Abstract
Aging affects several processes modulated by the 5-HT7 receptor subtype, including circadian rhythms, learning and memory, and depression. Previously, we showed that aging induces a decrease in the hamster dorsal raphe (DRN) in both 5-HT7 receptor binding and circadian phase resetting responses to 8-OH-DPAT microinjection. To elucidate the mechanisms underlying the aging decrease in 5-HT7 receptors, we investigated aging modulation of 5-HT7 receptor mRNA expression in the DRN, brain regions afferent to the DRN, and brain regions regulating circadian rhythms or memory. In situ hybridization for 5-HT7 receptor mRNA was performed on coronal sections prepared from the brains of young, middle-aged, and old male Syrian hamsters. 5-HT7 receptor mRNA expression was quantified by densitometry of X-ray film autoradiograms. The results showed that aging did not significantly affect 5-HT7 receptor mRNA expression in the DRN or most other brain regions examined, with the exception of the cingulate cortex and paraventricular thalamic nucleus. Within the suprachiasmatic nucleus, the site of the master circadian pacemaker in mammals, 5-HT7 receptor mRNA expression was localized in a discrete subregion resembling the calbindin subnucleus previously described. A second experiment using adjacent tissue sections showed that 5-HT7 receptor mRNA and calbindin mRNAs were concentrated in the same region of the SCN, and as well as in the same region of several other brain structures. The localization of 5-HT7 receptors and calbindin mRNAs within the same regions suggests that proteins they encode may interact to modulate processes such as circadian timekeeping.
Keywords: serotonin receptors, suprachiasmatic nucleus, hippocampus, raphe nuclei
1. Introduction
The 5-HT7 receptor subtype, the most recently cloned of the serotonin receptor subtypes, modulates many physiological and behavioral functions, including circadian rhythms (Ehlen et al., 2001; Duncan et al., 2004; Duncan and Davis, 2005), rapid eye movement (REM) sleep (Hagan et al., 2000; Thomas et al., 2003; Monti and Jantos, 2006), body temperature (Hedlund et al., 2003), learning and memory (Roberts et al., 2004), and behavior (Guscott et al., 2005; Hedlund et al., 2005). For example, administration of the 5-HT7 receptor-selective antagonists, SB-269970-A or DR-4004, to hamsters blocks phase advances of circadian locomotor activity rhythms induced by the serotonergic agonists, 8-OH-DPAT or 5-carboxamidotryptamine, demonstrating that pharmacological activation of 5-HT7 receptors stimulates circadian phase resetting (Ehlen et al., 2001; Duncan et al., 2004). Also, administration of 5-HT7 receptor-selective antagonists to guinea pigs or rats selectively decreases REM sleep, suggesting that endogenous serotonin acting at 5-HT7 receptors exerts a tonic inhibition on REM sleep (Hagan et al., 2000; Thomas et al., 2003; Monti and Jantos, 2006). Furthermore, studies in mice have shown that deletion of the 5-HT7 receptor gene impairs memory and learning (Roberts et al., 2004), reduces immobility in the Porsolt swim test, similar to antidepressants (Guscott et al., 2005; Hedlund et al., 2005), and inhibits hypothermia induced by 8-OH-DPAT (Hedlund et al., 2003).
Aging deleteriously affects some of the functions modulated by 5-HT7 receptors, most notably, circadian rhythms and memory. Furthermore, some age-related changes in these processes have been associated with decreases in 5-HT7 receptor expression. For example, significant attenuation of serotonergic induction of circadian phase shifts was observed in hamsters by 17–19 months of age (Penev et al., 1995; Duncan et al., 2004), the same age at which a significant reduction of specific 5-HT7 receptor binding was exhibited in the dorsal raphe nucleus (DRN) (Duncan et al., 1999). Also, age-related memory deficits are exhibited by rodents and humans, and decreased expression of 5-HT7 receptor mRNA in the ventral CA3 of the hippocampus has been observed in old rats (Kohen et al., 2000) [but see also (Yau et al., 1999)].
