Abstract
Exposure to reduced activity induces skeletal muscle atrophy. Oxidative stress might contribute to muscle wasting via proteolysis activation. This study aimed to test two hypotheses in rats. Firstly, supplementation of the antioxidant vitamin E, prior and during the phase of unloading, would partly counteract unloading-induced soleus muscle atrophy. Secondly, vitamin E supplementation would decrease the rate of muscle proteolysis by reducing expression of calpains, caspase-3, -9, -12 and E3 ubiquitin ligases (MuRF1 and MAFbx). Soleus muscle atrophy (− 49%) induced by fourteen days of hindlimb unloading was reduced to only 32 % under vitamin E. Vitamin E partly prevented the decrease in type I and IIa fiber size. Supplementation increased HSP72 content, suppressed the rise in muscle level of thiobarbituric acid-reactive substance caused by unloading but failed to modify the lower ratio of reduced vs. oxidized glutathione, the higher uncoupling proteins mRNA and the antioxidant enzyme activities (superoxide dismutase, catalase, glutathione peroxidase) observed after unloading. Vitamin E treatment abolished the large upregulation of caspase 9, 12 and MuRF1 transcripts in unloaded muscle and greatly decreased the upregulation of μ-calpain, caspase 3 and MAFbx mRNA. In conclusion, the protective effect of vitamin E might be due to modulation of muscle proteolysis-related genes rather than to its antioxidant function.
Keywords: Muscle atrophy, Vitamin E, Oxidative stress, Uncoupling proteins, Heat shock protein, Caspases, Ubiquitine ligases
INTRODUCTION
Exposure to reduced activity induces a disuse atrophy of postural muscles which results in a decrease in muscle volume and strength and in a compromised ability to deal with physical work capacity (1, 2, 3). Multiple rodent models, including hindlimb unloading, have been developed to better understand the factors involved in muscle wasting (4). The loss in muscle mass is caused primarily by a rapid decrease in myofibril protein synthesis rate followed by a slower transient increase in myofibril protein degradation rate (5). Muscle atrophy is accompanied by a general shift in the contractile and metabolic profiles of a slow-twitch oxidative muscle toward that of a fast-twitch glycolytic muscle (6, 7). Among potential triggers and molecular signalling events underlying such skeletal muscle plasticity, oxidative stress might contribute to muscle wasting (2, 8–10). Although early studies in rats suggested that vitamin E (alpha-tocopherol), a potent antioxidative nutrient, decreased skeletal muscle atrophy induced by limb immobilization or plaster cast (11, 12), Koesterer et al. (13) demonstrated that antioxidant supplementation failed to attenuate the soleus and gastrocnemius (GS) atrophy or the decrease in Gas force generation induced by hindlimb unloading. Presumably, these discrepancies could be caused by differences in experimental design related to various disuse models and/or potential dose-dependent effects of antioxidant supplementation. More importantly, cellular functions of vitamin E that are independent of its radical-scavenging properties, have been demonstrated in the last years (14, 15). Azzi et al. (14,15) reported that alpha–tocopherol mediates cell signalling and regulates the expression of a large number of genes. A protective effect of vitamin E might be due to modulation of muscle proteolysis-related genes rather than to its antioxidant function.
Currently, unresolved questions remain concerning the specific role of reactive oxygen species (ROS) in the regulation of hindlimb unloading-induced muscle atrophy and more specifically which protease system are controlled by ROS (9). There is evidence that both lysosomal and/or Ca2+ - activated (i.e. calpains) proteases contribute for a minor part to muscle wasting while the ATP-ubiquitin proteasome pathway is mainly responsible for the unloaded-soleus muscle proteolysis (16). Additionally, a cascade of cystein-dependent proteases called caspases might be involved in the loss of myonuclei by apoptosis in skeletal muscle atrophied fibers (17, 18). It therefore appeared promising to further explore the therapeutic potential of vitamin E supplementation in muscle atrophy and to advance in the understanding of signaling pathways that control muscle mass following vitamin E supplementation.
This study was designed to test two hypotheses in rats. Firstly, long-term supplementation of the lipid-soluble antioxidant vitamin E, prior (21 days) and during (14 days) the phase of unloading, would partly counteract unloading-induced soleus muscle atrophy. Secondly, vitamin E supplementation would downregulate genes involved in muscle proteolysis. To address this question, we measured biomarkers of oxidative stress (glutathione vs. glutathione disulfide ratio, level of thiobarbituric acid-reactive substance), antioxidant enzyme activities (superoxide dismutase, catalase and glutathione peroxidase) and other potent antioxidant defense systems (uncoupling proteins, UCP2, UCP3 mRNA and heat shock protein content, HSP72). We assessed muscle proteolysis by measuring the relative abundance of mRNA encoding calpains, caspase-3, -9, -12 and two atrophy-related ubiquitine ligases (Muscle Ring Finger 1, MuRF1 and Muscle Atrophy F-box, MAFbx). Here we show that prevention of muscle atrophy by vitamin E might be due to modulation of muscle proteolysis-related genes rather than to its antioxidant function.
