Abstract
The neurophysiological basis of therapeutic acupuncture is not well understood but is likely to consist of both specific and non-specific (e.g. placebo) effects. Data from animal studies suggest that endogenous anti-nociceptive networks may play a large role in therapeutic acupuncture. These networks have also been demonstrated to support placebo analgesia making differentiation between acupuncture specific and non-specific networks challenging. However, modern neuroimaging techniques such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET), electroencephalography (EEG) and magnetoencephalography (MEG) provide a means to safely monitor brain activity in humans and may be used to help map the neural correlates of acupuncture. Recent neuroimaging studies have explored brain activity during acupuncture stimulation and/or the analgesic effects of acupuncture on pain stimulus processing. Although controversy regarding appropriate control methodology (e.g. sham acupuncture) continues, data suggest that modulation of certain limbic brain networks may differentiate between specific and placebo components of acupuncture.
Keywords: acupuncture, placebo, pain, alternative medicine, fMRI, PET, MEG, EEG, neuroimaging, amygdala, hypothalamus, somatosensory, limbic, cingulate, brainstem, frontal cortex, complementary, opioidergic, monaminergic
Introduction
Acupuncture is an ancient Chinese healing modality with putative therapeutic effects for clinical pain management. However, it is often dismissed by mainstream allopathic medicine due to a paucity of data demonstrating its neurophysiological differentiation from placebo. Functional neuroimaging provides a means to determine which brain networks support acupuncture as well as map the differences between its specific and non-specific neural correlates. It is important to remember that although placebo effects can occur with all forms of medical treatment their neurophysiological basis may differ with the type of treatment being given (Colloca and Benedetti 2005). In general, placebo effects are believed to arise from unconscious conditioning (Voudouris et al. 1990; Wickramasekera 1999), changes in (verbal) expectancy (Montgomery and Kirsch 1997), and/or differences in practitioner suggestion and patient suggestibility (De Pascalis et al. 2002; Wickramasekera 1999). However, these effects likely overlap and there is strong evidence that expectancy is the major contributor to increasing treatment efficacy (Montgomery and Kirsch 1997). Thus, acupuncture specific brain activity must be differentiated from non-specific activity supporting subject expectations for treatment.
Therapeutic Acupuncture is at Least Partially Mediated by Endogenous Anti-nociceptive Brain Networks
Neuroimaging data demonstrate that acupuncture recruits a distributed cortical and subcortical brain network. Although some evidence exists for specificity of brain response to acupoint vs. non-acupoint treatment (Wu et al. 2002) as well as Traditional Chinese Medicine (TCM) applications (Cho et al. 1998; Li et al. 2003), clear acupoint specificity has been difficult to replicate (Gareus et al. 2002) and many points may elicit overlapping responses within multiple brain areas. Indeed, it is highly likely that acupuncture elicits a common, widely distributed network of brain regions and that the neurophysiological effects related to acupoint specificity are subtle as well as subject and/or state dependant.
Data from animal research provide strong support for the hypothesis that therapeutic acupuncture is mediated, at least partially, by opioidergic and/or monoaminergic neurotransmission involving the brainstem, thalamus, and/or hypothalamic as well as pituitary action (Pomeranz and Chiu 1976; Stux and Hammerschlag 2001; Zhou et al. 1981). Afferent spinal gating and, in the case of painful needling, stress induced analgesia and diffuse noxious inhibitory control (DNIC), may support short-term analgesic effects (Carlsson 2002). Human neuroimaging data demonstrate that acupuncture stimulation modulates a wide network of brain regions including the primary somatosensory (SI), secondary somatosensory (SII), anterior cingulate (ACC), prefrontal (PFC), and insular cortices, amygdala, hippocampus, hypothalamus, periaquaductal gray (PAG), rostral ventral medulla (RVM) and cerebellar vermis (Hui et al. 2000; Hui et al. 2005; Liu et al. 2004; Napadow et al. 2005; Wu et al. 1999; Yoo et al. 2004; Zhang et al. 2003). Furthermore, many investigators have noted fMRI deactivation in limbic regions when contrasting acupuncture needle stimulation with non-stimulation baseline (Figure 1), attributing this phenomenon to decreased neuronal activity (Hui et al. 2000; Napadow et al. 2005; Wu et al. 1999; Zhang et al. 2003). Whether these modulations result from opioidergic and/or monoaminergic anti-nociceptive neurotransmission is unclear. However, other imaging studies have not found limbic deactivations (Yoo et al. 2004) and it is possible that the lack of consensus arises from variability in needling technique, data processing methods, or perhaps most importantly, the type of ‘deqi1’ sensations elicited (Hui et al. 2005). Specifically, brain response may differ when deep needle stimulation evokes dull, aching sensations as opposed to sharp pain (Hui et al. 2005).
