Abstract
Infections due to extraintestinal pathogenic E. coli (ExPEC) result in significant morbidity, mortality and increased healthcare costs. An efficacious vaccine against ExPEC would be desirable. In this report we explore the use of killed-whole E. coli as a vaccine immunogen. Given the diversity of capsule and O-antigens in ExPEC we have hypothesized that alternative targets are viable vaccine candidates. We have also hypothesized that immunization with a genetically engineered strain that is deficient in the capsule and O-antigen will generate a greater immune response against antigens other than the capsular and O-antigen epitopes than a wild-type strain. Lastly, we hypothesize that mucosal immunization with killed E. coli has the potential to generate a significant immune response. In this study we demonstrated that nasal immunization with a formalin-killed ExPEC derivative deficient in capsule and O-antigen results in a significantly greater overall humoral response compared to its wild-type derivative (which demonstrates that capsule and/or the O-antigen impede the development of an optimal humoral immune response) and a significantly greater immune response against non-capsular and O-antigen epitopes. These antibodies also bound to a subset of heterologous ExPEC strains and enhanced neutrophil-mediated bactericidal activity against the homologous and a heterologous strain. Taken together these studies support the concept that formalin-killed genetically engineered ExPEC derivatives are whole cell vaccine candidates to prevent infections due to ExPEC.
Keywords: extraintestinal pathogenic E. coli, vaccine, capsule, O-antigen, antibody
Introduction
A wide variety of infections continue to be responsible for significant morbidity, mortality and healthcare costs. The development of new vaccines directed against the responsible pathogens will help minimize disease and should be highly cost-effective. Despite a recent emphasis on Gram-positive bacterial infections, Gram-negative bacterial infections, especially those due to extraintestinal pathogenic E. coli (ExPEC), continue to be extremely important. ExPEC are the most common enteric Gram-negative organisms to cause extraintestinal infection in the ambulatory, long-term-care, and hospital settings [1-5]. Typical extraintestinal infections due to E. coli include urinary tract infection, diverse intra-abdominal infections, pneumonia, surgical site infection, meningitis, intra-vascular device infection, osteomyelitis, and soft tissue infections, any of which can be accompanied by bacteremia and sepsis [6]. Sepsis is ranked as the tenth overall cause of death in the U.S. [7] and by using the conservative estimate that E. coli causes 17% of cases of severe sepsis [1, 8, 9], severe sepsis due to E. coli (the leading etiologic agent) was associated with an estimated 40,000 deaths in 2001.
In the past ExPEC typically have been highly antibiotic susceptible, hence readily eradicated with antibiotic therapy. Unfortunately, this situation has changed recently. Resistance to trimethoprim-sulfamethoxazole and fluoroquinolones has increased [10-12]. In addition, a significant minority of extraintestinal E. coli isolates from long-term care facilities and hospitals in the U.S. already have acquired plasmids encoding extended spectrum ß-lactamases that confer resistance to 3rd generation cephalosporins, and aztreonam, and frequently contain linked resistance determinants for aminoglycosides, tetracyclines, and trimethoprim-sulfamethoxasole. Furthermore, the incidence of serious extraintestinal infection due to E. coli increases with age [1, 13]. As the proportion of elderly patients increases in the U.S. and other developed countries, so likely will the number of extraintestinal E. coli infections.
ExPEC are typical extracellular bacterial pathogens. These strains are inherently resistant to innate host defense factors such as complement, cationic antimicrobial peptides, and phagocytosis in the absence of opsonization. Given the extracellular lifestyle of ExPEC, the development of bactericidal antibodies should lead to protective immunity [14]. However, despite the peaceful coexistence of extraintestinal pathogenic E. coli strains with humans (and other mammals and birds) on the intestinal (+ /− the vaginal and oropharynx) mucosal surface, the host is unable to develop a protective immune response as a result of colonization. In fact, not only is the host susceptible to an initial infection in an extraintestinal site, but it also is susceptible to recurrent infections from both homologous and heterologous strains [15]. This suggests that “natural” ExPEC infection does not always result in a protective immune response and/or that ExPEC have evolved mechanisms to subvert an acquired protective immune response from the host.
Nonetheless, despite the host's apparent inability to develop a protective immune response to natural infection, we hypothesize that a successful immunization strategy can be developed and used to confer protection against ExPEC. In animal models, passive or active immunization against capsule, O-specific antigen, and iron regulated outer membrane proteins have afforded protection against systemic infection [16-18], and immunization with capsule, O-antigen, P and type 1 fimbriae, and the siderophore receptor IroN are protective against urinary tract infection from ExPEC strains expressing these virulence factors [19-25]. However, a vaccine based on capsule and/or O-specific antigens is impractical because of the significant antigenic heterogeneity (>80 capsular and >150 O-antigen variants). Although only a fraction of these capsular and O-antigen variants are encountered among ExPEC, surface polysaccharides from ExPEC isolates nonetheless exhibit considerable antigenic diversity. Further, certain capsular polysaccharides (e.g. K1, K5) are poorly immunogenic, which has been speculated to be due to their antigenic similarities to host tissues. In addition, initial findings from human trials reportedly did not demonstrate efficacy of immunization with the type 1 fimbrial adhesin FimH against UTI. In contrast, oral immunization with lyophilized extracts of whole E. coli strains has been used in Europe to prevent recurrent UTI with some success [26]. Immunization with whole E. coli has certain potential advantages. First, given that commensal E. coli and ExPEC are part of the normal human flora, mucosal immunization with whole organisms will likely be safe. Second, utilization of whole organisms has the potential for the development of bactericidal antibodies to multiple antigenic targets. Third whole organisms may possess natural adjuvants[27, 28]. Lastly, immunization with whole organisms has the potential for development of bactericidal antibodies directed against conformational and linear epitopes.
However, given that natural infection or intestinal colonization does not appear to generate a protective response against subsequent infection due to homologous and heterologous ExPEC strains we hypothesize that: a) these routes of “natural” immunization do not result in an optimal immune response and b) ExPEC may possess virulence factors (e.g. capsule and O-antigen) that preclude the development of this response. Despite this, we hypothesize that an alternative route of immunization with a genetically engineered strain in which capsule and O-antigen are no longer expressed is needed for the development of an optimal immune response. In the study reported here we tested the hypotheses that: 1) nasal immunization with the wild-type (w.t.) ExPEC isolate CP9 (O4/K54) can be used to generate a humoral immune response, 2) nasal immunization with formalin-killed CP9 (w.t.) will generate an immune response that is similar to that achieved with live CP9, 3) CP9's surface polysaccharides capsule and the O-antigen moiety of lipopolysaccharide (LPS) impede the development of an optimal immune response, 4) a similar amount of antibodies that recognize surface epitopes on CP923 (capsule and O-antigen minus) will be generated after immunization with live and formalin-killed CP923, 5) CP923-specific antiserum is biologically active, and 6) opsonization of either the homologous parent strain (CP9) or a heterologous ExPEC strain with CP923-specific antiserum will enhance neutrophil-mediated bactericidal activity compared to the CP9 specific antiserum.
Methods
Bacterial strains
CP9 is an E. coli blood isolate from a patient with sepsis and has been previously described in detail [29, 30]. CP9 possesses many of the characteristics of typical ExPEC strains [31] and is highly virulent in a urinary tract infection model [32], an intra-peritoneal infection model [33], and a pneumonia model [34, 35]. CP923 (capsule and O-antigen minus) is an isogenic derivative of CP9 generated by transposon mutagenesis [36]. Sequence analysis established the precise location of the transposon insertions in the expected capsule and O-antigen gene clusters [36, 37]. As a result of their locations within these gene clusters, polar effects of the transposon insertions only affected genes involved in capsule or O-antigen biosynthesis, transport and assembly [36-38]. Additional ExPEC strains used in antibody binding studies are described in the methods section entitled random amplified polymorphic DNA (RAPD) analysis. All strains were maintained at -80°C in 50% Luria-Bertani (LB) broth and 50% glycerol. Bacterial strains were grown in LB broth (5 grams yeast extract, 10 grams tryptone, 10 grams NaCl per liter) in which iron was chelated with 0.2M 2,2'-dipyridyl (Sigma, St. Louis, MO) so that iron repressed proteins would be expressed as occurs within the host in vivo. Incubations were performed at 37°C unless otherwise described.
Animal Immunization
Mice
To assess for an acquired humoral immune response against whole bacteria, equal numbers of male and female C57 black6 mice (18-24 grams) were immunized with live or formalin-killed whole bacteria. Live bacteria were grown overnight in LB-iron-chelated medium, titered to determine cfus, and washed once with 1X phosphate buffered saline (PBS). Formalin-killed bacteria were generated by growth overnight in LB-iron-chelated medium, titered to determine cfus, resuspended in 1% formalin in 1X PBS and subsequently incubated at 37°C for 24 hours. After incubation with formalin bacteria were washed 3 times with the same volume of 1X PBS. After acquiring pre-immunization sera, mice were nasally immunized three times at two-week intervals with either approximately 1 × 109 cfu of live bacteria or formalin-killed bacteria, or received buffer alone (non-immunization controls). Ten to fourteen days after the third immunization, post-immunization serum was acquired. Comparison of pre and post immunization sera were used to evaluate the antibody response resulting from immunization with whole bacteria. Sera were obtained and measurements were made from 20 non-immunized control animals (4 independent experiments), 6 animals immunized with live CP9 (2 independent experiments), 18 animals immunized with formalin-killed CP9 (3 independent experiments), 11 animals immunized with live CP923 (2 independent experiments) and 28 animals immunized with formalin-killed CP923 (4 independent experiments). Limited quantities of serum were obtained from some animals, precluding use in some experiments.