EXPERIMENTAL PROCEDURES
Animal care and protocol
Thirty two pathogen-free male Wistar rats, weighting 350 g from Charles River, were housed in a temperature-controlled room (24 ± 2°C) with a light dark cycle (12:12h). After one week of acclimatation, rats were assigned randomly to one of four experimental conditions. Two groups of eight rats received six intraperitoneal injections of vitamin E at a dose of 60 mg/kg twice a week, for 35 days according to Appell et al. (11). Vitamin E was given in the form of α tocopherol acetate solubilized in soybean oil (Sigma). After 21 days of treatment, half of them were hindlimb suspended for 14 days in individual cages using Morey’s tail-suspension model (4). Other rats were kept as controls. Two other groups of eight rats received soybean injections during 35 days and half of them were suspended for 14 days. This protocol generated 4 groups of rats: controls rats (C), vitamin E-supplemented control rats (C + VE), hindlimb unloaded rats (HU), vitamin E-supplemented and unloaded rats (HU+VE). The present investigation was performed following the recommendations provided by the European Convention for the protection of Vertebrate Animals used for Experimental and Scientific purposes (Council of Europe N° 123, Strasbourg, 1985). The animals were anaesthetised with halothane. Soleus muscles were excised, weighed, frozen in isopentane chilled with liquid nitrogen and stored at −80°C until analysis.
Myosin ATPase
The midbelly region of each muscle was cut in serial transverse sections (10 μm) on a microtome at −25°C and stained for the myosin adenosine triphosphatase (ATPase) as previously described (6). Muscle fibers were classified into two major types (I, IIa). The fiber cross-sectional areas were calculated by means of a computerized planimetry coupled to a digitizer and the area of at least 100 fibers were measured.
Glutathione levels
Reduced (GSH) and oxidized glutathione (GSSG) were measured according to the method of Anderson (19) by monitoring the reduction by GSH of 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) to 5-thio-2-nitrobenzoate (TNB) at 412 nm (25°C). Proteins were precipitated with ice-cold 5 % metaphosphoric acid and centrifuged at 5000 g for 5 min. For GSH measurements, samples were derivated with 2-vinyl-pyridin. The assay was initiated by the addition of 10 μL of 50 U/mL glutathione reductase. GSH was used as a standard and was assayed in parallel under the same condition as the tissue samples.
Muscle lipid peroxidation
The concentration of lipid peroxide was estimated by the thiobarbituric acid-reactive substances (TBARS) method described by Ohkawa et al. (20).
Antioxidant enzymes activities
A portion of soleus muscle was homogenised with a potter Elvehjem at 4°C, in buffer containing KH2PO4 (100 mM), DTT (1mM) and EDTA (2 mM), pH 7.4. After centrifugation (3000 g/min, for 5 minutes), the supernatant was used for enzymatic assays. Superoxide dismutase (SOD) activity was assayed by monitoring the rate of acetylated cytochrome c reduction by superoxide radicals generated by the xanthine-xanthine oxidase system (21). One activity unit of SOD is defined as the amount of enzyme which inhibits the rate of acetylated cytochrome c reduction by 50 %. To distinguish mangano-SOD (MnSOD), exclusively located in mitochondrial matrix, from cuprozinc-SOD (CuZnSOD), which is primarily located in the cytosol, SOD activity was determined after incubation with NaCN (1 mM). At this concentration, cyanide inhibits the CuZn isoform of the enzyme, but does not affect the Mn isoform (20). The total activity of glutathione peroxidase (GPx) activity was assayed with cumene hydroperoxide as a substrate according to Tappel (22). The activity of catalase (CAT) was determined by the method of Aebi (23). This technique used the first-order rate constant of the decomposition of H2O2 by tissue CAT at 20°C. One unit of catalase activity was calculated by using k = (2.3/dt)(log A1/A2), where k is CAT activity, dt is change in time, A1 is initial absorbance, and A2 is final absorbance. All enzyme activities are expressed in U/mg of proteins.
HSP72 content
For HSP quantification, we performed polyacrylamide gel electrophoresis and immunoblotting as previously described (24). Briefly, 200 μL of muscle homogenate were mixed with 200 μL of buffer containing 40 mM Tris(hydroxymethyl)aminomethane pH 6.8, 1% SDS, 6% glycerol, and 1% β-mercaptoethanol. This mixture was then heated at 100°C for 10 min, and subjected to one-dimensional sodium dodecyl sulfate (SDS)-PAGE with a 5% stacking and 12.5% resolving gels for 12 hours. After electrophoretic separation, proteins were transferred at a constant voltage to nitrocellulose membranes. After protein transfer, the membranes were blocked for 2h, then incubated 2 h with a monoclonal antibody specific for HSP72 (SPA 810, StressGen, diluted 1:1000) and then exposed to the secondary antibody (goat anti-mouse immunoglobulin G conjugated to horseradish peroxidase, Bio-Rad at a 1:10000 dilution). HSP72 were visualized by the enhanced chemiluminescence detection method (RPN 2106, Amersham). Scanning with a densitometer performed quantification of bands from blots and the data were expressed numerically as integrated optical density arbitrary units.
