treated mice. (A) The total volume (in mm3) of metastases was estimated by volumetric CT and (B) the total metastasis count in vertebrae and extremities was counted at days 21 and 35 after the initial intracardiac injection of MDA-MB-231 cells. Data are mean±SD (n=5 mice/group). (C) Representative CT images from the lower extremities of a buffer-treated mouse and an Eap-treated mouse 35 days after initial intracardiac inoculation of MDA-MB-231 cells. Metastatic osteolytic lesions are depicted by arrows. *: P<0.05, as compared to buffer; **: P<0.01, as compared to buffer.
DISCUSSION
Osseous metastases are common for breast tumors and they are associated with a decrease in survival rate and a dramatic decline in life quality[1],[2]. Bone metastasis requires changes in cancer cells that permit the evasion from the primary tumor, entry into and extravasation from the circulation, as well as arrest and survival in the bone microenvironment. Such changes involve the elevated expression of αvβ3-integrin and/or of OPN in breast cancer cells, as the OPN/αvβ3-interaction is crucial in the initial breast cancer colonization in the bone[4],[6],[29],[30]. Consistently, increased levels of OPN in tumor tissue and blood correlate with poor prognosis in breast cancer patients[31], and elevated OPN expression is associated with higher metastasis rate in experimental animal models of breast cancer[32]. OPN has a protease-hypersensitive site that is susceptible to thrombin- or MMP-cleavage resulting in an N-terminal domain that primarily interacts with integrins and an C-terminal domain that binds to CD44[18],[19]. The N-terminal domain is pro-adhesive due to more efficient integrin engagement via an exposed integrin-binding motif[18]. We found here that Eap specifically interacts with the MMP-cleaved N-terminal fragment, whereas it hardly interacted with the C-terminal domain. Eap thereby interfered with αvβ3-integrin binding to the N-terminus of OPN and blocked αvβ3-integrin-dependent OPN adhesion and migration of MDA-MB-231 breast cancer cells in vitro. Moreover, Eap blocked blood-borne metastasis of breast cancer cells to the bone and we hypothesize that the Eap-mediated blockade of the OPN/αvβ3-integrin-interaction may account for this in vivo effect of Eap.
In addition to serving as a substrate for the adhesion and invasion of tumor cells in the bone microenvironment, OPN may also act in an autocrine or a paracrine manner to promote survival- and proliferation-related functions of metastatic tumor cells[14]. It has been documented that the OPN/αv-integrin-interaction may exert anti-apoptotic functions by activating the PI 3-kinase-Akt pathway in breast cancer cells[14],[28]. Other potential mechanisms of OPN-mediated malignancy, include induction of expression and activity of proteases, such as urokinase-type plasminogen activator or MMP-9, in a NF-κB- or AP-1-dependent manner, respectively[14],[28]. Thus, Eap may block tumor-progression- and metastasis-related events downstream of the OPN/αvβ3-integrin-interaction, and such a possibility needs to be addressed in future studies. Together, the αv-integrin/OPN system is instrumental in several aspects of the organotropic metastasis of breast cancer cells to the bone. Our new findings that Eap interferes with this system, and that Eap blocks bone metastasis in vivo, support the possibility that Eap-derived agents may be utilized as an attractive approach to prevent bone metastasis in patients.
Acknowledgments
This work was supported in part by the Intramural Research Program of the NIH, National Cancer Institute (T.C.) and the Deutsche Forschungsgemeinschaft (K.T.P., M.H). We acknowledge the technical assistance of Uwe Schubert, Astrid Sobke, Kathrin Respondek, Martin Obert and Sonke Bartling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kamby C. The pattern of metastases in human breast cancer: methodological aspects and influence of prognostic factors. Cancer Treat Rev. 1990;17:37–61. doi: 10.1016/0305-7372(90)90075-q. [DOI] [PubMed] [Google Scholar]
- 2.Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer. implications for management. Eur J Cancer. 2000;36:476–482. doi: 10.1016/s0959-8049(99)00331-7. [DOI] [PubMed] [Google Scholar]
- 3.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 4.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 5.Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, Mueller BM. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felding-Habermann B, Fransvea E, O’Toole TE, Manzuk L, Faha B, Hensler M. Involvement of tumor cell integrin alpha v beta 3 in hematogenous metastasis of human melanoma cells. Clin Exp Metastasis. 2002;19:427–436. doi: 10.1023/a:1016377114119. [DOI] [PubMed] [Google Scholar]
- 7.van der P, Vloedgraven H, Papapoulos S, Lowick C, Grzesik W, Kerr J, Robey PG. Attachment characteristics and involvement of integrins in adhesion of breast cancer cell lines to extracellular bone matrix components. Lab Invest. 1997;77:665–675. [PubMed] [Google Scholar]
- 8.Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res. 2005;25:79–83. [PubMed] [Google Scholar]
- 9.Teitelbaum SL. Osteoclasts and integrins. Ann N Y Acad Sci. 2006;1068:95–99. doi: 10.1196/annals.1346.017. [DOI] [PubMed] [Google Scholar]
- 10.Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 2004;26:179–184. [PubMed] [Google Scholar]