Figure 1. fMRI Evaluation of Acupuncture Stimulation and its Effects on Somatosensory processing: A) Acupuncture induced fMRI signal decreases in the amygdala within healthy adults.
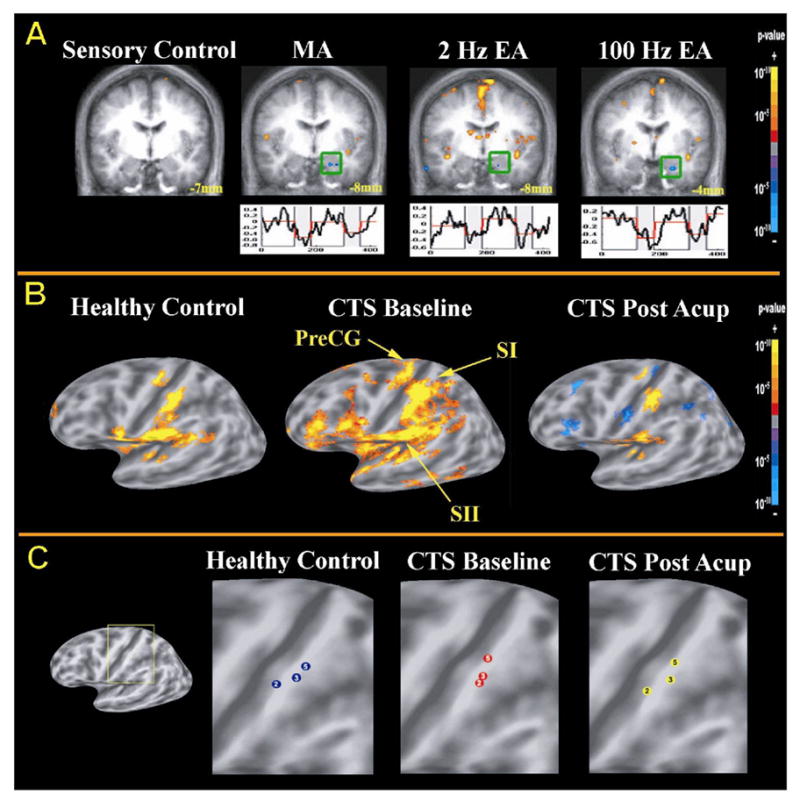
Both manual (MA) and electro-acupuncture (EA) but not tactile control stimulation at ST-36 induces fMRI signal decrease in the amygdala. (adapted from Napadow et al. 2005). B) fMRI of somatosensory processing in Carpal Tunnel Syndrome (CTS) patients before and after therapeutic acupuncture: In CTS, the brain demonstrates sensorimotor hyperactivation to innocuous stimulation of the 3rd finger (median nerve innervated) of the affected hand.. After a 5-week course of acupuncture treatment, CTS patients demonstrated less hyperactivation, and more focused SI finger representation (adapted from Napadow et al. 2006b). C) Effects of acupuncture treatment on somatotopy in CTS patients: Compared to healthy controls (HC), CTS patients demonstrated less separation of somatotopic representations for the 2nd and 3rd fingers (both median nerve innervated). After acupuncture treatment, the 2nd and 3rd finger representations were more separated, approximating normal somatotopy in HC (adapted from Napadow et al. 2006b).