In order to further elucidate the mechanisms responsible for the age-related reduction of 5-HT7 receptor binding in the aging hamster brain, we investigated 5-HT7 receptor mRNA expression in young, middle-aged and old hamsters. We focused on the DRN, based on our previous identification of age-associated loss of 5-HT7 receptor binding and functional responses in this region (Duncan et al., 1999; Duncan et al., 2004) and on brain regions known to send afferent projections to the DRN. We also investigated several other brain regions involved in the regulation of circadian rhythms, memory, or mood. In the course of investigating the expression of 5-HT7 mRNA in the hamster suprachiasmatic nucleus, we observed that its expression was concentrated in a small subregion previously reported to express calbindin mRNA. Therefore, in this project, we also investigated the effect of aging on calbindin mRNA expression in the SCN and other brain regions.
2. Results
2.1. Experiment 1. The effect of aging on 5-HT7 receptor mRNA expression in the dorsal raphe and other discrete regions of the midbrain and the forebrain
Expression of 5-HT7 receptor mRNA was observed in many brain regions, similar to previous findings in rats (Neumaier et al., 2001). The DRN exhibited a relatively low level of 5-HT7 receptor mRNA expression that was not significantly affected by aging (Fig. 1 and Table 1). Furthermore, no effect of aging on 5-HT7 receptor mRNA expression was observed in most of the midbrain or forebrain regions examined, with the exception of the cingulate cortex and paraventricular thalamic nucleus (Fig. 1 and Table 1). Distinct but faint labeling was detected in a small circular region of the ventral mid-caudal SCN of young hamsters, but appeared to be absent from this region in most of the middle-aged and old animals.
Figure 1.
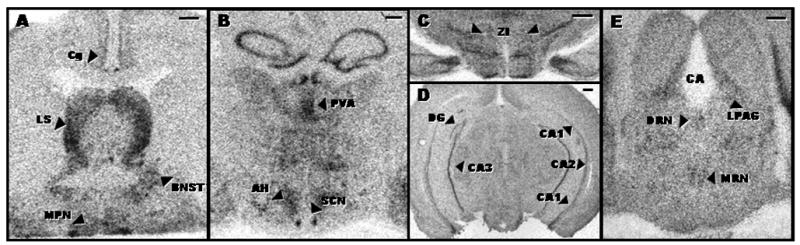
Autoradiograms representing expression of 5-HT7 receptor mRNA in the forebrain and midbrain of young adult hamsters. Scale bar depicts 500 microns.
Table 1.
5-HT7 receptor mRNA expression in brain regions of young, middle-aged, and old hamsters.
| Young | Middle-aged | Old | |||||
|---|---|---|---|---|---|---|---|
| Brain Region | Mean | SEM | Mean | SEM | Mean | SEM | P |
|
Cortex
Cg DG CA1 CA2 CA3 |
8.6* 6.53 39.0 50.8 76.8 |
1.1 1.8 6.1 17.8 4.8 |
8.7 7.4 45.0 50.2 74.1 |
1.2 1.0 9.3 8.8 4.3 |
5.4 8.4 27.0 40.8 78.9 |
0.6 1.0 4.1 3.2 4.3 |
0.04 0.58 0.19 0.77 0.74 |
|
Basal forebrain
LS BNST |
20.0 12.0 |
2.0 1.0 |
17.3 12.5 |
3.0 1.7 |
11.6 10.3 |
2.0 0.9 |
0.06 0.44 |
|
Hypothalamus
SCN AH |
76.4 61.9 |
13.6 4.0 |
72.9 56.3 |
29.0 5.9 |
108.0 54.4 |
16.8 3.2 |
0.44 0.48 |
|
Amygdala
Ce |
87.7 |
7.5 |
93.4 |
12.0 |
75.6 |
10.3 |
0.46 |
|
Thalamus
MHb PVA ZI IGL |
16.2 384.0 128.0 41.3 |
2.6 27.9 3.4 4.5 |
15.2 322.0 114.0 32.1 |
1.7 22.6 5.9 5.2 |
12.2 295.0 119.0 39.9 |
1.0 18.3 2.7 6.7 |
0.30 0.03 0.07 0.49 |
|
Mid-brain
MRN DRN LPAG |
10.4 11.1 17.7 |
1.3 1.6 2.0 |
12.0 13.4 22.2 |
2.4 2.4 1.8 |
14.5 11.0 18.7 |
2.2 2.2 1.7 |
0.34 0.70 0.22 |
Values represent the mean of 6–9 animals.