Rabbits
High titer, high volume rabbit anti-sera was obtained via subcutaneous immunization (four times at 2 week intervals) with formalin-killed CP9 (w.t.)(9.0 × 109 cfu) or CP923 (O-antigen and capsule-minus)(2.6 × 109 cfu) grown overnight in LB-iron-chelated medium without adjuvant. This sera was used for neutrophil-mediated bactericidal assays.
Antibody response to whole strains of E. coli
Quantitative measurement
The measurement of the serum IgG/IgA response after nasal immunization with CP9 (w.t.), the comparison of this response after immunization with live and formalin-killed CP9, and the comparison of mice nasally immunized with either formalin-killed CP9 or CP923 utilized a quantitative assay. Antibody levels were determined in duplicate by ELISA. The wells of ELISA plates (Dynatek Immulon II) were coated overnight at room temperature with either unlabelled goat anti-mouse IgG or IgA (100ng/well) or whole live bacteria (5 × 106 cfu/well of either CP9 or CP923) in borate buffered saline (pH 8.2). The homologous strain was used to test a given antiserum unless otherwise stated. The next day plates were washed x 3 with washing buffer (1X PBS-0.5% Tween 20(pH 7.2)) and dilutions (1X PBS- 0.15% Tween 20 (pH 7.2)) of the sera being tested (for CP9 1:100-1:2,500 for IgG/IgA and for CP923 1:100-1:2,500 for IgA and 1:350-1:12,600 for IgG) were added to the wells (100 μl/well) coated with the appropriate bacteria and incubated overnight at room temperature. On the third day plates were washed x 3 with washing buffer. Antibody levels were detected by the addition of a 1/5000 dilution of the appropriate horse-radish-peroxidase-conjugated goat anti-mouse Ig (Southern Biotechnology Associates, Inc., Birmingham, AL) for 4 hours at room temperature, followed by washing buffer x 3, and then followed by the addition of Ophenylenediamine dihydrochloride and 30% H2O2 in phosphate-citrate buffer (100μl/well). The reaction was stopped with 1M sulphuric acid (100μl/well) and read on an ELISA-plate reader at 490nm. For each experiment a standard curve was generated using a mouse immunoglobulin reference serum against wells coated with goat anti-mouse IgG or IgA. This curve established that the ELISA assay for a given experiment was linear up to an OD490nm of 1.5-2.0. Sample dilutions were chosen so that OD490nm measurements would fall within this OD490nm range. The CP9 or CP923-specific antibody response was presented as ng/ml, calculated from the standard curve, and the fold increase was calculated as the ratio of the post versus pre-immunization measurement from the CP9/CP923 ELISA.
Semi-quantitative measurement
The comparison of the serum IgG response after immunization with formalin-killed CP9 (w.t.) versus formalin-killed CP923 (capsule and O-antigen-minus) using multiple bacterial titers was done using a semi-quantitative assay. Antibody levels in pre and post-immunization pooled sera were determined by ELISA as described above with the following modifications. Eight sera pools were generated as follows: 1) males immunized with 1X PBS (CP9 controls, n= 10); 2) males immunized with formalin-killed CP9 (n = 10); 3) males immunized with 1X PBS (CP923 controls, n= 17); 4) males immunized with formalin-killed CP923 (n = 18); 5) females immunized with 1X PBS (CP9 controls, n= 5); 6) females immunized with formalin-killed CP9 (n = 5); 7) females immunized with 1X PBS (CP923 controls, n= 10); 8) females immunized with formalin-killed CP923 (n = 10). The wells of ELISA plates were coated overnight at room temperature with whole live bacteria, in borate buffered saline (pH 8.2), over a range of titers (1 × 102 - 1 × 108 cfu/well of either CP9 or CP923) since CP9 and CP923 may bind antibody differently. Because capsular polysaccharide and O-antigen affects antibody binding (Russo, TA unpublished data) both CP9 and CP923 were used to test antisera generated by immunization with formalin-killed CP9 and formalin-killed CP923. The next day plates were washed, 1: 100 and 1:1000 dilutions of CP9 and CP923-specific antisera were added to the wells coated with various titers of either CP9 or CP923, and incubated overnight. On the third day plates were washed and antibody levels were detected. The reaction was stopped and read on an ELISA-plate reader at 490nm. A standard curve was not generated for this experiment. Differences in the IgG response after immunization with either formalin-killed CP9 or formalin-killed CP923 or in animals that received 1X PBS were determined by differences in the optical density at 490nm at an equivalent antiserum dilution and titer of bacteria coating the ELISA plate.
Antibody binding to live E. coli
The appropriate E. coli strains were grown in iron chelated LB medium, diluted to 1×106 cfu, washed in 100 μl of 1X PBS (pH 7.2), resuspended in 100 μl of 1: 100 diluted mouse serum (complement inactivated), and incubated rotating for 60 minutes at 37°C. Preliminary experiments established that this incubation time and dilution resulted in maximal antibody binding. Post-incubation the bacteria were washed once with 0.5ml of 1X PBS, resuspended in 100μl of goat anti-mouse IgG/IgM fluorescein isothiocyanate (FITC)-labeled conjugate (Caltag Laboratories, Burlingame, CA), and incubated rotating at 37°C for 30 minutes. After labeling bacteria were washed, resuspended in 500 μl of 1X PBS and transferred to 5ml flow cytometry tubes (Falcon 352054). Antibody binding was analyzed by flow cytometry. Ten-thousand bacterial events were gated in the forward-scatter versus side-scatter plot, discriminating them from “debris”, and antibody-bound-bacteria events were assessed in the FL-1 channel and reported as geometric mean fluorescence (GMF). The mean GMF for binding of sera obtained from animals immunized with 1X PBS was 4.6 ± 2.3 (S.D.). Therefore, a GMF of ≥10 was considered significant (> 2 S.D. above the mean). For studies that assessed antibody binding to heterologous ExPEC strains, CP923-specific antiserum, generated by nasal immunization of mice with formalin-killed CP923 was utilized.
Random amplified polymorphic DNA (RAPD) analysis
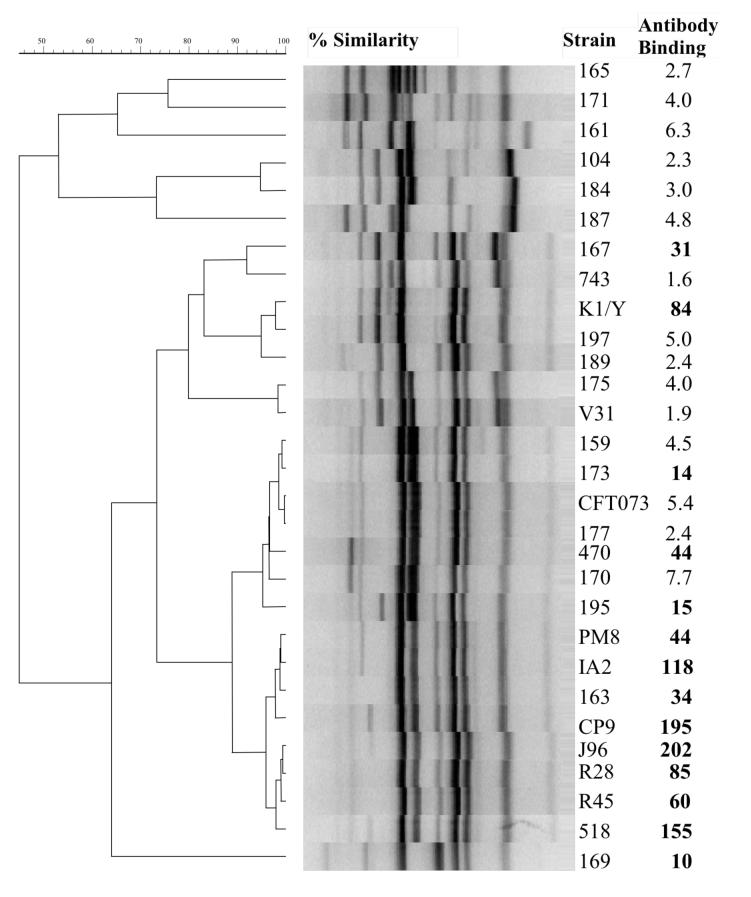
Twenty-nine ExPEC strains underwent RAPD analysis to assess genomic relatedness, including strain CP9(O4:K54) and 28 heterologous strains. These 28 strains consisted of 17 isolates of unknown serotypes from patients with bacteremia from Buffalo, NY (104, 159, 161, 163, 165, 167, 169, 170, 171, 173, 175, 177, 184, 187, 189, 195, 197), 2 serotyped bacteremic isolates kindly provided by Alan Cross (470(O25:K5), 743(O6:K2)), 3 strains previously established to be highly related to CP9 (O4 J96-like strains; J96(O4:K-), R28(O4:K3), 518(O4:K10, K54:96) [30], 3 strains previously established to be less related to CP9 (other O4 strains; R45(O4:K12, V31(O4:K12), PM8(O4:K12)) [30], and 3 strains that have served as model pathogens for investigations on ExPEC pathogenesis K1/Y (O7:K1)[39], IA2 (O4:K12)[40], CFT073(O6:K2)[41]. These isolates underwent genomic profiling by random amplified polymorphic DNA (RAPD) analysis using the arbitrary decamer 1254 [42], with boiled lysates used as template DNA. Within the application Molecular Analyst (BioRad), profiles were compared digitally in a pairwise fashion as analog densitometric scans by using Pearson's correlation coefficient [43]. A dendrogram was inferred from the resulting similarity matrix according to the unweighted pair group method with arithmetic means (UPGMA) [43].