mRNA concentration in soleus muscle
Total RNA were extracted from muscle samples (50 mg) of rats using Trizol® (Invitrogen). Concentration and purity were verified by measuring optimal density at 260 and 280 nm. Their integrity was checked by 1% agarose gel electrophoresis (Eurobio). MRNA relative abundance was measured by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) using cyclophilin as reference. Primer sequences are shown in table 1. For each sample, a RT was performed from 1 μg of total RNA with 100 U of M-MLV Reverse Transcriptase (Promega), 5μL of M-MLV RT 5X buffer, 20 U of RNasin Ribonuclease Inhibitor, 15 nmoles of deoxynucleotide triphosphate and 1 μg of oligo dT, in a final volume of 25 μL. The reaction consisted of 5 min at 70°C (RNA and oligo dT), then 90 min at 42°C (all mix) followed by 10 min at 70°C. After chilling, 2.5 μL was used for PCR. The 2.5 μL of RT medium was added to 47.5 μL of PCR mix containing 5 μL of 10X EurobioTaq PCR buffer, 75 nmoles of MgCl2, 15 nmoles of deoxynucleoside triphosphate, 2.5 U of EurobioTaq DNA polymerase, 22.5 pmoles of corresponding antisense and sense primers. The PCR conditions were: 2 min at 94 °C followed by n cycles of PCR (1 cycle = 1 min at 94°C, 1 min at 60°C, 1 min at 72°C). The numbers of cycles is indicated in table 1 for each target. PCR was ended by 10 min at 72°C. Products were analysed on 1.5% agarose gel prestained with ethidium bromide. For quantitation of relative bands intensities, pictures were taken with a Camera DC120 (Kodak) and the ratio of each target to cyclophilin was determined for each sample with Kodak Digital Science 1D 2.0 (Kodak Scientific Imaging System).
Table 1.
Primer sequences used for RT-PCR
| Sense (5′→3′) | Antisense (5′→3′) | Size (bp) | n cycles | |
|---|---|---|---|---|
| cyclophilin (NM_017101) | GTG GCA AGT CCA TCT ACG GAG | CCA CAG TCG GAG ATG GTG ATC | 265 | 25 |
| MAFbx (AY059628) | GCC TGA ACT ACG ATG TTG CAG | GCT GGT CTT CAA GAA CTT TC | 323 | 29 |
| MuRF1 (AY059627) | AGG TGC CTA CTT GCT CCT TGT | GCT GTT TCC ACA AGC TTG GTC | 378 | 27 |
| caspase 3 (NM_012922) | CAC TGG AAT GTC AGC TCG CAA | CCA CTG TCT GTC TCA ATA CCG | 359 | 32 |
| caspase 9 (NM_031632) | GCA GGA TCC AGA AGC TGT TAC | GCC ATA TCT GCA TGT CTC TCG | 281 | 36 |
| caspase 12 (NM_130422) | CCG ACA AAC AGC TGA GTT TAC | GTC AGT CTC AGC ATC ATC TCT | 361 | 32 |
| μ-calpaine (NM_019152) | CCG ATA TCC GTG ATT TGG AGG | TCG ACT GTC ATA GTC GTC TGC | 478 | 36 |
| m-calpaine (NM_017116) | CAT CGC GAT GAA ACT GGC CAA | GGA AGC TCT GGT CTA GAG GCA CA | 388 | 36 |
| UCP2 (NM 019354) | AGC AGT TCT ACA CCA AGG GC | AGC ATG GTC AGG GCA CAG TG | 471 | 28 |
| UCP3 (NM_013167) | ATG CAT GCC TAC AGA ACC AT | CTG GGC CAC CAT CCT CAG CA | 314 | 28 |
Statistical analysis
All data reported are means ± SE. A multifactorial analysis of variance was used for intergroup comparisons. The Fisher paired least significant difference was used to identify specific means differences. Values were considered statistically different when P < 0.05.
RESULTS
Muscle mass and fiber size
Soleus muscle to body mass ratio and cross-sectional areas of type I and IIa fibers are shown in Table 2. Soleus muscle atrophy (− 49%) induced by 14 days of hindlimb unloading was reduced to only 32 % under vitamin E. Unloading led to a decrease in type I (− 59 %) and IIa (− 42 %) fiber size. Vitamin E partly prevented this decrease as type I and IIa fiber cross-sectional areas were only reduced by 38 and 32 % respectively.
Table 2.
Soleus muscle to body mass ratio and cross-sectional areas of type I and IIa fibers in soleus muscle
| Parameters | Soleus (mg/100g) | Fiber Areas (μm2)
|
|
|---|---|---|---|
| Type I | Type IIa | ||
| Control + saline (C) | 44.7 ± 1.7 | 4078 ± 224 | 3866 ± 227 |
| Control + vitamin E (C+VE) | 40.7 ± 1.2 | 3763 ± 158 | 4299 ± 178 |
| Hindlimb unloading + saline (HU) | 22.9 ± 1.4 * | 1661 ± 65* | 2251 ± 105* |
| Hindlimb unloading + vitamin E (HU+VE) | 27.8 ± 1.1 †# | 2348 ± 139 †# | 2937 ± 230 †# |
Values are means ± SE for 8 animals.
P < 0.05, significantly different from control + saline rats.
P < 0.05, significantly different from vitamin E-supplemented control rats.
P < 0.05, significantly different from hindlimb unloaded rats
GSH/GSSG
In control rats, there was no significant change in reduced to oxidized glutathione ratio (GSH/GSSG) after vitamin E supplementation (Fig. 1a). Unloading resulted in a significant decrease (− 23 %) of GSH/GSSG in HU rats but vitamin E supplementation failed to modify the lower ratio of reduced vs. oxidized glutathione.