While many of these studies have mapped brain response to acupuncture stimulation, other studies have explored how brain response to a pain stimulus is altered by acupuncture. For example, both verum and sham acupuncture have been found to reduce fMRI pain responses in the thalamus and insula of fibromyalgia patients; PET data using carfentanil in this same population also supports μ-opioid receptor involvement in acupuncture and/or sham analgesia (Harris et al. 2005). Other studies demonstrate similar fMRI activity reductions in pain response within the sensory thalamus, ACC and premotor cortex after acupuncture stimulation at either real or sham (non-classical) acupoints (Cho et al. 2002). Furthermore, EEG studies have found that acupuncture modulates painful somatosensory evoked potential amplitude at both short and long latencies following stimulation, suggesting that acupuncture may have both humorally and neurally mediated effects on pain (Xu et al. 1993). Collectively, there is an abundance of neuroimaging data showing that acupuncture modulates brain responses in distributed cortical, limbic and brainstem centers. Many of these areas including SI, SII, ACC, PFC, insula, thalamus, hypothalamus, amygdala, and hippocampus, support both sensory and affective pain perception and are also implicated in endogenous anti-nociceptive signaling (Zubieta et al. 2006). Thus, questions remain as to which brain responses support acupuncture specific vs. non-specific effects.
Neuroimaging Placebo Effects in the Brain
Early work with naloxone suggests that placebo analgesia is partially mediated by opioidergic (Levine et al. 1978) limbic and brainstem networks (Hoffman et al. 2005) which may be activated during sustained pain and modulated by sensory and affective dimensions of pain perception (Zubieta et al. 2005; Zubieta et al. 2006). Recently, Wager et al. used fMRI to explore how the cortical and subcortical pain neuromatrix is modulated by expectancy (i.e. placebo disguised as viable pain treatment) (Wager et al. 2004). Decreased pain rating during covert placebo was accompanied by decreased brain activity in the insula, ACC and thalamus. Placebo effects following conditioning with surreptitious variation of heat pain also resulted in decreased activity within the pain neuromatrix. Furthermore, the anticipation of pain was associated with increased activity in the prefrontal cortex. The authors hypothesized that placebo analgesia may arise from changes in the expectation of pain within higher cognitive centers such as the prefrontal cortex and ACC. A similar study with PET used noxious thermal stimuli with covert placebo (saline I.V.) and active treatment I.V. (opioid receptor agonist remifentanil) (Petrovic et al. 2002). In both conditions, analgesia was accompanied by changes in rostral ACC activity that correlated with activity in the brainstem PAG and pons. Increased activity was also noted in orbitofrontal regions. The authors suggested that the prefrontal cortex and ACC may support top down regulation of pain. FMRI data also suggest that placebo induced rostral ACC activity is correlated with response within bilateral amygdala as well as the PAG (Bingel et al. 2006). However, differences in ACC response during placebo analgesia, for example decreased fMRI activity (Wager et al.) versus increased rCBF (Petrovic et al.), are difficult to explain but may be due to differences in task conditions and/or simply imaging modality.
In short, neuroimaging data demonstrate that placebo analgesia recruits subcortical and cortical opioid sensitive brain regions including the PAG, rostral ACC, thalamus, insula, amygdala, and in some studies PFC. Many of these areas overlap with those modulated by acupuncture. Thus, to better understand how acupuncture specific effects differ from placebo it may be necessary to study expectancy in the context of verum (real) and sham acupuncture.
Dissociating Acupuncture Specific Effects from Placebo Effects in the Brain
Several neuroimaging studies of acupuncture employing expectancy manipulations have utilized sham needles and surreptitious manipulation of pain stimuli in attempts to dissociate specific and non-specific effects of acupuncture stimulation. Sham needles, such as the Streitberger needle, employ a blunt tip which recedes into a hollow shaft when pressed against the skin thus, simulating penetration (Streitberger and Kleinhenz 1998). Streitberger needles may be important in clinical trials where the acupuncture intervention can be observed but less important in fMRI/PET studies during which subjects are typically unable to see the intervention performed. Thus, without explicitly manipulating expectancy, several studies have used non-magnetic von Frey monofilaments (a similarly blunt tipped probe) for somatosensory control stimulation and found more extensive modulation of limbic and paralimbic regions for verum compared to control stimulation (Hui et al. 2000; Hui et al. 2005; Napadow et al. 2005; Yoo et al. 2004).