Bactericidal Assay
A neutrophil-mediated bactericidal assay was performed essentially as described [44]. In brief, either 5 × 105 purified neutrophils [45] in 500 μl of 20% plasma pre-heated at 56°C for 30 minutes (Δ6°C)-1X PBS pH 7.4 (plasma-PBS) or plasma-PBS alone were added to each well of a tissue culture plate (Costar 3472-clear, Corning Inc., Corning, NY). Neutrophils were allowed to adhere at room temperature for 30 minutes. To assess the effect of opsonization with CP923-specific antisera against the homologous strain CP9 an estimated 1 × 105 cfu of CP9 (quantified by a subsequent titer) in 500 μl of plasma-PBS that were either: 1) not pre-opsonized (n=8), 2) pre-opsonized with a 1:100 dilution of pre-immune mouse serum (Δ56°C)(n=5) or, 3) opsonized with a 1:100 dilution of CP923-specific mouse antiserum (Δ56°C)(n=12) were added to wells that did or did not contain 5 × 105 purified human neutrophils. To compare the effects of opsonization with CP923-specific antisera versus CP9-specific antisera against the heterologous ExPEC strain K1/Y (55) an estimated 1 × 105 cfu of K1/Y (quantified by a subsequent titer) in 500 μl of plasma-PBS that were either: 1) not pre-opsonized (n=9), 2) pre-opsonized with a 1:100 dilution of CP9-specific mouse or rabbit antiserum(Δ56°C)(n=9) or, 3) opsonized with a 1:100 dilution of CP923-specific mouse or rabbit antiserum (Δ56°C)(n=9) were added to wells with and without neutrophils. To expedite bacterial-neutrophil contact the tissue culture plate was spun at 262xG for 10 minutes, and placed at 37°C in a CO2 incubator. At 60 minutes 20μl of saponin (from a 25mg/ml stock solution in H2O) was added to each well and the titer of CP9 or K1/Y was determined. Bactericidal activity was calculated as the difference between bacterial log titers in the presence and absence of neutrophils.
Results
Nasal immunization with a live wild-type ExPEC strain (CP9) results in a significant humoral immune response
Male and female C57 black6 mice were nasally immunized with the live ExPEC strain CP9 or received buffer (nonimmunized controls), and the humoral immune response was measured by ELISA. The mean IgG and IgA concentrations were measured in serum (Figure 1). Results were similar from male and female mice and therefore were pooled.
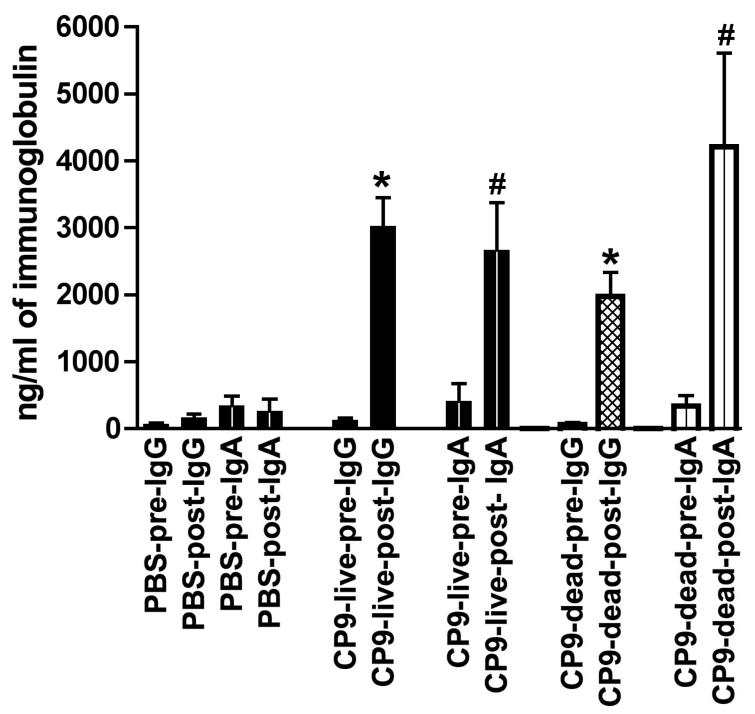
Figure Legend 1.
The serum IgG and IgA antibody response after nasal immunization with CP9 (w.t.). After pre-immunization serum was obtained, C57 black6 mice were nasally immunized (2 week intervals × 3) with 1 × 109 cfu live CP9, 1 × 109 formalin-killed CP9, or received 1X PBS (non-immunized controls). IgG and IgA were measured in pre and post-immunization sera by ELISA using the homologous strain CP9, as described in Methods. There was a significant increase in IgG and IgA concentrations between the pre and post CP9 (live) and pre and post CP9 (formalin-killed) groups (* P < 0.0001; # P = 0.01, paired t-test). There was no significant difference in the post-immunization concentrations of IgG and IgA between the CP9 (live) and CP9 (formalin-killed) groups (P > 0.1, unpaired t-test).
Assessment of the systemic compartment immune response demonstrated that in mice immunized with live CP9, the mean serum concentration of CP9-specific-IgG was significantly increased post-immunization (3019 ng/ml ± 432 S.E.M.) compared with pre-immunization (122 ± 41)(P < 0.0001, paired t-test). As expected, in mice that received buffer, the mean serum concentration of CP9-specific-IgG was not significantly increased post-nasal administration of buffer (163 ± 57) compared with pre-nasal administration (68 ± 18) (P > 0.1, paired t-test). These mean serum concentrations of CP9-specific-IgG represented an increase of 24.7-fold in mice immunized with live CP9 compared to an increase of 2.4-fold in mice that received buffer (non-immunized controls). In mice immunized with live CP9, the mean serum concentration of CP9-specific-IgA was also significantly increased post-immunization (2661 ng/ml ± 715 S.E.M.) compared with pre-immunization (407 ± 267)(P = 0.01, paired t-test). Again as expected, in mice that received buffer, the mean serum concentration of CP9-specific-IgA was not significantly increased post-nasal administration of buffer (258 ± 190) compared with pre-administration (337 ± 152) (P > 0.1, paired t-test). These mean serum concentrations of CP9-specific-IgA represented an increase of 6.5-fold in mice immunized with live CP9 compared to an increase of 0.8-fold in mice that received buffer (non-immunized controls). These data demonstrate that nasal immunization with a live ExPEC strain results in significant serum IgG and IgA responses directed against the immunizing strain.
Nasal immunization with a live or formalin-killed wild-type ExPEC strain (CP9) results in a similar humoral immune response
Immunization with a killed strain is more practical and safer than immunization with a live strain. Therefore the mean serum IgG and IgA concentrations directed against the ExPEC strain CP9 were compared in male and female C57 black6 mice that were nasally immunized with either live or formalin-killed CP9 (Figure 1). Results were similar from male and female mice and therefore were pooled.
In mice immunized with formalin-killed CP9, the mean serum concentration of CP9-specific-IgG was significantly increased post-immunization (1978 ng/ml ± 355 S.E.M.) compared with pre-immunization (69 ± 25)(P < 0.0001, paired t-test). These mean serum concentrations of CP9-specific-IgG represented an increase of 28.7-fold in mice immunized with formalin-killed CP9. In mice immunized with formalin-killed CP9, the mean serum concentration of CP9-specific-IgA was also significantly increased post-immunization (4205 ng/ml ± 1408 S.E.M.) compared with pre-immunization (337 ± 158)(P = 0.01, paired t-test). These mean serum concentrations of CP9-specific-IgA represented an increase of 12.5-fold in mice immunized with formalin-killed CP9. Although the mean serum concentration of CP9-specific-IgG was lower after immunization with formalin killed CP9 compared to live CP9 (1978 ng/ml ± 355 S.E.M. versus 3019 ng/ml ± 432 S.E.M respectively) and the mean serum concentration of CP9-specific-IgA was greater after immunization with formalin killed CP9 compared to live CP9 (4205 ng/ml ± 1408 S.E.M. versus 2661 ng/ml ± 715 S.E.M. respectively), these differences were not statistically significant (P > 0.1, unpaired t-test). These data demonstrate that nasal immunization with a formalin-killed ExPEC strain results in a significant serum IgG and IgA response directed against the immunizing strain that is comparable to that observed after immunization with a live strain.