Fig. 1. Effect of unloading and vitamin E supplementation on reduced to oxidized glutathione ratio (GSH/GSSG) and thiobarbituric acid reactive substances (TBARS) content.

C: control rats, C+VE: vitamin E-supplemented control rats, HU: hindlimb unloaded rats, HU+VE: vitamin E-supplemented and unloaded rats. Values are means ± SE for eight animals. * Significantly different from control rats.
Muscle lipid peroxidation
Thiobarbituric acid reactive substances (TBARS) content, an index of lipid peroxidation, was similar in C and C+VE (34.9 ± 3.6 and 33.8 ± 1.5 nmole MDA/g tissue, respectively, Fig. 1b). Vitamin E supplementation abolished the increase (+ 35 %) in TBARS content caused by unloading.
Antioxidant capacities
There was no significant difference in cytosolic (ZnCu-SOD), mitochondrial (Mn-SOD), GPx activities between C and C+VE rats (Fig. 2abc). Hindlimb unloading similarly increased ZnCu-SOD activity (+ 47 %), GPx (+ 43%) and CAT (+ 90 %) activities in HU rats and HU+VE rats (Fig. 2acd). CAT activity was elevated in the vitamin E supplemented control rats (+48 %) and there was a further significant increase on unloading (Fig. 2d).
Fig. 2. Effect of unloading and vitamin E supplementation on activity of a) cytosolic (SOD Zn2+, Cu2+) and b) mitochondrial (Mn2+) superoxide dismutase, c) glutathione peroxidase (GPx) and d) catalase (CAT).
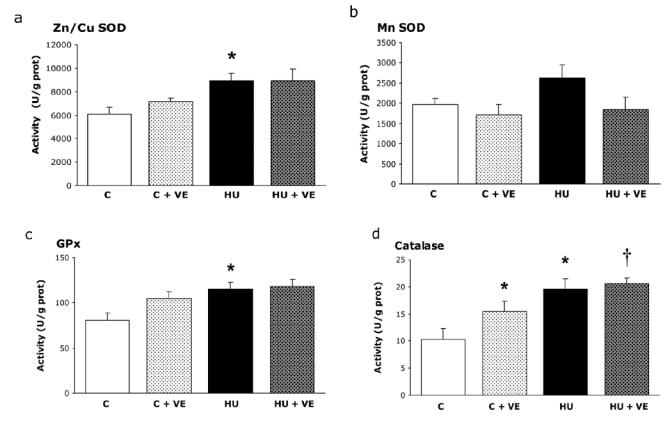
C: control rats, C+VE: vitamin E-supplemented control rats, HU: hindlimb unloaded rats, HU+VE: vitamin E-supplemented and unloaded rats. Values are means ± SE for eight animals. * Significantly different from control rats. † Significantly different from vitamin E-supplemented control rats.
Complementary defences: uncoupling proteins, heat shock proteins
Similar patterns were seen for UCP2 and UCP3 mRNA. Unloading led to higher UCP2 (+30 %) and UCP3 mRNA (+ 400%) in HU and HU+VE rats, but no obvious effect of vitamin E was observed in C rats (Fig 3ab). No change occurred in basal HSP72 level in various treatments whether supplementation or hindlimb unloading (Fig 3c). However, high levels of HSP72 (+ 50 %) were found in soleus muscle of HU+VE rats (Fig 3c).
Fig. 3. Influence of unloading and vitamin E supplementation on UCP 2 (a) and UCP3 (b) mRNA and HSP 72 protein content (c). Note that HSP72 contents are expressed as a percentage of HSP72 levels in control group.
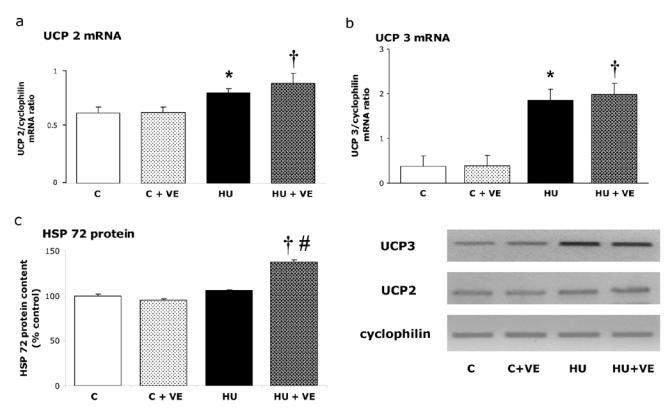
C: control rats, C+VE: vitamin E-supplemented control rats, HU: hindlimb unloaded rats, HU+VE: vitamin E-supplemented and unloaded rats. Values are means ± SE for eight animals.* Significantly different from control rats. † Significantly different from vitamin E-supplemented control rats.
Calpains, proapoptotic caspases, E3 ubiquitine ligases
In control rats, the relative abundance of caspases 3, 9, 12, MAFbx and MuRF1 mRNA were not affected by vitamin E supplementation (Fig 4, 5ab). μ-calpain mRNA was increased to 29 % after unloading, an effect that was 93 % prevented by vitamin E supplementation (P = 0.055, Fig 5c). m-calpain mRNA was neither affected by unloading nor by vitamin E supplementation (Fig 5d).