AH, anterior hypothalamus; BNST, bed nucleus of the stria terminalis; CA1, CA1 region of the hippocampus; CA2, CA2 region of the hippocampus; CA3, CA3 region of the hippocampus; Ce, central nucleus of the amygdala; Cg, cingulate cortex; DG, dentate gyrus; DRN, dorsal raphe nucleus; IGL, intergeniculate leaflet; LPAG, lateral periaqueductal gray; LS, lateral septum; MHb, medial habenula; MRN, median raphe nucleus; PVA, anterior paraventricular thalamic nucleus; SCN, suprachiasmatic nucleus; ZI, zona incerta
2.2. Experiment 2. The effect of aging on 5-HT7 receptor mRNA expression in the calbindin-expressing region of the SCN and on calbindin mRNA expression
Previous studies have indicated that the ventral mid-caudal region of the hamster SCN is unique in its expression of the calcium-binding protein calbindin, and furthermore, this region is essential for generation of circadian rhythms (LeSauter et al., 2002; Hamada et al., 2003; Kriegsfeld et al., 2004a; Antle and Silver, 2005). This experiment investigated if the expression of 5-HT7 receptor mRNA occurs in the same region of the SCN that expresses calbindin mRNA, and if aging effects the expression of 5-HT7 receptor mRNA or calbindin mRNA within this region. The results showed that the ventral mid-caudal region of the hamster SCN expressed calbindin mRNA, as expected. Furthermore, 5-HT7 receptor mRNA was co-expressed in the calbindin mRNA-expressing subnucleus (Fig. 2). The apparent age-related change in SCN 5-HT7 receptor mRNA expression suggested in Experiment 1 was not confirmed in this study, as aging did not significantly affect 5-HT7 receptor mRNA expression in the calbindin subnucleus of the SCN or calbindin mRNA expression itself (Fig. 2). (A third experiment investigating 5-HT7 receptor mRNA expression in the SCN of a separate set of hamsters also did not detect significant age-related differences [data not shown].) In contrast to the SCN and DRN, significant age-related changes in calbindin mRNA expression were observed in the cingulate cortex, MRN, and LPAG (Table 2).
Figure 2.
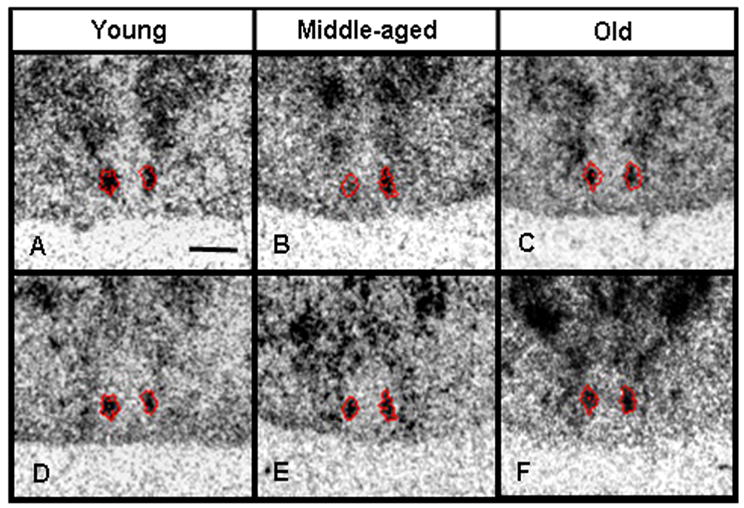
Autoradiograms demonstrating expression of 5-HT7 receptor mRNA and calbindin mRNA in the same region of the hamster SCN. The red traces represent the border of the calbindin mRNA expression. Scale bar depicts 500 microns.
Table 2.
Calbindin D28K mRNA expressions in brain regions of young, middle-aged, and old hamsters.