One recent study explored brain processing during verum, covert sham and overt sham needling at acupoint LI-4 in pain patients using PET (Pariente et al. 2005). Subjects were reportedly unable to distinguish between verum and covert sham interventions. However, verum acupuncture induced greater increases in brain response within ipsilateral insular cortex than covert sham. Verum acupuncture and covert sham differed from overt sham in dorsolateral PFC, rostral ACC, and the midbrain activation. The authors hypothesized that activity within the insular cortex may support acupuncture specific effects while modulation of the DLPFC, rACC and midbrain (PAG) may be related to expectancy. However, there were no analgesic effects of any of these treatments on experimental or ongoing pain which leaves open the question of whether the insula activity is related to acupuncture efficacy.
Another recent study evaluated brain processing supporting verum and sham acupuncture stimulation in carpal tunnel syndrome (CTS) patients compared to healthy controls (Napadow et al. 2006a). This study found that, in comparison to healthy controls, CTS patients responded to verum acupuncture with more pronounced signal decrease in the amygdala and signal increase in the hypothalamus. The authors hypothesized that these limbic areas may reduce pain through cooperative modulation of affective/cognitive and autonomic nervous system (ANS) activity. Others have suggested that amygdala deactivation may support altered encoding of affective/cognitive dimensions of pain and foster more effective coping strategies (Petrovic et al. 2004).
In an fMRI study of experimental pain processing in healthy subjects, Kong et al. determined that placebo needle induced analgesia was associated with increased activity during a pain stimulus. These increases occurred within multiple pain processing regions including bilateral rostral ACC, lateral PFC, right anterior insula, supramarginal gyrus and the left inferior parietal lobe (Kong et al. 2006). Furthermore, pain ratings correlated negatively with bilateral PFC, rostral ACC, cerebellum, right fusiform, parahippocampus and pons. These results differ from Wager et al. which showed decreased activity within similar brain regions for placebo. The authors concluded that placebo needling may evoke different types of brain responses than those typically seen in studies utilizing creams/pills. Thus, differences in brain response with comparison to Wager et al. may be due to conditioning. However, the study did not directly image the acupuncture intervention, nor compare sham with a verum treatment.
To further investigate the specific and non-specific effects of acupuncture, it may be most useful to map brain networks supporting both positive and negative expectancy conditions for both verum and placebo treatments. In particular, a finding that verum acupuncture given in the guise of “placebo acupuncture” has significantly greater analgesic effects than placebo/sham needling would lend strong support for the existence of acupuncture specific analgesic effects.
Possible Acupuncture Specific Effects in the Brain
So, do the neural correlates of acupuncture and placebo differ? Neuroimaging studies in humans have validated that acupuncture modulates a widely distributed network of brain regions also involved in pain perception including limbic areas, sensorimotor and prefrontal cortices, brainstem nuclei and the cerebellum. Although these networks demonstrate overlap with those supporting placebo analgesia, differences in modulation of the DLPFC and rACC may support non-specific pain expectancy while amygdala, insula, and hypothalamus modulation may demonstrate some acupuncture specificity (Napadow et al. 2006a; Pariente et al. 2005). However, future studies that image verum and sham acupuncture under positive and negative expectancy conditions may more clearly dissociate between specific and non-specific effects.
It is important to remember that placebo effects, in general, are short lived and that most neuroimaging work described above involves brain response to a single application in healthy subjects. However, the clinically relevant effects of acupuncture analgesia (brain response) may in fact be cumulative over multiple treatments and gradually extend beyond the length of each session (Carlsson 2002; Price et al. 1984). Thus, acupuncture may modulate similar networks as placebo during the treatment session (short-term) but have prolonged effects on specific brain areas following multiple treatments. Indeed, long-term acupuncture treatment in chronic neuropathic pain patients has been found to alter cortical somatosensory processing and produce beneficial changes in somatotopy (Figure 1, Napadow et al. 2006b).