Fig. 4. Effect of vitamin E on Caspase 3, 9, 12 mRNA in control and unloaded soleus muscles.
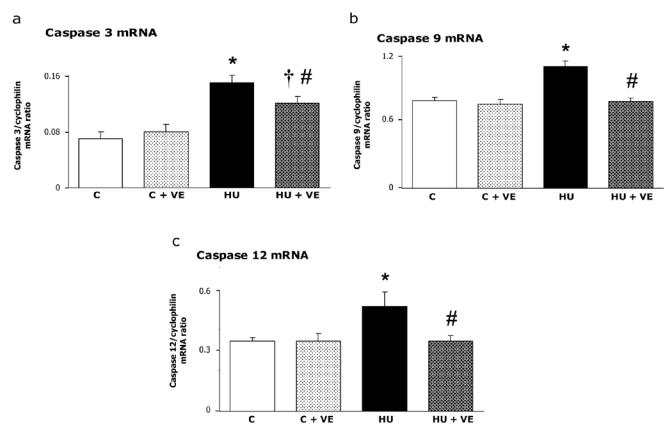
C: control rats, C+VE: vitamin E-supplemented control rats, HU: hindlimb unloaded rats, HU+VE: vitamin E-supplemented and unloaded rats. Values are means ± SE.
* Significantly different from control rats. † Significantly different from vitamin E- supplemented control rats. # Significantly different from hindlimb unloaded rats.
Fig. 5. Effect of vitamin E on expression of two atrophy-related ubiquitin ligases, MAFbx and MuRF1.
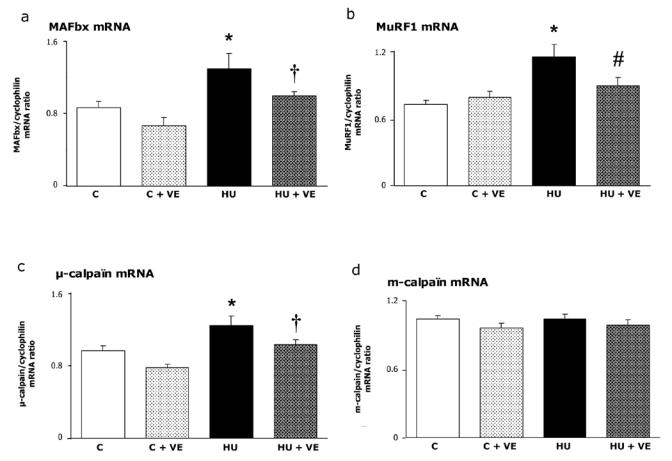
C: control rats, C+VE: vitamin E-supplemented control rats, HU: hindlimb unloaded rats, HU+VE: vitamin E-supplemented and unloaded rats. Values are means ± SE.
* Significantly different from control rats. † Significantly different from vitamin E-supplemented control rats. # Significantly different from hindlimb unloaded rats.
Vitamin E supplementation abolished the unloading-induced increase in caspase -9 and -12 mRNA (+39 and +50%, respectively, Fig. 4b, c) and decreased the upregulation (+ 114 %) of caspase -3 mRNA by 50 % (Fig. 4a). MAFbx and MuRF1 mRNA were increased in unloaded rats by 50 and 59 % respectively (Fig 5). This effect was significantly prevented by vitamin E supplementation for MuRF1 and at the limit of statistical significance for MAFbx (P = 0.07).
DISCUSSION
Supplementation of Vitamin E prevented soleus muscle atrophy by 20 % after 14 days of hindlimb unloading. The type I and IIa fiber cross-sectional areas were only decreased by 38 and 32 % respectively after Vitamin E treatment (vs 59 and 42 % respectively for the untreated-unloaded rats). Similar results were previously observed in rats submitted to various disuse models: plaster cast (11) or immobilization of the ankle joint of one hindlimb in the extended position (10). However, using the same experimental design and the same duration of hindlimb unloading, Koesterer et al (13) reported that vitamin E supplementation was not an effective countermeasure to soleus muscle disuse, suggesting that the mechanisms of atrophy induced by immobilization and hindlimb unloading might be fundamentally different. The dose-dependent effects, the potential composition and duration of the pretreatment (three weeks vs one) of supplementation might explain these discrepancies. In the present study, unloading, as expected (13), resulted in a lower ratio of reduced vs. oxidized glutathione and in an increased TBARS content, two biomarkers of oxidative stress and vitamin E supplementation restored the increased TBARS content caused by unloading. In fact, we have clearly demonstrated, as it was reported with other disuse models (10, 11), that vitamin E supplementation partly prevents soleus atrophy.
A complex cytoprotective system that includes antioxidant enzymes is recruited against free radical damage. Unloading significantly increased activities of ZnCu-SOD (primarily localized in the cytosol) as previously observed (8) but failed to induce a significant rise (P = 0.11) in Mn-SOD (localized in the mitochondrial matrix) activity. Unloading induces a rise in GPx and CAT activities contrary to the decrease observed by Lawler et al. (8). Vitamin E supplementation did not confer an additionnal protection against oxidative damage through a further increase in antioxidant enzymes activities.