Nasal immunization with formalin-killed ExPEC strain CP923 that does not produce a capsule or the O-antigen moiety of LPS results in an overall significantly greater immune response as well as a greater immune response against non-capsule and O-antigen polysaccharides compared to nasal immunization with formalin-killed ExPEC strain CP9(w.t.)
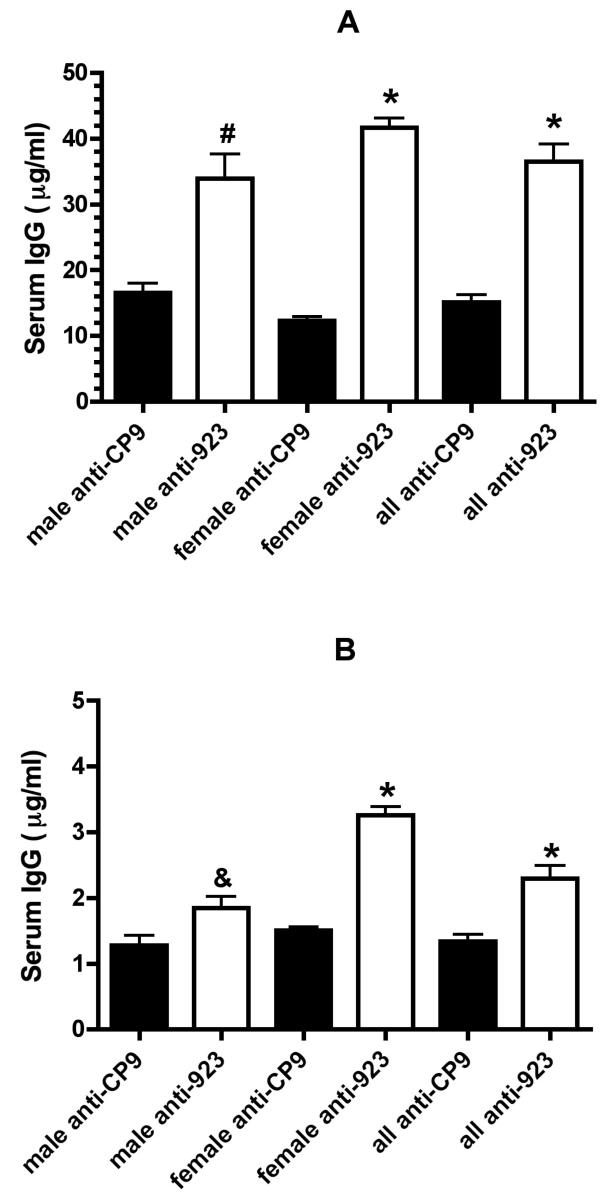
To compare the humoral immune response after nasal immunization with formalin-killed CP9 (wt) and CP923 (capsule and O-antigen minus), male and female C57 black6 mice were nasally immunized with each strain or received only buffer (non-immunized controls). First, a quantitative assessment was performed using pooled sera from 5 female and 10 male mice immunized with formalin-killed CP9 and 9 female and 10 male mice immunized with formalin-killed CP923 (Figure 2). Data from our laboratory have demonstrated that capsule and the O-antigen impede antibody binding (Russo, TA unpublished data). Therefore, assessing the CP9-specific-IgG response by ELISA against CP9 alone might underestimate the amount of non-surface polysaccharide antibodies present. To control for this CP9 and CP923-specific antisera were tested against both CP9 and CP923. Surprisingly these data demonstrated that immunization with formalin-killed CP923 compared to formalin-killed CP9 resulted in a significantly higher humoral immune response when both CP923 (36.5 ± 2.7 versus 15.1 ± 1.1 μg/ml IgG respectively; P < 0.0001; unpaired t-test) and CP9 (2.3 ± 0.2 versus 1.3 ± 0.1 μg/ml IgG respectively; P < 0.0001; unpaired t-test) were used as antigens. These data support the concept that capsule and/or O-antigen impede an optimal humoral immune response. Further, when CP923 is used as an antigen, antibodies directed against epitopes other than the capsular and O antigen epitopes are detected. This demonstrates that immunization with CP923 results in the generation of significantly greater amount of IgG antibodies directed against non-capsular and O-antigen epitopes. This antibody response is desirable for protection against heterologous strains.
Figure Legend 2.
The serum IgG antibody response after nasal immunization of mice with formalin-killed CP9 (w.t.) and CP923 (capsule and O-antigen minus). C57 black6 mice were nasally immunized (2 week intervals × 3) with 1 × 109 cfu formalin-killed CP9 or 1 × 109 formalin-killed CP923. A quantitative assessment of serum IgG (by ELISA, as described in Methods) was performed using pooled post-immunization sera from 5 female and 10 male mice immunized with formalin-killed CP9 and 9 female and 10 male C57 mice immunized with formalin-killed CP923. In panels A and B the ELISA plates were coated with 1 × 107 cfu of CP923 and CP9 respectively. In all instances, a significantly greater amount of CP923-specific IgG was present after CP923 was used as the immunogen compared to CP9-specific IgG when CP9 was used. (* P < 0.0001, # P = 0.0003, & P = 0.02; unpaired t-test).
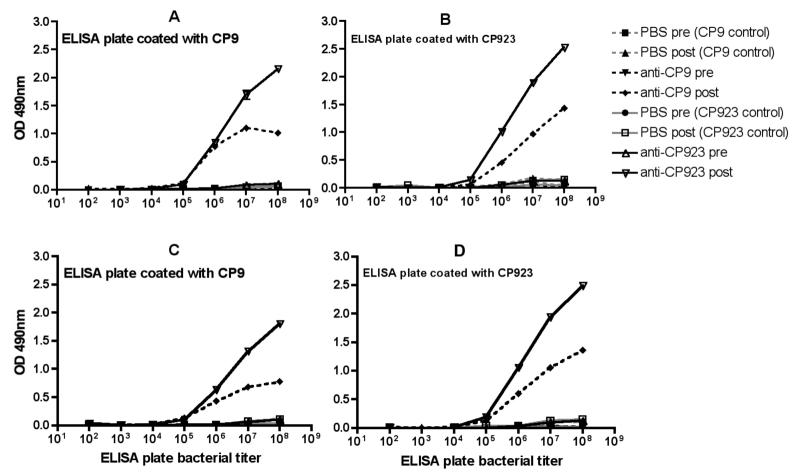
Next, to control for possible differences in antibody binding by CP9 and CP923, 1:100 and 1:1000 dilutions of CP9 and CP923-specific antisera were measured against a range of bacterial titers (102-108). The serum IgG pre and post-immunization response was semi-quantitatively measured by ELISA. As can be seen in Figure 3 (1:1000 dilution), a greater amount of antibody was generated after immunization with formalin-killed CP923 compared to formalin-killed CP9. A similar immune response was observed in males (panels A, B) and females (panels C, D). Antibody responses were similar at a 1:100 dilution (data not shown). These data further substantiate that nasal immunization with the formalin-killed ExPEC strain CP923 (capsule and O-antigen-minus) results in a greater serum IgG response compared to immunization with its isogenic wild-type parent CP9, which supports the concept that capsule and the O-antigen moiety of LPS impede the development of an optimal immune response.
Figure Legends 3.
The serum IgG antibody response after nasal immunization of mice with formalin-killed CP9 (w.t.) and CP923 (capsule and O-antigen minus). After pre-immunization serum was obtained, C57 black6 mice were nasally immunized (2 week intervals × 3) with 1 × 109 cfu formalin-killed CP9, 1 × 109 formalin-killed CP923, or received 1X PBS. IgG concentrations were measured against varying concentrations (102-108) of both CP9 and CP923 in pre and post-immunization sera by ELISA, as described in Methods. 1:1000 dilutions of CP9 and CP923-specific anti-sera were used. Panels A and B depict the response in male mice and panels C and D depict the response in female mice. The serum IgG response was greater in mice immunized with formalin-killed CP923 compared to formalin-killed CP9.
A similar amount of antibodies that recognize surface epitopes on CP923 (capsule & O-antigen-minus) is generated after immunization with live and formalin-killed CP923
Formalin was chosen as a method to kill the ExPEC strains because it is expected to maintain the bacterium's surface architecture. To test this, male and female C57 black6 mice were nasally immunized with either live or formalin-killed CP923. The resultant sera were assessed for the amount of antibody each contained that bound to the surface of live CP923 using a flow cytometry-based methodology. Results were similar from male and female mice and therefore were pooled.
First, however, ELISA assays were performed on the mouse sera generated to establish that the concentration of CP923-specific-IgG was similar when live and formalin-killed CP923 were used for immunization. The mean post-immunization sera concentration of CP923-specific-IgG was similar when either live or formalin-killed CP923 was used for immunization (22859 ng/ml ± 2208 S.E.M. versus 24745 ± 2167 S.E.M. respectively, P > 0.1, unpaired t-test).