| Young | Middle-aged | Old | |||||
|---|---|---|---|---|---|---|---|
| Brain Region | Mean | SEM | Mean | SEM | Mean | SEM | P |
|
Cortex
Cg CA1 CA2 |
81.9* 207.0 128.0 |
6.9 12.0 9.5 |
53.5 166.0 97.2 |
4.3 15.1 11.6 |
51.7 179.0 101.0 |
5.5 15.1 15.7 |
0.002 0.15 0.22 |
|
Basal forebrain
LS ventral TS BNST |
87.1 135.0 66.8 |
9.8 40.4 12.7 |
68.0 166.0 93.8 |
8.8 42.3 18.0 |
84.6 193.0 73.2 |
7.2 29.6 9.9 |
0.27 0.57 0.37 |
|
Basal ganglia
CPu |
58.2 |
4.5 |
48.3 |
5.8 |
53.0 |
4.8 |
0.42 |
|
Hypothalamus
SCN PMV |
84.5 299.0 |
9.0 65.8 |
83.3 266.0 |
8.9 44.9 |
60.0 287.0 |
5.0 39.4 |
0.10 0.90 |
|
Mid-brain
MRN DRN LPAG |
38.1 162.0 84.8 |
10.0 25.7 8.6 |
10.3 144.0 56.0 |
5.2 29.1 9.8 |
12.0 141.0 53.3 |
3.4 21.1 6.9 |
0.02 0.80 0.03 |
Values represent the mean of 6–9 animals.
BNST, bed nucleus of the stria terminalis; CA1, CA1 region of the hippocampus; CA2, CA2 region of the hippocampus; Cg, cingulate cortex; CPu, caudate putamen; DRN, dorsal raphe nucleus; LPAG, lateral periaqueductal gray; LS ventral, ventral lateral septum; MRN, median raphe nucleus; PMV, ventral premammillary nucleus; SCN, suprachiasmatic nucleus; TS, triangular septal nucleus
3. Discussion
Aging did not significantly alter 5-HT7 receptor mRNA expression in the hamster DRN at ZT 6, and thus changes in transcription of this mRNA do not account for the age-associated decreases in 5-HT7 receptor binding or functional responses previously observed in this brain structure (Duncan et al., 1999; Duncan et al., 2004). Furthermore, the relatively low levels of expression of 5-HT7 receptor mRNA observed in the DRN (and MRN) suggest that some of the 5-HT7 receptor protein identified in these regions by binding studies or functional studies may be located on afferent terminals of nerve fibers originating in other brain regions. Some of the brain regions that have been demonstrated to be afferent to the DRN in the rat, such as the bed nucleus of the stria terminalis, the medial preoptic nucleus, the anterior hypothalamic area, the ventral pontine periaqueductal gray, the cingulate cortex, and the paraventricular thalamic nucleus (Peyron et al., 1998; Gervasoni et al., 2000; Brown et al., 2002; Lee et al., 2005), exhibited 5-HT7 receptor mRNA expression in the hamster brain, although other afferent regions, such as the lateral preoptic area, lateral hypothalamic area, lateral habenula, perifornical nucleus, posterior hypothalamic area, and medial tuberal nucleus, did not show 5-HT7 receptor mRNA signal in this study. Of the DRN-afferent regions that expressed 5-HT7 receptor mRNA, only two, the cingulate cortex and the paraventricular thalamic nucleus, exhibited age-related decreases in expression of this mRNA. If decreased 5-HT7 receptor mRNA expression leads to decreased 5-HT7 receptor protein on efferent projections from these regions to the DRN, then it could contribute to the age-related reduction in 5-HT7 receptor mediated phase shifts that we have previously observed (Duncan et al., 2004). However, the lack of detectable age-related difference in 5-HT7 receptor mRNA expression in most of the brain regions afferent to the DRN seems to suggest an alternative hypothesis, that age-related decrease in 5-HT7 receptors in the DRN may be the result of some process other than decreased 5-HT7 receptor mRNA expression, possibly neurotransmitter-induced down regulation. As shown in a previous study, aging is associated with a decrease in serotonin transporter binding in the hamster DRN (Duncan and Hensler, 2002), which would be likely to contribute to reduced serotonin reuptake and therefore increased extracellular serotonin concentrations that could induce 5-HT7 receptor down regulation. This possibility is supported by findings that chronic treatment with serotonin selective reuptake inhibitors decreases 5-HT7 receptor binding sites in rats (Mullins et al., 1999; Sleight et al., 1995).