One of the main adaptations of mitochondria to oxidative stress would be a mild uncoupling of oxidative phosphorylation that might reduce the mitochondrial production of ROS by lowering mitochondrial membrane potential (25, 26). The UCPs might attenuate cellular oxidative damage in some pathophysiological states leading to increased oxidative stress such as cancer cachexia (27). Body weight loss and muscle atrophy are commonly associated with this syndrome. An activation of UCP2 and UCP3 gene expression associated with an increased catalase protein content was reported in atrophied gastrocnemius muscles of tumour-bearing rats (28, 29). Busquet et al. (29) postulate that UCPs might confers protection against muscle oxidative injury. We have previously shown a marked upregulation of UCP3 gene in unloaded soleus muscle suggesting that the rise in UCP3 expression might contribute to protect less active skeletal myocytes from oxidative damage by stimulating oxygen consumption, depleting the local concentration of oxygen and decreasing the generation of ROS (30). The current study is the first to report a similar upregulation of UCP2 and UCP3 mRNA in unloaded soleus muscle following vitamin E supplementation compared to untreated rats. These results clearly show that the effects of unloading and vitamin E treatment were found to be non-additive. Assuming a role of UCP2 as a regulator of ROS production by reducing the mitochondrial proton gradient (31–33), overexpression of UCP2 would be also beneficial for preventing unloaded cells from free-radical damage.
Supplementation of Vitamin E failed to modify the lower ratio of reduced vs. oxidized glutathione, the increased antioxidant enzyme activities (superoxide dismutase, catalase, glutathione peroxidase) and the higher uncoupling proteins mRNA observed after unloading. Consequently, our data suggest that Vitamin E acts in a non-antioxidant way on the prevention of muscle atrophy which is consistent with the findings of previous studies (14, 15). Since 15 years, it has been well documented that Vitamin E exerts cellular functions that are independent of its radical-scavenging properties (14, 15).
To our knowledge this is the first report to demonstrate an increase in the stress-inducible form of HSP70 (HSP72) content as a result of Vitamin E supplementation in unloaded rats. In non-supplemented rats, the maintained basal HSP72 level in soleus muscle after two weeks of unloading is in accordance with our previous data (30) and those of Oishi et al. (34). The induction of HSP72 protein suggests that HSP72 acts as a complementary protective mechanism against muscle atrophy. Given the role of HSP72 as molecular chaperones preventing aggregation and facilitating the refolding of denaturated proteins, increased expression of HSP72 may prevent oxidative damage and assist in repair of damaged proteins (35, 36). Moreover stress-inducible form of Hsp70 prevents cell apoptosis by interfering with the ability of cytochrome c and Apaf-1 (apoptosis protease activator protein) to recruit procaspase-9 (37, 38).
In a recent review, Powers et al. (9), raised the interesting question of whether the redox control of protease activity during muscle disuse atrophy occurs by allosteric regulation (ie control of intracellular calcium levels) and/or via increased gene expression of proteases. The present study characterizes some signaling pathways that control unloading-induced muscle proteolysis following antioxidant supplementation. Vitamin E supplementation was found to prevent the upregulation of μ-calpain mRNA while m-calpain mRNA remained unaffected during muscle wasting. Ubiquitous calpains are non-lysosomal Ca2+ activated cystein proteases that cleave titin and nebulin at sites near the Z-disk, leading to a progressive disruption of the Z-disk (39, 40). They are involved in the initial breakdown of myofibrils, releasing actin and myosin from the sarcomere before they undergo ubiquitination and degradation by the proteasome. The ubiquitous μ and m-calpains (also called 1 and 2) may have different role in the development of soleus atrophy since they have different in vitro calcium sensibility (in the range of 3–50 μM for μ-calpain and 400–800 μm for m-calpain, 36). In parallel to the upregulation of μ-calpain in our study, a 36 % increase in resting intracellular calcium (from 17 to 23 nM) was reported by Ingalls et al. (41) in mice soleus muscle after 14 days of hindlimb suspension. Goll et al. (39) postulated that the increase in intracellular calcium is not sufficiently large to activate the calpains directly but it seems probable that it alters the regulation of calpain activity by calpastatin, phosphorylation or still unknown mechanisms. Indeed, a previous study reported a sustained activation of m-calpain activity after 9 days of hindlimb suspension (16).
Vitamin E partly controls the soleus muscle proteolysis mediated by the ubiquitin proteasome pathway via two muscle-specific ubiquitin ligases (E3): MAFbx (muscle atrophy Fbox or atrogin-1) and MuRF1, (muscle ring finger, 42, 43). MAFbx and MuRF1 are critical regulators in the enhanced proteolysis leading to muscle atrophy in various diseases (2, 42). As Murf1 has been shown to bind titin (44), MurF1 might participate to the disruption of the Z disk in addition to the calpain system. Vitamin E supplementation prevented MurF1 induction whereas MAFbx expression was greatly reduced (P = 0.07). It has been well documented that Vitamin E has important non-antioxidant effects and mediate cell signalling and regulation of gene expression (14, 15). For example, vitamin E downregulates a number of genes involved in acute inflammation. Given the role played by cytokines such as TNFα (tumor necrosis factor) which activate NF-KB in muscle cells (45) and the pronounced anti-inflammatory effects of vitamin E (15), one could possibly explain the prevention of MurF1 upregulation. However, if cytokines are involved in cachexia, Hunter et al. (46) did not find any change in TNFα - protein levels after a short period of muscle unloading (seven days).