Indeed, more work is needed to understand acupuncture specific effects in the brain. Future studies using PET imaging may help elucidate the relative contributions of opioidergic and monaminergic transmission in therapeutic acupuncture. Studies mapping hypersensitization in chronic pain populations and its modification by successive acupuncture treatments may also prove useful. Importantly, in addition to more studies in chronic pain populations, longitudinal control studies in healthy adults that utilize the same acupuncture treatment regimen are needed. Furthermore, imaging studies should address how activity differs when studies map acupuncture intervention vs. acupuncture’s effects on brain response to a pain stimulus. Additional work evaluating the effects of both positive and negative expectancy manipulations in clinical trials must also be done. Finally, concurrent physiological measurements (e.g. electrocardiography, pupillometry, electro-dermal activity) during neuroimaging sessions may help correlate acupuncture related changes in ANS function to brain activity. Additional neuroimaging research may lead to a better understanding of acupuncture mechanisms and help determine acupuncture’s potential as a treatment for chronic pain.
Acknowledgments
We thank R. Gollub, R. Harris, K.K. Hui, J. Kong for helpful discussion. This research was supported by grants from NCCAM, NIH (K01-AT002166-01, P01-AT002048-02).
Footnotes
Translates as “obtaining qi” and traditionally refers to sensations (e.g. soreness, aching, warmth etc.) that have been used to indicate accurate localization of an acupoint.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Carlsson C. Acupuncture mechanisms for clinically relevant long-term effects--reconsideration and a hypothesis. Acupunct Med. 2002;20:82–99. doi: 10.1136/aim.20.2-3.82. [DOI] [PubMed] [Google Scholar]
- Cho ZH, Chung SC, Jones JP, Park JB, Park HJ, Lee HJ, Wong EK, Min BI. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci U S A. 1998;95:2670–3. doi: 10.1073/pnas.95.5.2670. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cho ZH, Oleson TD, Alimi D, Niemtzow RC. Acupuncture: the search for biologic evidence with functional magnetic resonance imaging and positron emission tomography techniques. J Altern Complement Med. 2002;8:399–401. doi: 10.1089/107555302760253577. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Placebos and painkillers: is mind as real as matter? Nat Rev Neurosci. 2005;6:545–52. doi: 10.1038/nrn1705. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Chiaradia C, Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain. 2002;96:393–402. doi: 10.1016/S0304-3959(01)00485-7. [DOI] [PubMed] [Google Scholar]
- Gareus IK, Lacour M, Schulte AC, Hennig J. Is there a BOLD response of the visual cortex on stimulation of the vision-related acupoint GB 37? J Magn Reson Imaging. 2002;15:227–32. doi: 10.1002/jmri.10059. [DOI] [PubMed] [Google Scholar]
- Harris R, Naylor G, Weinzimmer D, Scott D, Gracely R, Zubieta J, Daniel C. Neuroimaging of Pain and Its Modulation by Acupuncture. NCCAM Neurobiological Correlates of Acupuncture Conference; Bethesda, MD. 2005. [Google Scholar]
- Hoffman GA, Harrington A, Fields HL. Pain and the placebo: what we have learned. Perspect Biol Med. 2005;48:248–65. doi: 10.1353/pbm.2005.0054. [DOI] [PubMed] [Google Scholar]
- Hui KK, Liu J, Makris N, Gollub RL, Chen AJ, Moore CI, Kennedy DN, Rosen BR, Kwong KK. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp. 2000;9:13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage. 2005;27:479–96. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–8. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2:654–7. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- Li G, Cheung RT, Ma QY, Yang ES. Visual cortical activations on fMRI upon stimulation of the vision-implicated acupoints. Neuroreport. 2003;14:669–73. doi: 10.1097/00001756-200304150-00002. [DOI] [PubMed] [Google Scholar]
