Primary angle closure glaucoma is common in certain regions of the world and is an important cause of blindness worldwide. Currently accepted treatment for patients with anatomically narrow angles is laser peripheral iridotomy (LPI).
Gonioscopy is currently the standard clinical tool for detecting occludable angles. However, this subjective technique is limited by interobserver bias and is difficult to perform in a reproducible fashion. Ultrasound biomicroscopy (UBM) is a more objective and reproducible method of angle assessment,1, 2 but the contact or immersion requirement is inconvenient and may cause artifactual widening of the angle.
Optical coherence tomography (OCT)3 is a new imaging modality that allows high-resolution, cross-sectional imaging of the eye.4-5 OCT requires no contact or immersion and has higher spatial resolution than ultrasound. In this study, we used OCT to quantify changes in anterior segment morphology after LPI.
PATIENTS AND METHODS
This study was approved by the Institutional Review Board of University of Southern California and was HIPAA compliant.
AC angle width was graded in four quadrants according to the Shaffer6 grading system. Angles with grading score ≤ 1 were classified as narrow. Ten eyes of 10 patients were enrolled in the study after obtaining informed consent and underwent manifest refraction, axial length measurement and baseline OCT imaging before LPI. Imaging was again performed 1 week post LPI.
We used an anterior segment OCT prototype provided by Carl Zeiss Meditec, Inc. (Dublin, California). This model uses a 1.3-μm wavelength light source to acquire 2000 axial scans/second. Cross-sectional angle images were obtained in the nasal and temporal quadrants. OCT images were analyzed using custom MATLAB software version 7.0 (The MathWorks, Inc., Natick, MA). AC parameters measured were: angle opening distance1 at 500 μm (AOD 500), angle recess area2 at 500 μm and 750 μm (ARA 500 and 750) and trabecular-iris space area5 at 500 μm and 750 μm (TISA 500 and 750).
Statistical analysis
Changes in mean values between baseline and post-LPI visits were assessed using paired Student's t test and Wilcoxon signed-rank test. P value < 0.05 was considered statistically significant.
RESULTS
Ten eyes of 10 patients with occludable angles were enrolled in the study. Patients included five males and five females. Six patients were Hispanic, two were white, and two were Asian. Average patient age was 63.6 years (± 15.85; 41-83 years). Spherical equivalent refraction was +0.8 diopters (D) (± 2.2 D; −2.75 D to +2.25 D). Average axial length was 21.8 mm (± 2.0; 20.87 - 23.24 mm).
The gonioscopy grades increased from 0.56 ± 0.8 before LPI to 2.6 ± 0.8 after LPI (P < 0.001). AOD 500, ARA 500, ARA 750, TISA 500, and TISA 750 all were significantly increased after LPI (Table).
Table.
Changes in OCT parameters and gonioscopy before and after LPI
| AOD 500 (μm) |
ARA 500 ( × 10−2 mm2) |
ARA 750 ( × 10−2 mm2) |
TISA 500 ( × 10−2 mm2) |
TISA 750 ( × 10−2 mm2) |
Gonioscopy | |
|---|---|---|---|---|---|---|
| Pre LPI | 83.4 ± 80.9 | 4.7 ± 4.0 | 7.5 ± 6.8 | 3.8 ± 3.3 | 6.0 ± 5.6 | 0.56 ± 0.8 |
| Post LPI | 220.6 ± 141.6 | 14.5 ± 11.5 | 17.1 ± 6.3 | 7.8 ± 4.1 | 14.6 ± 9.2 | 2.6 ± 0.8 |
| P value | 0.02 | 0.02 | 0.004 | 0.03 | 0.02 | <0.001 |
OCT = optical coherence tomography; LPI = laser peripheral iridotomy; AOD = angle opening distance; ARA = angle recess area; TISA = trabecular-iris space area.
DISCUSSION
Several studies have used UBM to look at the effects of LPI on AC angle morphology,1, 2 but few have used anterior segment OCT to quantify such changes.7 OCT has several advantages over UBM. It is a light-based system that produces images with high spatial resolution. Its non-contact nature enhances patient comfort and allows rapid image acquisition in the sitting position without mechanical distortion of the angle. Our OCT images show that after LPI, the convex configuration of the iris flattens and the AC angle widens (Figure). The degree of angle widening can be quantified using custom software that has been previously validated5 in comparison with gonioscopy and UBM. These LPI-induced changes in angle parameters agree well with gonioscopic angle opening.
Figure.
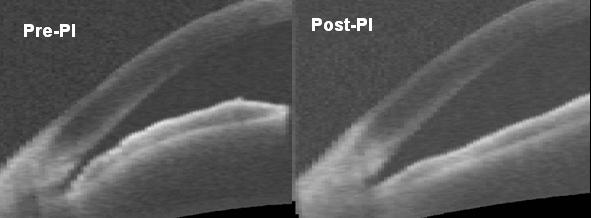
Cross-sectional OCT image of an angle before and after laser peripheral iridotomy (LPI). Note flattening of the convex iris configuration and widening of the angle after the procedure.
We believe that the ease of image acquisition and analysis with OCT, together with its non-contact nature, make it a desirable tool for large-scale screening for narrow angles. By using cut-off values on the OCT parameters we can determine which angles are occludable and would benefit from LPI. Future studies with larger numbers of subjects are needed to more clearly define the role of OCT for this potential application.
ACKNOWLEDGEMENTS/DISCLOSURE
Grant support from NIH R24 EY 13015, National Eye Institute, Bethesda, MD and an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY. Research equipment and grant support from Carl Zeiss Meditec, Inc., Dublin, CA. David Huang receives royalties from OCT patents licensed to Carl Zeiss Meditec, Inc., Dublin, CA. David Huang and Yan Li receive research grant support from Carl Zeiss Meditec, Inc. None of the other authors has any proprietary interest in the subject of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992;113:381–389. doi: 10.1016/s0002-9394(14)76159-8. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa H, Liebmann JM, Ritch R. Quantitative assessment of the anterior segment using ultrasound biomicroscopy. Curr Opin Ophthalmol. 2000;11:133–139. doi: 10.1097/00055735-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radhakrishnan S, Rollins AM, Roth JE, et al. Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch Ophthalmol. 2001;119:1179–1185. doi: 10.1001/archopht.119.8.1179. [DOI] [PubMed] [Google Scholar]
- 5.Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005;123:1053–1059. doi: 10.1001/archopht.123.8.1053. [DOI] [PubMed] [Google Scholar]
- 6.Shaffer RN. Primary glaucomas. Gonioscopy, ophthalmoscopy and perimetry. Trans Am Acad Ophthalmol Otolaryngol. 1960;64:112–127. [PubMed] [Google Scholar]
- 7.Chalita MR, Li Y, Patil C, et al. High-speed optical coherence tomography of laser iridotomy. Am J Ophthalmol. 2005;140:1133–6. doi: 10.1016/j.ajo.2005.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]