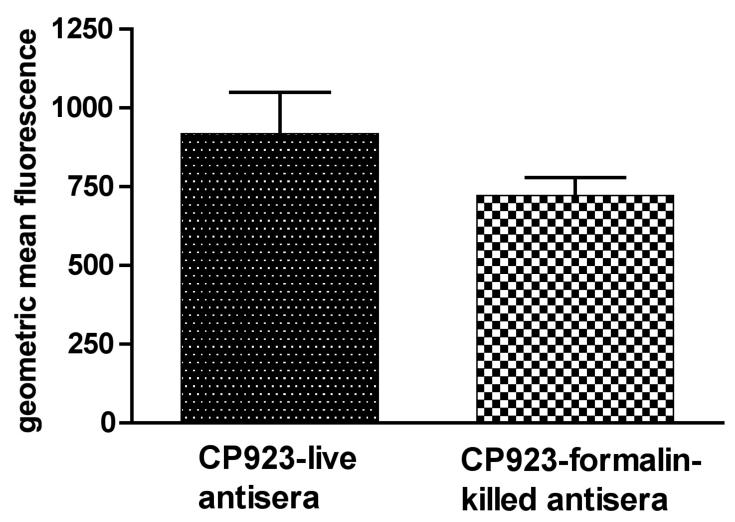
Next, these antisera were used to assess binding of IgG/IgM to the surface of live bacteria (Figure 4). Antisera generated by immunization with either live or formalin–killed CP923 were incubated with live CP923 and antibody binding was analyzed by flow cytometry. Ten-thousand bacterial events were gated and antibody bound bacteria events were assessed in the FL-1 channel by measuring the geometric mean fluorescence (GMF). The GMF was similar when mouse antiserum generated from nasal immunization with either live CP923 (918 ± 130 S.E.M.) or formalin-killed CP923 (721 ± 58 S.E.M.) was used (P > 0.1), unpaired t-test). These data demonstrate that when ExPEC are used for nasal immunization, live and formalin-killed bacteria generate antibodies that bind comparably to the surface of the live homologous strain, which supports the contention that formalin-killed ExPEC maintain their surface architecture.
Figure Legend 4.
Binding of antibodies generated by immunization with either live or formalin-killed CP923 to CP923 (capsule and O-antigen minus). C57 black6 mice were nasally immunized (2 week intervals × 3) with either 1 × 109 cfu of live CP923 or 1 × 109 of formalin-killed CP923. Binding of these antisera to live CP923 (homologous strain) was measured by flow cytometry as described in Methods and was expressed as geometric mean fluorescence. The binding of antibodies generated by immunization with either live or formalin-killed CP923 was similar (P > 0.1).
CP923-specific antiserum generated by immunization with formalin-killed CP923 binds to some but not all ExPEC isolates
To test whether CP923-specific antiserum generated from immunization with formalin-killed CP923 recognizes non-homologous ExPEC strains, antibody binding was measured via flow cytometry and the phylogenetic relatedness of these strains was assessed by RAPD analysis (Figure 5). Binding of antibodies present in CP923-specific antiserum above non-specific background levels (GMF ≥10) occurred in 13/28 ExPEC isolates. GMF was greatest for strains most closely related to CP9 by RAPD analysis (PM8, IA2, 163, J96, R28, R45, 518), but specific antibody binding also occurred in 6 other strains (167, K1/Y, 173, 470, 195, 169). These data demonstrate that CP923-specific antiserum binds non-homologous ExPEC strains.
Figure Legend 5.
Random amplified polymorphic DNA (RAPD)-based phylogenetic analysis of 29 ExPEC strains and the binding of CP923-specific antiserum to these strains. Genomic profiles, as generated for each isolate using RAPD primer 1254, were used for cluster analysis. Antibody binding was performed on live bacteria via flow cytometry and was reported as the geometric mean fluorescence (GMF). A GMF ≥10 (in bold) was above non-specific background levels and therefore represents specific binding. Antibody binding is listed to the right of each strain. CP9 is the homologous strain. The heterologous strains are: 104, 159, 161, 163, 165, 167, 169, 170, 171, 173, 175, 177, 184, 187, 189, 195, 197 (bacteremic isolates from Buffalo, NY); 470(O25/K5), 743(O6/K2) (bacteremic isolates kindly provided by Alan Cross); J96(O4/K-), R28(O4/K3), 518(O4/K10, K54/96) (3 strains previously established to be highly related to CP9 (O4 J96-like strains)(17); R45(O4/K12, V31(O4/K12), PM8(O4/K12)(3 strains previously established to be less related to CP9 (other O4 strains)(17); and K1/Y(O7/K1)(55), IA2 (O4/K12)(15), CFT073(O6/K2)(56)(3 strains that have served as model pathogens for investigations on ExPEC pathogenesis).
Opsonization with CP923-specific mouse antiserum generated from nasal immunization with formalin-killed CP923 enhances neutrophil-mediated bactericidal activity
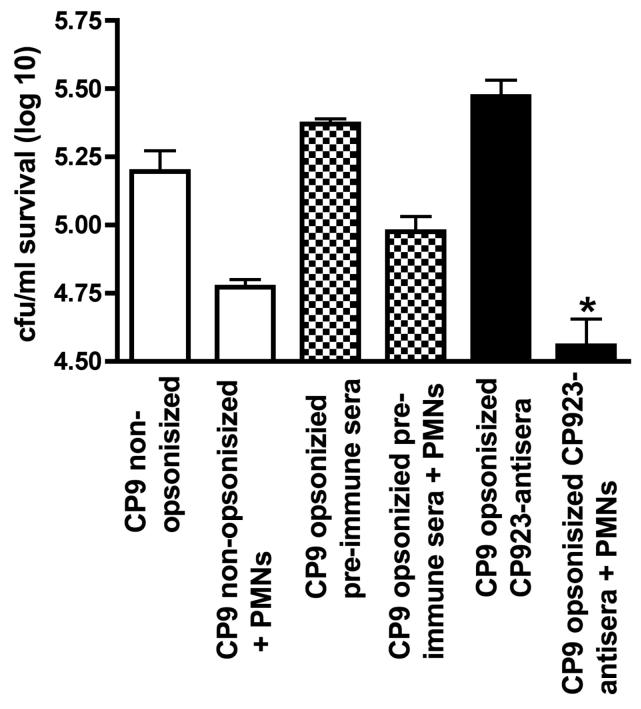
To test if CP923-specific antiserum was biologically active against its homologous wild-type parent, bactericidal assays in the presence and absence of neutrophils were performed against non-opsonized CP9 (w.t.), CP9 opsonized with pre-immune antiserum, and CP9 opsonized with CP923-specific antiserum (Figure 6). Compared to non-opsonized CP9 and CP9 opsonized with pre-immune antiserum, a significant increase in neutrophil-mediated bactericidal activity occurred when CP9 was opsonized with CP923-specific antiserum (P < 0.0006 and P < 0.002 respectively; unpaired t-test). These data demonstrated that CP923-specific antiserum contains biologically active antibodies directed against CP9, which enhance neutrophil-mediated bactericidal activity.
Figure Legend 6.
Bactericidal assay assessing the effects of CP923-specific antiserum on neutrophil-mediated bactericidal activity against the homologous strain CP9 (w.t.). Approximately 1 × 105 cfu of CP9 that were: 1) not pre-opsonized (n=8), 2) preopsonized with a 1:100 dilution of pre-immune serum (Δ56°C) (n =5) or, 3) opsonized with a 1:100 dilution of CP923-specific antiserum (Δ56°C) (n = 12) were added to wells did or did not contain 5 × 105 purified human neutrophils. Bacterial titers were determined at 60 minutes and bactericidal activity was calculated as the difference between bacterial log titers in the presence and absence of neutrophils. * P = 0.002 compared to CP9 pre-opsonized with pre-immune sera; * P = 0.0006 compared to CP9 that was not pre-opsonized.
Neutrophil mediated bactericidal activity against the heterologous E. coli strain K1/Y is significantly greater when CP923-specific antiserum is used for opsonization compared to CP9-specific antiserum
As demonstrated above, a greater amount of antibodies directed against non capsular and O-antigen epitopes were generated after immunization with CP923 compared to CP9 (O4:K54). Therefore, this experiment was designed to test the proof of principle that opsonization of a heterologous, O7:K1 ExPEC strain with CP923-specific antiserum resulted in greater neutrophil-mediated bactericidal activity compared to CP9-specific antiserum. First, bactericidal assays were performed against non-opsonized K1/Y, K1/Y opsonized with pooled CP923-specific antiserum, and K1/Y opsonized with pooled CP9-specific antiserum generated by nasal immunization of mice with formalin-killed strains. However opsonization of K1/Y with either of these antisera did not result in a significant increase in neutrophil-mediated bactericidal activity compared to non-opsonized K1/Y (data not shown).