As well as affecting circadian rhythms, the age-related decrease in 5-HT7 receptor mRNA expression in the cingulate cortex may be related to age-associated changes in memory or affective state, because these processes that have been shown to be regulated by the cingulate cortex and modulated by 5-HT7 receptors (Frankland et al., 2004; Maviel et al., 2004; Caetano et al., 2006; Mayberg, 2006; Roberts et al., 2004; Hedlund et al., 2005; Guscott et al., 2005). For example, the cingulate cortex participates in the regulation of contextual fear conditioning (Frankland et al., 2004) and in the affective response to pain (Johansen et al., 2001). Aging leads to deficits in contextual fear conditioning (Gemma et al., 2004; Mesches et al., 2004; Gemma et al., 2006), as does mutation of the 5-HT7 receptor (Roberts et al., 2004). If the age-related decrease in 5-HT7 receptor mRNA expression in the cingulate cortex is also associated with a decrease in 5-HT7 receptors, it would likely contribute to aging deficits in contextual fear conditioning.
In contrast to the cingulate cortex, the possible functional significance of age-related changes in 5-HT7 receptor mRNA expression in the paraventricular thalamic nucleus is less clear. The processes that are regulated by the paraventricular thalamic nucleus, such as arousal and attention, as well as in the effects of stress on body temperature and energy balance (van der Werf et al., 2002; Kirouac et al., 2005; Bhatnagar and Dallman, 1999; Huang et al., 2006), have not been reported to be affected by 5-HT7 receptor activation.
The lack of effect of aging on 5-HT7 receptor mRNA expression in the hamster hippocampal ventral CA3 region in the current study contrasts with a previous report in rats, in which middle-aged and old animals exhibited significantly lower 5-HT7 receptor mRNA expression than young animals (Kohen et al., 2000). The present results are consistent, however, with an earlier study in rats, which did not observe any age-associated changes in 5-HT7 receptor mRNA expression in any hippocampal subfield (Yau et al., 1999).
The most interesting finding of the present study was the observation that 5-HT7 receptor mRNA expression in the hamster SCN is concentrated in the calbindin subnucleus. This region is crucial for the generation of circadian rhythmicity, as shown by the loss of circadian rhythms in behavior (locomotor activity, drinking, gnawing) and circulating concentrations of hormones (melatonin and cortisol) in hamsters after discrete lesions of this area (LeSauter and Silver, 1999; Kriegsfeld et al., 2004b). Also, the calbindin subnucleus receives direct retinal innervation (Bryant et al., 2000) and mediates photic phase shifts, as demonstrated by the finding that intracerebroventricular administration of calbindin antisense oligonucleotides attenuates light induction of Per1 mRNA expression in the SCN and circadian phase shifts (Hamada et al., 2003). The present identification of 5-HT7 receptor mRNA within the calbindin subnucleus supports other findings that post-synaptic 5-HT7 receptors in the SCN modulate the effects of serotonergic agonists on responses to light or to glutamate, the major neurotransmitter released by the retinohypothalamic tract that innervates the SCN (Ying and Rusak, 1997; Quintero and McMahon, 1999). Furthermore, the discrete localization of 5-HT7 receptor mRNA within the calbindin mRNA expressing region of the SCN suggests that this receptor may modulate photic phase shifts through an interaction with calbindin.
The calbindin subnucleus is also innervated by serotonin- and neuropeptide Y- immunoreactive fibers (LeSauter et al., 2002; Antle and Silver, 2005). Because NPY projections from the IGL and serotonin projections from the median raphe are known to communicate nonphotic information to the suprachiasmatic nucleus (Meyer-Bernstein and Morin, 1998; Cutrera et al., 1994; Schuhler et al., 1999; Janik and Mrosovsky, 1994), it is possible that the calbindin subnucleus may be involved in mediating nonphotic resetting of the circadian pacemaker. One study investigating this possibility found that administration of calbindin antisense oligonucleotides did not affect phase shifts induced by injections of the 5-HT1A/7/5 agonist, 8-OH-DPAT, although a small sample size (N=3) was used (Hamada et al., 2003). However, it should be noted that photic phase shifts involve an increase in Per1 mRNA expression in the SCN, especially in the calbindin subregion, and that this increase is attenuated by administration of calbindin antisense oligonucleotides (Hamada et al., 2003). Because induction of phase shifts by 8-OH-DPAT injection or other nonphotic signals is associated with decreased Per1 mRNA expression (Horikawa et al., 2000; Duncan et al., 2005; Fukuhara et al., 2001; Hamada et al., 2004), it is not surprising that calbindin antisense oligonucleotide attenuation of SCN Per1 expression does not prevent phase shifts to 8-OH-DPAT (Hamada et al., 2003).