Previous studies have shown that apoptosis contributes to the unloading-induced soleus muscle atrophy (17, 18). In the present study, vitamin E supplementation prevents the upregulation of caspases-9 and -12 but failed to totally abolished the increased caspase-3 mRNA. Sarcoplasmic reticulum can induce apoptosis through a calpain-mediated activation of caspase-12 and subsequent activation of caspase-9 and the key effector protease caspase-3 (47). A second intrinsic pathway of apoptosis, dependent of mitochondria, is partly initiated by reactive oxygen species (47). Release of cytochrome c into the cytosol induced the formation of an apoptosome complex with Apaf-1, ATP and procaspase-9, leading to the activation of caspase 3. Recently, Leeuwenburgh et al.(18) reported an increase in caspase-3 activity in young unloaded muscles suggesting that disuse-induced apoptosis was likely caspase-dependent whereas additional pathways such as the caspase-independent translocation of a proapoptotic endonuclease (EndoG, a mitochondrion endo-specific nuclease) might be involved in old rats. The implication of these different patterns in unloading-induced atrophy and in prevention by vitamin E should be further investigated.
The present study demonstrates that acute dosing of vitamin E, prior and during unloading, partly prevents soleus muscle atrophy. Vitamin E failed to modify markers of oxidative stress (GSH/GSSG, SOD, GPx, CAT, UCPs), suggesting a non-antioxidant mechanism for Vitamin E action. A number of genes involved in muscle proteolysis such as μcalpain, caspases-3,-9,-12 and two atrophy-related ubiquitine ligases (MuRF1 and MAFbx), were downregulated by Vitamin E while HSP72 was upregulated. These results provide an alternative signaling mechanism for understanding the muscle protective effects of Vitamin E during unloading, via muscle proteolysis-related genes regulation. Vitamin E may be regarded as an adjuvant to protect muscle cells from atrophy.
Acknowledgments
This work was supported by CNES (Centre National de la Recherche Spatiale) Grant 071299 to D. Desplanches. The authors thank Marie-Hélène Sornay-Mayet and Anne Sophie Rouziere for excellent technical assistance and Christiaan Leeuwenburgh for stimulating discussions.
LISTE OF ABBREVIATIONS
- CAT
catalase
- GPx
glutathione peroxidase
- GSH
glutathione
- GSSG
glutathione disulfide
- HSP72
heat shock protein of 72 Kda
- MAFbx
muscle atrophy F- box/atrogin-1
- MuRF1
muscle ring finger 1
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid-reactive substance
- UCP
uncoupling protein
References
- 1.Desplanches D. Structural and functional adaptations of skeletal muscle to weightlessness. Int J Sports Med. 1997;18:S 259–S264. doi: 10.1055/s-2007-972722. [DOI] [PubMed] [Google Scholar]
- 2.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 3.McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 2004;119:907–910. doi: 10.1016/j.cell.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 5.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Desplanches D, Mayet MH, Sempore B, Flandrois R. Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol. 1987;63:558–563. doi: 10.1152/jappl.1987.63.2.558. [DOI] [PubMed] [Google Scholar]
- 7.Wittwer M, Flück M, Hoppeler H, Muller S, Desplanches D, Billeter R. Prolonged unloading of rat soleus muscle causes distinct adaptations of the gene profile. Faseb J. 2002;16:884–886. doi: 10.1096/fj.01-0792fje. [DOI] [PubMed] [Google Scholar]
- 8.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 9.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol. 2005;288:R337–R344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 10.Kondo H, Nakagaki I, Sasaki S, Hori S, Itokawa Y. Mechanism of oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol. 1993;265:E839–E844. doi: 10.1152/ajpendo.1993.265.6.E839. [DOI] [PubMed] [Google Scholar]
- 11.Appell HJ, Duarte JAR, Soares JMC. Supplementation of Vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med. 1997;18:157–160. doi: 10.1055/s-2007-972612. [DOI] [PubMed] [Google Scholar]
- 12.Kondo H, Miura M, Nakagaki I, Sasaki S, Itokawa Y. Trace element movement and oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol. 1992;262:E583–E590. doi: 10.1152/ajpendo.1992.262.5.E583. [DOI] [PubMed] [Google Scholar]
- 13.Koesterer TJ, Dodd SL, Powers S. Increased antioxydant capacity does not attenuate muscle atrophy caused by unweighting. J Appl Physiol. 2002;23:1959–1965. doi: 10.1152/japplphysiol.00511.2002. [DOI] [PubMed] [Google Scholar]
- 14.Azzi A, Gysin R, Kempna P, Munteanu A, Negis Y, Villacorta L, Visarius T, Zingg JM. Vitamin E mediates cell signalling and regulation of gene expression. Ann NY Acad Sci. 2004;1031:86–95. doi: 10.1196/annals.1331.009. [DOI] [PubMed] [Google Scholar]
- 15.Zingg JM, Azzi A. Non-antioxidant activities of vitamin E. Curr Med Chem. 2004;11:1113–1133. doi: 10.2174/0929867043365332. [DOI] [PubMed] [Google Scholar]
- 16.Taillandier D, Aurousseau E, Denis-Meynial D, Bechet D, Ferrara M, Collins P, Ducastaing A, Bigard X, Guezennec CY, Schmid HP, Attaix D. Coordinate activation of lysosomal Ca2+ - activated and ATP-ubiquitin -dependent proteinases in the unweighted rat soleus muscle. Biochem J. 1996;316:65–72. doi: 10.1042/bj3160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol. 1997;273:C 579–C 587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- 18.Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol. 2005;288:R1288–R1296. doi: 10.1152/ajpregu.00576.2004. [DOI] [PubMed] [Google Scholar]