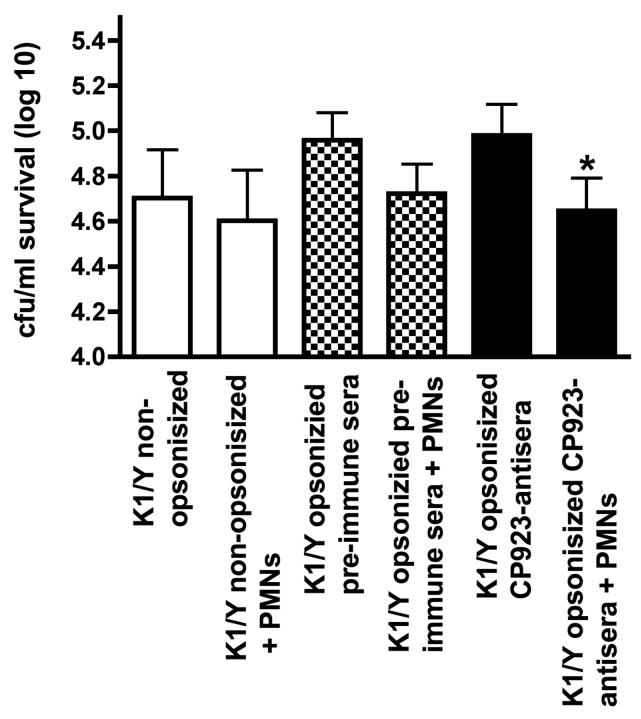
We speculated that the lack of enhancement of neutrophil-mediated bactericidal activity by these antisera may be antibody titer related. Therefore, next we performed the same experiment using higher titer rabbit CP9 and CP923-specific antisera. Similar to what was observed with CP9 and CP923-specific antisera generated from nasal immunization of mice, immunization of rabbits with formalin-killed CP923 compared to formalin-killed CP9 generated antiserum that had significantly greater binding to non-capsule and O-antigen epitopes from the homologous strain CP923 (binding to CP923 was 1838 ± 169 versus 312 ± 28 GMF respectively; P < 0.0009). Compared to antisera generated from nasal immunization of mice, binding of rabbit CP923 and CP9-specific antisera to CP923 is increased 2.7 and 1.6-fold respectively. When bactericidal assays were performed, a significant increase in neutrophil-mediated bactericidal activity occurred when K1/Y was opsonized with CP923-specific antiserum compared to non-opsonized K1/Y and K1/Y opsonized with CP9-specific antiserum (P < 0.0001 and P < 0.0003 respectively)(Figure 7). Taken together these data establish the proof of principle that CP923-specific antiserum significantly enhances neutrophil mediated bactericidal activity compared to CP9 antiserum against a heterologous ExPEC isolate.
Figure Legend 7.
Bactericidal assay comparing the effects of CP9-specific antiserum to CP923-specific antiserum on neutrophil-mediated bactericidal activity against the heterologous strain K1/Y. Approximately 1 × 105 cfu of K1/Y that were: 1) not pre-opsonized (N=9), 2) pre-opsonized with a 1:100 dilution of CP9-specific mouse or rabbit antiserum(Δ56°C)(N=9) or, 3) opsonized with a 1:100 dilution of CP923-specific mouse or rabbit antiserum (Δ56°C)(N=9) were added to wells with and without neutrophils. Bacterial titers were determined at 60 minutes and bactericidal activity was calculated as the difference between bacterial log titers in the presence and absence of neutrophils. * P < 0.0003 compared to K1/Y opsonized with CP9-specific antiserum sera; * P < 0.0001 compared to K1/Y that was not pre-opsonized.
Discussion
In this report we demonstrated that: 1) nasal immunization with the wild-type ExPEC isolate CP9(O4/K54) results in the generation of a significant humoral immune response, 2) nasal immunization with formalin-killed CP9 generated an humoral immune response similar to that achieved with live CP9, 3) immunization with the formalin-killed CP9 derivative deficient in capsule and O-antigen (CP923) results in a significantly greater overall humoral response compared to CP9 (which demonstrates that capsule and the O-antigen moiety of lipopolysaccharide, impede the development of an optimal humoral immune response) and a significantly greater immune response against epitopes other than capsular and O-antigen polysaccharides, 4) similar levels of antibodies that recognize surface epitopes on CP923 (capsule and O-antigen minus) were generated after immunization with live and formalin-killed CP923, 5) CP923-specific antiserum was biologically active, binding to heterologous ExPEC strains and enhancing neutrophil-mediated bactericidal activity, and 6) opsonization of the homologous strain (CP9) with CP923-specific antiserum enhanced neutrophil-mediated bactericidal activity and opsonization of the heterologous, O7:K1 ExPEC strain K1/Y with CP923-specific antiserum resulted in greater neutrophil-mediated bactericidal activity compared to CP9-specific antiserum. Taken together these studies support the concept that formalin-killed ExPEC derivatives deficient in capsule and O-antigen are whole cell vaccine candidates for the development of a polyclonal immune response directed against surface exposed epitopes other than capsular and O-antigen polysaccharides.
Development of a successful vaccine designed to protect against infections due to ExPEC presents several challenges. First, although surface polysaccharides such as capsule have formed the basis for several successful vaccines directed against extracellular pathogens such as Haemophilus influenzae type b, Streptococcus pneumoniae, and certain serotypes of Neisseria meningitidis [46-48], this approach is less practical for ExPEC. More than 80 capsular serotypes exist for E. coli strains [49, 50]. Further, certain K antigens (e.g. K1, K5) are poorly immunogenic, which has been speculated to be due to their structural similarities to host antigens [51]. The O-antigen moiety of LPS is also problematic since >150 variants have been described in E. coli strains [52]. Although only a fraction of these capsular and O-antigen variants are encountered among ExPEC, surface polysaccharides from ExPEC isolates nonetheless exhibit considerable antigenic diversity. These considerations would be predicted to make the development of an ExPEC vaccine based on surface polysaccharides extremely challenging. Given these issues, our laboratory has pursued OMPs as potential vaccine candidates. However OMPs possess their own challenges, which includes difficulties in purification and in maintaining their conformational structure. Further, in ExPEC strains, although there is far less variation in OMPs compared to surface polysaccharides, an OMP-based vaccine will still likely need to be polyvalent. Lastly, given the diverse nature of infections due to ExPEC, a systemic and perhaps a mucosal (e.g. against cystitis) immune response may be required for optimal protection.
The findings in this study suggest an alternative approach in the development of a vaccine designed to protect against ExPEC infections. Immunization with whole organisms has several advantages. First, although not surprising, nasal immunization with live wild-type ExPEC generated a significant systemic antibody response (Figure 1). However, despite ExPEC being part of the normal human intestinal flora [6, 53] and the likelihood that humans ingest these strains on a daily basis [54], the use of live strains for nasal vaccination incurs a small but finite risk of microaspiration and the subsequent development of pneumonia. Further, live strains would not be appropriate for systemic immunization. Therefore, we compared the immune response after nasal immunization with live and formalin-killed wild-type ExPEC and demonstrated the antibody response was similar (Figure 1). Although immunization with killed strains affords certain advantages, the method of killing the bacterium may affect antigen presentation, and conformational epitopes could be lost. To try to maintain the bacterium's surface architecture we used formalin to kill the bacteria prior to immunization. Subsequently, we compared the binding of antibodies to the surface of CP923 (capsule and O-antigen minus) generated after immunization with live and formalin-killed preparations of this strain (Figure 4). The same degree of binding was observed, which supports the contention that that formalin-killed ExPEC maintain their surface architecture.
A surprising but important finding in this study was that an overall greater antibody response was observed after nasal immunization with formalin-killed CP923 (capsule and O-antigen minus) than with the formalin-killed isogenic parent strain CP9 (capsule and O-antigen positive) (Figures 2-3). The mechanism by which capsule and/or O-antigen decrease the antibody response has not been established, but one could hypothesize that capsule and/or O-antigen inhibits the presentation of OMPs, lipidA-core, or other epitopes to B-cells, macrophages, or dendritic cells. Alternatively, the absence of capsule and/or O-antigen may enhance the effect of unidentified OMPs or other components of ExPEC that serve as natural adjuvants [27, 28]. It is tempting to speculate that this effect of capsule and/or O-antigen is one of the reasons why intestinal colonization or natural infection from ExPEC strains does not appear to result in a protective immune response. Less surprising was the finding that a significantly greater antibody response to non-capsular and O-antigen epitopes was observed after nasal immunization with formalin-killed CP923 than with the formalin-killed isogenic parent strain CP9. Establishing this fact is important, since the development of bactericidal antibodies directed against non-capsular and O-antigen epitopes have the potential to protect against heterologous strains.