In the present study, neither calbindin mRNA expression in the SCN nor 5-HT7 receptor mRNA expression in the calbindin subnucleus of the SCN were significantly affected by aging. Thus, the age-related attenuation of photic induction of phase shifts and SCN Per mRNA expression that has been previously reported in hamsters (Kolker et al., 2003; Zhang et al., 1996) apparently is not mediated by decreased calbindin mRNA expression in the SCN. Furthermore, the present findings in the hamster brain are consistent with previous studies in rats indicating that age-associated changes in calbindin mRNA occur only in discrete regions. In aging rats, decreases in calbindin mRNA or protein expression were observed only in the cerebellum, corpus striatum and nucleus basalis and not in most brain regions examined (Iacopino and Christakos, 1990). Age-related decreases in calbindin mRNA expression in the hamster brain were observed only in the cingulate cortex, the median raphe nucleus, and the lateral periaqueductal gray. The significance of these age-related changes is not known, because the function of calbindin in these brain regions has not been elucidated.
The findings presented here show that 5-HT7 receptor mRNA is expressed in regions of the SCN and other brain structures where calbindin mRNA is expressed. Because a specific antibody that distinctly labels 5-HT7 receptors in hamster brain tissue is not available, we have been unable to investigate whether 5-HT7 receptor protein is co-localized at the cellular level with calbindin protein. It is interesting to note that immunohistochemical studies have shown that another serotonin receptor subtype, the 5-HT1A receptor, is co-localized with calbindin in several rat brain regions, including the hippocampus, the thalamus and the septum (Aznar et al., 2003). As well as modulating photic phase shifts (Hamada et al., 2003), as described above, calbindin has been implicated in several other functions, including spatial memory, long-term potentiation in the hippocampus, synaptic plasticity, and either neuroprotection or enhanced vulnerability to neurodegeneration (Jouvenceau et al., 1999; Chard et al., 1995; Molinari et al., 1996; Nagerl et al., 2000). Some of these functions, such as memory, long-term potentiation, and synaptic plasticity are also modulated by 5-HT7 receptors (Perez-Garcia et al., 2006; Perez-Garcia and Meneses, 2005; Roberts et al., 2004; Kvachnina et al., 2005).
4. Conclusions
5-HT7 receptor mRNA expression in the DRN and most other hamster brain regions examined is not significantly affected by aging. Within the SCN, 5-HT7 receptor mRNA expression is concentrated in the subregion that expresses calbindin mRNA. Expression of calbindin mRNA was also observed in several other brain regions that express 5-HT7 receptor mRNA, including the cingulate cortex, median raphe nucleus, and hippocampal CA1 and CA2 subfields.
Localization of mRNAs for both the 5-HT7 receptor and calbindin within the same regions suggests the potential for interaction of these two proteins for modulation of processes such as circadian rhythms and memory. The present findings show that aging affects expression of 5-HT7 mRNA and calbindin mRNA differentially and in a brain region specific manner. If decreases in mRNA expression predict decreases in protein expression, then these decreases may contribute to age-related decrements in the functions subserved by these proteins in discrete brain regions.
5. Experimental procedures
5.1. Animals and tissue preparation
Male Syrian hamsters (Harlan HSD) of three ages (young, 3–5 months; middle-aged, 12–14 months; old, 17–19 months; N=6–9/age/experiment) were used. After exposure to a light:dark cycle (lights on from 0600–2000 h) for at least ten days, the hamsters were decapitated at 1400 h (i.e., zeitgeber time [ZT] 6, where ZT 12 is conventionally considered the time of lights off), the time at which serotonergic drugs induce maximal circadian phase advances (Tominaga et al., 1992). After dissection, brains were frozen on crushed dry ice and stored at −80°C. Coronal sections (20 μm thick) through the hypothalamus and midbrain were cut with a cryostat and mounted onto negatively charged slides. The experimental procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee prior to implementation.
5.2. In situ hybridization
Partial cDNA sequences of hamster 5-HT7 receptor (nt 1–765) and calbindin-D28K (nt 179–658) were synthesized by RT-PCR and cloned into pBluescript SK(+). Plasmid DNA was purified, linearized, and subjected to c-RNA synthesis using 35S-UTP as the label. Four tissue sections per animal per anatomical region were used for each in situ hybridization experiment. Slide-mounted tissue sections were equilibrated to room temperature, fixed for 15 min in 4% PFA/0.1M PB (pH 7.4) and washed with PBS. The sections were acetylated in 0.1 M triethanolamine/0.25% acetic anhydride, dehydrated and delipidated, and air dried.