- 11.Chellaiah MA, Kizer N, Biswas R, Alvarez U, Strauss-Schoenberger J, Rifas L, Rittling SR, Denhardt DT, Hruska KA. Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression. Mol Biol Cell. 2003;14:173–189. doi: 10.1091/mbc.E02-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemoto H, Rittling SR, Yoshitake H, Furuya K, Amagasa T, Tsuji K, Nifuji A, Denhardt DT, Noda M. Osteopontin deficiency reduces experimental tumor cell metastasis to bone and soft tissues. J Bone Miner Res. 2001;16:652–659. doi: 10.1359/jbmr.2001.16.4.652. [DOI] [PubMed] [Google Scholar]
- 13.Harms JF, et al. A small molecule antagonist of the alpha(v)beta3 integrin suppresses MDA-MB-435 skeletal metastasis. Clin Exp Metastasis. 2004;21:119–128. doi: 10.1023/b:clin.0000024763.69809.64. [DOI] [PubMed] [Google Scholar]
- 14.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Bautista DS, Xuan JW, Hota C, Chambers AF, Harris JF. Inhibition of Arg-Gly-Asp (RGD)-mediated cell adhesion to osteopontin by a monoclonal antibody against osteopontin. J Biol Chem. 1994;269:23280–23285. [PubMed] [Google Scholar]
- 16.Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, Saitoh Y, Yamakido M, Taooka Y, Sheppard D. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 17.Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001;276:28261–28267. doi: 10.1074/jbc.M103608200. [DOI] [PubMed] [Google Scholar]
- 18.Gao YA, Agnihotri R, Vary CP, Liaw L. Expression and characterization of recombinant osteopontin peptides representing matrix metalloproteinase proteolytic fragments. Matrix Biol. 2004;23:457–466. doi: 10.1016/j.matbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Bayless KJ, Davis GE. Identification of dual alpha 4beta1 integrin binding sites within a 38 amino acid domain in the N-terminal thrombin fragment of human osteopontin. J Biol Chem. 2001;276:13483–13489. doi: 10.1074/jbc.M011392200. [DOI] [PubMed] [Google Scholar]
- 20.Chavakis T, Wiechmann K, Preissner KT, Herrmann M. Staphylococcus aureus interactions with the endothelium: the role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thromb Haemost. 2005;94:278–285. doi: 10.1160/TH05-05-0306. [DOI] [PubMed] [Google Scholar]
- 21.McGavin MH, Krajewska-Pietrasik D, Ryden C, Hook M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect Immun. 1993;61:2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonsson K, McDevitt D, McGavin MH, Patti JM, Hook M. Staphylococcus aureus expresses a major histocompatibility complex class II analog. J Biol Chem. 1995;270:21457–21460. doi: 10.1074/jbc.270.37.21457. [DOI] [PubMed] [Google Scholar]
- 23.Chavakis T, Hussain M, Kanse SM, Peters G, Bretzel RG, Flock JI, Herrmann M, Preissner KT. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat Med. 2002;8:687–693. doi: 10.1038/nm728. [DOI] [PubMed] [Google Scholar]
- 24.Athanasopoulos AN, Economopoulou M, Orlova VV, Sobke A, Schneider D, Weber H, Augustin HG, Eming SA, Schubert U, Linn T, Nawroth PP, Hussain M, Hammes HP, Herrmann M, Preissner KT, Chavakis T. The Extracellular Adherence Protein (Eap) of Staphylococcus aureus Inhibits Wound Healing by Interfering with Host Defense and Repair Mechanisms. Blood. 2005;107:2720–7. doi: 10.1182/blood-2005-08-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavakis T, Kanse SM, Lupu F, Hammes HP, Muller-Esterl W, Pixley RA, Colman RW, Preissner KT. Different mechanisms define the antiadhesive function of high molecular weight kininogen in integrin- and urokinase receptor-dependent interactions. Blood. 2000;96:514–522. [PubMed] [Google Scholar]
- 26.Tester AM, Sharp JA, Dhanesuan N, Waltham M, Thompson EW. Correlation between extent of osteolytic damage and metastatic burden of human breast cancer metastasis in nude mice: real-time PCR quantitation. Clin Exp Metastasis. 2002;19:377–383. doi: 10.1023/a:1016381416463. [DOI] [PubMed] [Google Scholar]
- 27.Kiessling F, Greschus S, Lichy MP, Bock M, Fink C, Vosseler S, Moll J, Mueller MM, Fusenig NE, Traupe H, Semmler W. Volumetric computed tomography (VCT): a new technology for noninvasive, high-resolution monitoring of tumor angiogenesis. Nat Med. 2004;10:1133–1138. doi: 10.1038/nm1101. [DOI] [PubMed] [Google Scholar]
- 28.Das R, Mahabeleshwar GH, Kundu GC. Osteopontin induces AP-1-mediated secretion of urokinase-type plasminogen activator through c-Src-dependent epidermal growth factor receptor transactivation in breast cancer cells. J Biol Chem. 2004;279:11051–11064. doi: 10.1074/jbc.M310256200. [DOI] [PubMed] [Google Scholar]
- 29.Furger KA, Menon RK, Tuckl AB, Bramwelll VH, Chambers AF. The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med. 2001;1:621–632. doi: 10.2174/1566524013363339. [DOI] [PubMed] [Google Scholar]
- 30.Pecheur I, Peyruchaud O, Serre CM, Guglielmi J, Voland C, Bourre F, Margue C, Cohen-Solal M, Buffet A, Kieffer N, Clezardin P. Integrin alpha(v)beta3 expression confers on tumor cells a greater propensity to metastasize to bone. Faseb J. 2002;16:1266–1268. doi: 10.1096/fj.01-0911fje. [DOI] [PubMed] [Google Scholar]
- 31.Rudland PS, Platt-Higgins A, El-Tanani M, De Silva Rudland S, Barraclough R, Winstanley JH, Howitt R, West CR. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62:3417–3427. [PubMed] [Google Scholar]
- 32.Wai PY, Kuo PC. The role of Osteopontin in tumor metastasis. J Surg Res. 2004;121:228–241. doi: 10.1016/j.jss.2004.03.028. [DOI] [PubMed] [Google Scholar]