- Liu WC, Feldman SC, Cook DB, Hung DL, Xu T, Kalnin AJ, Komisaruk BR. fMRI study of acupuncture-induced periaqueductal gray activity in humans. Neuroreport. 2004;15:1937–40. doi: 10.1097/00001756-200408260-00021. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–13. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Napadow V, Liu J, Li M, Kettner N, Kwong K, Vangel M, Makris N, Audette J, Hui KKS. Hypothalamus and Amygdala Response to Acupuncture Stimuli in Carpal Tunnel Syndrome. In Review. 2006a doi: 10.1016/j.pain.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Liu J, Li M, Kettner N, Ryan A, Kwong KK, Hui KKS, Audette J. Somatosensory Cortical Plasticity in Carpal Tunnel Syndrome Treated by Acupuncture. Human Brain Mapping. 2006b doi: 10.1002/hbm.20261. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KKS. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2005;24:193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente J, White P, Frackowiak RS, Lewith G. Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture. Neuroimage. 2005;25:1161–7. doi: 10.1016/j.neuroimage.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. J Cogn Neurosci. 2004;16:1289–301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Chiu D. Naloxone blockade of acupuncture analgesia: endorphin implicated. Life Sci. 1976;19:1757–62. doi: 10.1016/0024-3205(76)90084-9. [DOI] [PubMed] [Google Scholar]
- Price DD, Rafii A, Watkins LR, Buckingham B. A psychophysical analysis of acupuncture analgesia. Pain. 1984;19:27–42. doi: 10.1016/0304-3959(84)90062-9. [DOI] [PubMed] [Google Scholar]
- Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet. 1998;352:364–5. doi: 10.1016/S0140-6736(97)10471-8. [DOI] [PubMed] [Google Scholar]
- Stux G, Hammerschlag R. Clinical Acupuncture Scientific Basis. Springer-Verlag; Berlin: 2001. p. 227. [Google Scholar]
- Voudouris NJ, Peck CL, Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43:121–8. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wickramasekera I. How does biofeedback reduce clinical symptoms and do memories and beliefs have biological consequences? Toward a model of mind-body healing. Appl Psychophysiol Biofeedback. 1999;24:91–105. doi: 10.1023/a:1022201710323. [DOI] [PubMed] [Google Scholar]
- Wu MT, Hsieh JC, Xiong J, Yang CF, Pan HB, Chen YC, Tsai G, Rosen BR, Kwong KK. Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain--preliminary experience. Radiology. 1999;212:133–41. doi: 10.1148/radiology.212.1.r99jl04133. [DOI] [PubMed] [Google Scholar]
- Wu MT, Sheen JM, Chuang KH, Yang P, Chin SL, Tsai CY, Chen CJ, Liao JR, Lai PH, Chu KA, Pan HB, Yang CF. Neuronal specificity of acupuncture response: a fMRI study with electroacupuncture. Neuroimage. 2002;16:1028–37. doi: 10.1006/nimg.2002.1145. [DOI] [PubMed] [Google Scholar]
- Xu X, Shibasaki H, Shindo K. Effects of acupuncture on somatosensory evoked potentials: a review. J Clin Neurophysiol. 1993;10:370–7. doi: 10.1097/00004691-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Teh EK, Blinder RA, Jolesz FA. Modulation of cerebellar activities by acupuncture stimulation: evidence from fMRI study. Neuroimage. 2004;22:932–40. doi: 10.1016/j.neuroimage.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Zhang WT, Jin Z, Cui GH, Zhang KL, Zhang L, Zeng YW, Luo F, Chen AC, Han JS. Relations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: a functional magnetic resonance imaging study. Brain Res. 2003;982:168–78. doi: 10.1016/s0006-8993(03)02983-4. [DOI] [PubMed] [Google Scholar]
- Zhou ZF, Du MY, Wu WY, Jiang Y, Han JS. Effect of intracerebral microinjection of naloxone on acupuncture- and morphine-analgesia in the rabbit. Sci Sin. 1981;24:1166–78. [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–62. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Yau WY, Scott DJ, Stohler CS. Belief or Need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain Behav Immun. 2006;20:15–26. doi: 10.1016/j.bbi.2005.08.006. [DOI] [PubMed] [Google Scholar]