The sections were hybridized for 20 h at 55°C with riboprobes diluted in hybridization cocktail (250 mg/ml salmon sperm DNA, 625 mg/ml yeast total RNA, 20 mM Tris-HCl, 1 mM EDTA, 300 mM NaCl, 50% v/v deionized formamide, 10% v/v dextran sulphate, 1X Denhardt’s solution, 100 mM dithiothreitol, 0.1% SDS, and 0.1% Na-thiosulfate). After hybridization, the sections were rinsed in 2X SSC/10 mM DTT at 22°C, treated with RNAse (40 mg/ml) at 37°C, washed in 1X SSC and incubated for 1 h at 63°C in 0.1X SSC. Then the sections were washed (0.1X SSC, 22°C), dehydrated and air dried. Experimental slides and 14C-standards (Amersham Biosciences) were apposed to X-ray film (Kodak Biomax-MR) for 2–4 weeks. The X-ray films were developed using standard procedures. The resulting autoradiograms were analyzed using computer-assisted microdensitometry (MCID, Imaging Research Inc.), as described previously (Duncan et al., 2001).
5.3. Experiment 1. The effect of aging on 5-HT7 mRNA expression in the dorsal raphe and other discrete regions of the midbrain and the forebrain
This study investigated whether aging is associated with changes in 5-HT7 receptor mRNA expression in the DRN, and other circadian substrates, e.g., the suprachiasmatic nucleus (SCN), the intergeniculate leaftlet (IGL), and the median raphe (MRN). Additionally, brain regions afferent to the DRN were measured, including the cingulate cortex, bed nucleus of the stria terminalis (BNST), lateral septum, central nucleus of the amygdala, anterior hypothalamus, anterior paraventricular thalamic nucleus, zona incerta, and lateral periaqueductal gray (LPAG) (Gervasoni et al., 2000; Rampon et al., 1999). Finally, the dentate gyrus (DG) and fields CA1, CA2, and CA3 of the hippocampus were also studied. The brain sections used corresponded to plates 18–20 (lateral septum, BNST, and cingulate cortex), 24–25 (SCN, central nucleus of the amygdala, anterior hypothalamus, and anterior paraventricular thalamic nucleus), 29–31 (IGL and zona incerta), plates 33–35 (hippocampus) or plates 41–42 (DRN, MRN, and LPAG) shown in A Stereotaxic Atlas of The Golden Hamster Brain, by L.P. Morin and R.I. Wood (Academic Press, 2001).
5.4. Experiment 2. The effect of aging on 5-HT7 mRNA expression in the calbindin-expressing region of the suprachiasmatic nucleus
The results of Experiment 1 indicated that expression of 5-HT7 receptor mRNA is localized to a small subregion of the SCN that resembled the calbindin subnucleus previously described in the hamster SCN (LeSauter et al., 2002; Hamada et al., 2003; Kriegsfeld et al., 2004a; Antle and Silver, 2005). Therefore, this experiment investigated if the expression of 5-HT7 receptor mRNA occurs in the same region of the SCN that expresses calbindin mRNA, and if aging effects the expression of 5-HT7 receptor mRNA or calbindin mRNA within this region. Adjacent sections of the mid-caudal SCN were used for in situ hybridization for these two mRNA species. For each animal, the boundary of the autoradiogram representing calbindin mRNA expression was traced and superimposed over the autoradiogram representing 5-HT7 receptor mRNA expression in an adjacent SCN section, and 5-HT7 receptor mRNA expression within this region was determined.
5.5 Data and statistical analyses
The data for each brain region were subjected to one-way ANOVA, assessing the effect of age. In the case of significant P values (P<0.05), Bonferroni’s test was conducted.
Acknowledgments
The authors gratefully acknowledge Verda A. Davis for assistance with brain sectioning and the National Institutes of Health for financial support (R01 AG013418).