Antibodies in CP923-specific antiserum bound to some, but not all, heterologous ExPEC strains. Perhaps not surprisingly but importantly, binding was greatest to ExPEC strains most closely related to w.t. parent strain CP9, as defined by RAPD analysis (Figure 5). These data suggest that a single formalin-killed ExPEC strain does not possess the antigenic diversity needed to develop an immune response sufficient to protect against 90-100% of ExPEC isolates. A polyvalent vaccine will undoubtedly be necessary. However, these data also suggest a logical strategy to identify and test candidate strains as potential components of a polyvalent vaccine. A molecular epidemiologic approach, such as one using RAPD analysis, has the potential to identify prototypic strains from genomic relatedness groups. The exact number of such prototypic strains needed to comprise a polyvalent vaccine that would protect against 90-100% of ExPEC isolates remains to be defined. However, such an approach is a reasonable strategy for developing an efficacious vaccine against an antigenically diverse group of pathogens such as ExPEC.
Finally we demonstrated that opsonization with CP923-specific antiserum enhanced neutrophil-mediated bactericidal activity against its homologous parent CP9 and the heterologous ExPEC strain K1/Y (Figures 6-7). These data established the proof of principle that opsonization of a heterologous ExPEC strain with CP923-specific antiserum resulted in greater neutrophil-mediated bactericidal activity compared to CP9-specific antiserum. This supports the concept of using formalin-killed, capsule and O-antigen-minus ExPEC derivatives as potential vaccine candidates. Although opsonization of the heterologous strain K1/Y with CP923-specific antiserum generated from nasal immunization of mice did not increase neutrophil-mediated bactericidal activity, bactericidal activity increased when higher titer CP923-specific antiserum generated from immunization of a rabbit was used (Figure 7). Therefore a nasal immunization strategy that results in higher antibody titers (e.g. adjuvants) will be critical for optimal vaccine design and development.
In future studies we envision the potential for additional improvement of the humoral immune response. First, since the genetics of capsule and O-antigen production in ExPEC are well-described [55], construction of additional capsule and O-antigen deficient derivatives should be straightforward. Our data support that a molecular epidemiologic approach, such as RAPD analysis, has the potential to identify prototypic strains from relatedness groups for use in a polyvalent vaccine containing multiple formalin-killed capsule and O-antigen deficient constructs. The goal in vaccine design and development will be for such a vaccine to generate a polyclonal antibody response against surface exposed epitopes in 90-100% of ExPEC isolates. Second, we hypothesize that other ExPEC factors may adversely modulate the host's acquired immune response or that a dominant antigen may narrow the immune response. If/when identified, disruption of these genes may further enhance or improve the host's antibody response. Third, adjuvants have the potential to enhance the immune response. Fourth, although lipidA toxicity should be less of an issue with mucosal immunization, if necessary, constructs can be generated in which the bioactive properties of lipidA have been minimized [56]. Lastly, if certain epitopes/proteins are shown in future studies to be critical in conferring protection against ExPEC infection, they can be over-expressed in the genetically engineered background that results in the optimal antibody response.
In summary, we have established that with the use of nasal immunization, in our model system the best humoral immune response obtained to date is with formalin-killed CP923 (capsule and O-antigen minus). Although only the systemic antibody response was assessed in this study, immunization via the nasal route has the potential for inducing a mucosal antibody response as well [57]. An additional advantage of using a formalin killed capsule/O-antigen deficient derivative is that the antibody response will not be directed against surface polysaccharide epitopes, but presumably OMPs, other surface exposed protein, and perhaps the lipidA-core moieties of LPS. This is desirable since, as described above, we believe that capsule and O-antigen are not practical or optimal vaccine candidates for protection against infection due to ExPEC. Studies are presently underway to define the degree of protection observed after immunization with CP923 in a variety of infection models.
Acknowledgements
Financial support: This material is based upon work supported by Office of Research and Development, Medical Research Service, Department of Veterans Affairs (TAR and JRJ); National Institutes of Health grant HL69763 (TAR); and The John R. Oishei Foundation (TAR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McBean M, Rajamani S. Increasing rates of hospitalization due to septicemia in the US elderly population, 1986-1997. J Infect Dis. 2001;183:596–603. doi: 10.1086/318526. [DOI] [PubMed] [Google Scholar]
- 2.Gransden W, Eykyn S, Phillips I, Rowe B. Bacteremia due to Escherichia coli: A study of 861 episodes. Rev Infect Dis. 1990;12:1008–18. doi: 10.1093/clinids/12.6.1008. [DOI] [PubMed] [Google Scholar]
- 3.Roberts F, Geere I, Coldman A. A three-year study of positive blood cultures, with emphasis on prognosis. Rev Infect Dis. 1991;13:34–46. doi: 10.1093/clinids/13.1.34. [DOI] [PubMed] [Google Scholar]
- 4.Vastag B. New vaccine decreases rate of nosocomial infections. JAMA. 2001;285:1565. [PubMed] [Google Scholar]
- 5.Siegman-Igra Y, Fourer B, Orni-Wasserlauf R, Golan Y, Noy A, Schwartz D, et al. Reappraisal of comminity-acquired bacteremia: A proposal of a new classification for the spectrum of acquisition of bacteremia. Clin Infect Dis. 2002;34:1431–39. doi: 10.1086/339809. [DOI] [PubMed] [Google Scholar]
- 6.Russo T, Johnson J. Medical and Economic Impact of Extraintestinal Infections Due to Escherichia coli: An Overlooked Epidemic. Microbes and Infect. 2003;5:449–56. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 7.Minino A, Smith B. Deaths: Preliminary data for 2000. National Vital Statistics Reports. 2001;49:1–40. [PubMed] [Google Scholar]
- 8.Fluit A, Schmitz F, Verhoef J, Group. ESP Frequency of isolation of pathogens from bloodstream, nosocomial pneumonia, skin and soft tissue, and urinary tract infections occurring in European patients. Eur J Clin Microbiol Infect Dis. 2001;20:188–91. doi: 10.1007/s100960100455. [DOI] [PubMed] [Google Scholar]
- 9.Bernard G, Vincent J, Laterre P, LaRosa S, Dhainaut J, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 10.Gupta K, Scholes D, Stamm W. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736–38. doi: 10.1001/jama.281.8.736. [DOI] [PubMed] [Google Scholar]
- 11.Gupta K, Hooton T, Stamm W. Increasing antimicobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135:41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 12.Talan D, Stamm W, Hooton T, Moran G, Burke T, Iravani A, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA. 2000;283:1583–90. doi: 10.1001/jama.283.12.1583. [DOI] [PubMed] [Google Scholar]
- 13.Angus D, Linde-Zwirble W, Lidlicker J, Clermont G, Carcillo J, Pinsky M. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Ahned R, Lanier J, Pamer E. Immunological memory and infection. In: Kaufmann S, Sher A, Ahmed R, editors. Immunology of Infectious Diseases. ASM Press; Washington D.C.: 2002. pp. 175–89. [Google Scholar]
- 15.Russo T, Stapleton A, Wenderoth S, Hooton T, Stamm W. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infection. J Infect Dis. 1995;172:440–45. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 16.Kaijser B, Ahlstedt S. Protective capacity of antibodies against Escherichia coli O and K antigens. Infect Immun. 1977;17:286–89. doi: 10.1128/iai.17.2.286-289.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salles M, Mandine E, Zalisz R, Guenounou M, Smets P. Protective effects of murine monoclonal antibodies in experimental septicemia: E. coli antibodies protect against different serotypes of E. coli. J Infect Dis. 1989;159:641–47. doi: 10.1093/infdis/159.4.641. [DOI] [PubMed] [Google Scholar]
- 18.Bolin C, Jensen A. Passive immunization with antibodies against iron-regulated outer membrane proteins protects turkeys from Escherichia coli septicemia. Infect Immun. 1987;55:1239–42. doi: 10.1128/iai.55.5.1239-1242.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaijser B, Larsson P, Olling S. Protection against ascending Escherichia coli pyelonephritis in rats and significance of local immunity. Infect Immun. 1978;20:78–81. doi: 10.1128/iai.20.1.78-81.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaijser B, Larsson P, Nimmich W, Soderstrom T. Antibodies to Escherichia coli K and O antigens in protection against acute pyelonephritis. Prog Allergy. 1983;33:275–88. doi: 10.1159/000318336. [DOI] [PubMed] [Google Scholar]