- 19.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 20.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Flohe L, Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- 22.Tappel AL. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978;52:506–513. doi: 10.1016/s0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- 23.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 24.Desplanches D, Ecochard L, Sempore B, Mayet-Sornay MH, Favier R. Skeletal muscle HSP72 response to mechanical unloading: influence of endurance training. Acta Physiol Scand. 2004;180:387–394. doi: 10.1111/j.1365-201X.2003.01255.x. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B, Gutteridge JMC Oxford University press. Free radical in biology and medicine. New York: 1999. [Google Scholar]
- 26.Papa S, Skulatchev VP. Reactive oxygen species mitochondria apoptosis and aging. Mol Cell Biochem. 1997;174:305–319. [PubMed] [Google Scholar]
- 27.Argiles JM, Busquets S, Lopez-Soriano FJ. The role of uncoupling proteins in pathophysiological states. Biochem Biophy Res Commun. 2002;293:1145–1152. doi: 10.1016/S0006-291X(02)00355-8. [DOI] [PubMed] [Google Scholar]
- 28.Sanchis D, Busquets S, Alvarez B, Ricquier D, Lopez-Soriano FJ, Argiles JM. Skeletal muscle UCP2 and UCP3 gene expression in a rat cancer cachexia model. FEBS Lett. 1998;436:415–418. doi: 10.1016/s0014-5793(98)01178-8. [DOI] [PubMed] [Google Scholar]
- 29.Busquets S, Almendro V, Barreiro E, Figueras M, Argiles JM, Lopez-Soriano FJ. Activation of UCPs gene expression in skeletal muscle can be independent on both circulating fatty acids food intake. Involvement of ROS in a model of a mouse cancer cachexia. FEBS Lett. 2005;579:717–722. doi: 10.1016/j.febslet.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 30.Denjean F, Desplanches D, Lachuer J, Cohen-Adad F, Mayet MH, Duchamp C. Muscle-specific up-regulation of rat UCP3 mRNA expression by long-term hindlimb unloading. Biochem Biophys Res Commun. 1999;266:518–522. doi: 10.1006/bbrc.1999.1847. [DOI] [PubMed] [Google Scholar]
- 31.Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity reactive and oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 32.Li LX, Skorpen F, Egeberg K, Jorgensen IH, Grill V. Uncoupling protein-2 participates in cellular defense against oxidative stress in clonal beta-cells. Biochem Biophys Res Commun. 2001;282:273–277. doi: 10.1006/bbrc.2001.4577. [DOI] [PubMed] [Google Scholar]
- 33.Negre-Salviayre A; Hirtz AC, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteila LA. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. Faseb J. 1997;11:809–815. [PubMed] [Google Scholar]
- 34.Qishi Y, Taniguchi K, Matsumoto H, Kawano F, Ishihara A, Ohira Y. Upregulation of HSP72 in reloading rat soleus muscle after prolonged hindlimb unloading. Jpn J Physiol. 2003;253:281–286. doi: 10.2170/jjphysiol.53.281. [DOI] [PubMed] [Google Scholar]
- 35.Georgopoulos C; Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 36.Smolka MB, Zoppi CC, Alves AA, Silveira LR, Marangoni S, Pereira-Dasilva L, Novello JC, Macedo DV. HSP72 as a complementary protection against oxidative stress induced by exercise in soleus muscle of rats. Am J Physiol. 2000;279:R1539–R1545. doi: 10.1152/ajpregu.2000.279.5.R1539. [DOI] [PubMed] [Google Scholar]
- 37.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI; Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 38.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the apaf-1 apoptosome by hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 39.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 40.Bartoli M, Richard I. Calpains in muscle wasting. Int J Biochem Cell Biol. 2005;37:2115–2133. doi: 10.1016/j.biocel.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Ingalls CP, Warren GL, Armstrong RB. Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J Appl Physiol. 1999;87:386–390. doi: 10.1152/jappl.1999.87.1.386. [DOI] [PubMed] [Google Scholar]
- 42.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA; Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, Dechiara TM, Stitt TN, Yancopoulos GD, Glass D J. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 43.Gomes MD, Lecker SH, Jagoe RT, Navon A; Goldberg AL. Atrogin-1, a muscle specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;25:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S. Identification of muscle ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol. 2001;306:710–726. doi: 10.1006/jmbi.2001.4448. [DOI] [PubMed] [Google Scholar]
- 45.Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-KB activation in response to tumor necrosis factor α̃. Faseb J̃. 1998;12:871–880. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 46.Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-KB pathway in skeletal muscle during disuse atrophy. Faseb J. 2002;16:529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- 47.Dupont-Veigsteegden EE. Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol. 2005;40:473–481. doi: 10.1016/j.exger.2005.04.003. [DOI] [PubMed] [Google Scholar]