- 21.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J, Burlein J, et al. Prevention of mucosal Escherichia coli infection by Fim-H-adhesin-based systemic vaccination. Science. 1997;276:607–11. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 22.Langermann S, Mollby R, Burlein J, Palaszynski S, Auguste C, De Fusco A, et al. Vaccination with FimH adhesin protects cynomologus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000;181:774–78. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 23.O'Hanley P, Lalonde G, Ji G. Alpha-hemolysin contributes to the pathogenicity of piliated digalactoside-binding Escherichia coli in the kidney: efficacy of an alpha-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect Immun. 1991;59:1153–61. doi: 10.1128/iai.59.3.1153-1161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pecha B, Low D, O'Hanley P. Gal-Gal pili vaccines prevent pyelonephritis by piliated Escherichia coli in a murine model: single component Gal-Gal pili vaccines prevent pyelonephritis by homologous and heterologous piliated E.coli strains. J Clin Invest. 1989;83:2102–08. doi: 10.1172/JCI114123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo TA, McFadden CD. Carlino-MacDonald UB, Beanan JM, Olson R, Wilding GE. The Siderophore receptor IroN of extraintestinal pathogenic Escherichia coli is a potential vaccine candidate. Infect Immun. 2003;71(12):7164–9. doi: 10.1128/IAI.71.12.7164-7169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer HW, Alloussi S, Egger G, Blumlein HM, Cozma G, Schulman CC. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur Urol. 2005;47(4):542–8. doi: 10.1016/j.eururo.2004.12.009. discussion 48. Epub 2005 Jan 21. [DOI] [PubMed] [Google Scholar]
- 27.Jeannin P, Magistrelli G, Goetsch L, Haeuw J, Thieblemont N, Bonnefoy J, et al. Outer membrane protein A (OmpA): a new pathogen-associated molecular pattern that interacts with antigen presenting cells-impact on vaccine strategies. Vaccine. 2002;20:A23–A27. doi: 10.1016/s0264-410x(02)00383-3. [DOI] [PubMed] [Google Scholar]
- 28.Jeannin P, Magistrelli G, Herbault N, Goetsch L, Godefroy S, Charbonnier P, et al. Outer membrane protein A renders dendritic cells and macrophages responsive to CCL21 and triggers dendritic cell migration to secondary lymphoid organs. Eur J Immunol. 2003;33:326–33. doi: 10.1002/immu.200310006. [DOI] [PubMed] [Google Scholar]
- 29.Russo T, Guenther J, Wenderoth S, Frank M. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Molec Microbiol. 1993;9:357–64. doi: 10.1111/j.1365-2958.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 30.Johnson J, Stapleton A, Russo T, Scheutz F, Brown J, Maslow J. Characteristics and prevalence within serogroup O4 of a J96-like clonal group of uropathogenic Escherichia coli O4:H5 containing the Class I and Class III alleles of papG. Infect Immun. 1997;65:2153–59. doi: 10.1128/iai.65.6.2153-2159.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson J, Delavari P, Kuskowski M, Stell A. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J Infect Dis. 2001;183:78–88. doi: 10.1086/317656. [DOI] [PubMed] [Google Scholar]
- 32.Russo T, Brown J, Jodush S, Johnson J. The O4 specific antigen moiety of lipopolysaccharide but not the K54 group 2 capsule is important for urovirulence in an extraintestinal isolate of Escherichia coli. Infect Immun. 1996;64:2343–48. doi: 10.1128/iai.64.6.2343-2348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo T, Liang Y, Cross A. The Presence of K54 Capsular Polysaccharide Increases the Pathogenicity of Escherichia coli In Vivo. J Infect Dis. 1994;169:112–18. doi: 10.1093/infdis/169.1.112. [DOI] [PubMed] [Google Scholar]
- 34.Russo TA, Davidson BA, Carlino-MacDonald UB, Helinski JD, Priore RL, Knight PR., 3rd The effects of Escherichia coli capsule, O-antigen, host neutrophils, and complement in a rat model of Gram-negative pneumonia. FEMS Microbiol Lett. 2003;226(2):355–61. doi: 10.1016/S0378-1097(03)00636-0. [DOI] [PubMed] [Google Scholar]
- 35.Russo T, Bartholomew L, Davidson B, Helinski J, Carlino U, Knight P, III, et al. Total extracellular surfactant is increased but abnormal in a rat model of gram-negative bacterial pneumonia. Am J Physiol-Lung Cell Molec Physiol. 2002;283:L655–63. doi: 10.1152/ajplung.00071.2002. [DOI] [PubMed] [Google Scholar]
- 36.Russo T, Sharma G, Brown C, Campagnari A. The loss of the O4 antigen moiety from the lipopolysaccharide of an extraintestinal isolate of Escherichia coli has only minor effects on serum sensitivity and virulence in vivo. Infect Immun. 1995;63:1263–69. doi: 10.1128/iai.63.4.1263-1269.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo T, Wenderoth S, Carlino U, Merrick J, Lesse A. Identification, genomic organization and analysis of the group III capsular polysaccharide genes kpsD, kpsM, kpsT, and kpsE from an extraintestinal isolate of Escherichia coli (CP9, O4/K54/H5) J Bacteriol. 1998;180:338–49. doi: 10.1128/jb.180.2.338-349.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Qu W, Reeves P. Sequence analysis of four Shigella boydii O-antigen loci: Implication for Escherichia coli and Shigella relationships. Infect Immun. 2001;69:6923–30. doi: 10.1128/IAI.69.11.6923-6930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss J, Elsbach P, Shu C, Castillo J, Grinna L, Horwitz A, et al. Human bactericidal/permeability-increasing protein and a recombinant NH2-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria. J Clin Invest. 1992;90(3):1122–30. doi: 10.1172/JCI115930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johanson I, Lindstedt R, Svanborg C. Roles of the pap- and prs-encoded adhesins in Escherichia coli adherence to human uroepithelial cells. Infect Immun. 1992;60(8):3416–22. doi: 10.1128/iai.60.8.3416-3422.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welch RA, Burland V, Plunkett G, 3rd, Redford P, Roesch P, Rasko D, et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 2002;99(26):17020–4. doi: 10.1073/pnas.252529799. Epub 2002 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg D, Akopyants N, Kersulyte D. Fingerprinting microbial genomes using the RAPD or AP-PCR method. Methods Mol Cell Biol. 1994;5:13–24. [Google Scholar]
- 43.Johnson JR, Manges AR, O'Bryan TT, Riley LW. A disseminated multidrug-resistant clonal group of uropathogenic Escherichia coli in pyelonephritis. Lancet. 2002;359(9325):2249–51. doi: 10.1016/S0140-6736(02)09264-4. [DOI] [PubMed] [Google Scholar]
- 44.Mayer-Scholl A, Hurwitz R, Brinkmann V, Schmid M, Jungblut P, Weinrauch Y, et al. Human Neutrophils Kill Bacillus anthracis. PLoS Pathog. 2005;1(3):e23. doi: 10.1371/journal.ppat.0010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo TA, Davidson BA, Genagon SA, Warholic NM, Macdonald U, Pawlicki PD, et al. E. coli virulence factor hemolysin induces neutrophil apoptosis and necrosis/lysis in vitro and necrosis/lysis and lung injury in a rat pneumonia model. Am J Physiol Lung Cell Mol Physiol. 2005;289(2):L207–16. doi: 10.1152/ajplung.00482.2004. Epub 2005 Apr 1. [DOI] [PubMed] [Google Scholar]
- 46.Smit P, Oberholzer D, Hayden-Smith S, Koornhof HJ, Hilleman MR. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA. 1977;238(24):2613–6. [PubMed] [Google Scholar]
- 47.Lepow ML, Beeler J, Randolph M, Samuelson JS, Hankins WA. Reactogenicity and immunogenicity of a quadrivalent combined meningococcal polysaccharide vaccine in children. J Infect Dis. 1986;154(6):1033–6. doi: 10.1093/infdis/154.6.1033. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. Recommendation of the Immunization Practices Advisory Committee (ACIP) Haemophilus b conjugate vaccines for prevention of Haemophilus influenzae type b disease among infants and children two months of age and older. MMWR Morb Mortal Wkly Rep. 1991;40:1–17. [Google Scholar]
- 49.Jann B, Jann K. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol and Immunol. 1990;150:19–42. doi: 10.1007/978-3-642-74694-9_2. [DOI] [PubMed] [Google Scholar]
- 50.Orskov F, Orskov I, Jann B, Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977;41:667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts IS. Bacterial polysaccharides in sickness and in health. The 1995 Fleming Lecture. Microbiology. 1995;141(Pt 9):2023–31. doi: 10.1099/13500872-141-9-2023. [DOI] [PubMed] [Google Scholar]
- 52.Whitfield C, Valvano M. Biosynthesis and expression of cell-surface polysaccharides in Gram-negative bacteria. Advances Micro Physiol. 1993;35:135–246. doi: 10.1016/s0065-2911(08)60099-5. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JR, Russo TA. Extraintestinal pathogenic Escherichia coli: “the other bad E. coli”. J Lab Clin Med. 2002;139(3):155–62. doi: 10.1067/mlc.2002.121550. [DOI] [PubMed] [Google Scholar]
- 54.Johnson JR, Kuskowski MA, Smith K, O'Bryan TT, Tatini S. Antimicrobial-resistant and extraintestinal pathogenic Escherichia coli in retail foods. J Infect Dis. 2005;191(7):1040–9. doi: 10.1086/428451. Epub 2005 Mar 1. [DOI] [PubMed] [Google Scholar]
- 55.Russo T. Capsule and Lipopolysaccharide. In: Donnenberg M, editor. Escherichia coli: Virulence Mechanisms of a Versatile Pathogen. Academic Press; London, UK: 2002. pp. 379–403. [Google Scholar]
- 56.Somerville JJ, Cassiano L, Bainbridge B, Cunningham M, Darveau R. A novel Escherichia coli lipid A mutant that produces an antiinflammaory lipopolysaccharide. J Clin Invest. 1996;97:359–65. doi: 10.1172/JCI118423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russell M, Mestecky J. Humoral immune response to microbial infections in the genital tract. Microbes and Infection. 2002;4:667–77. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]