Abbreviations
- Cg
cingulate cortex
- LS/LSV
lateral septum (ventral)
- TS
triangular septal nucleus
- BNST
bed nucleus of the stria terminalis
- CPu
caudate putamen
- SCN
suprachiasmatic nucleus
- AH
anterior hypothalamus
- Ce
central amygdaloid nucleus
- MHb
median habenula
- PVA
anterior paraventricular thalamic nucleus
- ZI
zona incerta
- PMV
ventral premammillary nucleus
- IGL
intergeniculate leaflet
- DG
dentate gyrus
- CA1
CA1 region of the hippocampus
- CA2
CA2 region of the hippocampus
- CA3
CA3 region of the hippocampus
- MRN
median raphe nucleus
- DRN
dorsal raphe nucleus
- LPAG
lateral periaqueductal gray
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. T I N S. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM. The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Res. 2003;959:58–67. doi: 10.1016/s0006-8993(02)03727-7. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman MF. The paraventricular nucleus of the thalamaus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain Res. 1999;851:66–75. doi: 10.1016/s0006-8993(99)02108-3. [DOI] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DN, LeSauter J, Silver R, Romero MT. Retinal innervation of calbindin-D28K cells in the hamster suprachiasmatic nucleus: ultrastructural characteristics. J Biol Rhythms. 2000;15:103–111. doi: 10.1177/074873040001500204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano SC, Kaur S, Brambilla P, Nicoletti M, Hatch JP, Sassi RB, Mallinger AG, Keshavan MS, Kupfer DJ, Frank E, Soares JC. Smaller cingulate volumes in unipolar depressed patients. Biol Psychiatry. 2006;59:702–706. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Chard PS, Jordan J, Marcuccilli CJ, Miller RJ, Leiden JM, Roos RP, Ghadge GD. Regulation of excitatiory transmission at hippocampal synapses by calbindin D28K. Proc Natl Acad Sci. 1995;92:5144–5148. doi: 10.1073/pnas.92.11.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrera RA, Kalsbeek A, Pévet P. Specific destruction of the serotonergic afferents to the suprachiasmatic nuclei prevents triazolam-induced phase advances of hamster activity rhythms. Behav Brain Res. 1994;62:21–28. doi: 10.1016/0166-4328(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Davis VA. Cyclic AMP mediates circadian phase shifts induced by microinjection of serotonergic drugs in the hamster dorsal raphe nucleus. Brain Res. 2005;1058:10–16. doi: 10.1016/j.brainres.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Franklin KM, Davis VA, Grossman GH, Knoch ME, Glass JD. Short-term constant light potentiation of large-magnitude circadian phase shifts induced by 8-OH-DPAT: effects on serotonin receptors and gene expression in the hamster suprachiasmatic nucleus. Eur J Neurosci. 2005;22:2306–2314. doi: 10.1111/j.1460-9568.2005.04399.x. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Grear KE, Hoskins MA. Aging and SB-269970-A, a selective 5-HT7 receptor antagonist, attenuate circadian phase advances induced by microinjections of serotonergic drugs in the hamster dorsal raphe nucleus. Brain Res. 2004;1008:40–48. doi: 10.1016/j.brainres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Hensler JG. Aging alters in a region-specific manner serotonin transporter sites and 5-HT1A receptor-G protein interactions in hamster brain. Neuropharmacol. 2002;43:36–44. doi: 10.1016/s0028-3908(02)00072-2. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Hill SA, Herron JM. Aging selectively suppresses vasoactive intestinal peptide messenger RNA expression in the suprachiasmatic nucleus. Mol Brain Res. 2001;87:196–203. doi: 10.1016/s0169-328x(01)00015-8. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Short J, Wheeler DL. Comparison of the effects of aging on 5-HT7 and 5-HT1A receptors in discrete regions of the circadian timing system in hamsters. Brain Res. 1999;829:39–45. doi: 10.1016/s0006-8993(99)01311-6. [DOI] [PubMed] [Google Scholar]
- Ehlen JC, Grossman GH, Glass JD. In vivo resetting of the hamster circadian clock by 5-HT7 receptors in the suprachiasmatic nucleus. J Neurosci. 2001;21:5351–5357. doi: 10.1523/JNEUROSCI.21-14-05351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Sci. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Brewer JM, Dirden JC, Bittman EL, Tosini G, Harrington ME. Neuropeptide Y rapidly reduces Period 1 and Period 2 mRNA levels in the hamster suprachiasmatic nucleus. Neurosci Lett. 2001;314:119–122. doi: 10.1016/s0304-3940(01)02304-7. [DOI] [PubMed] [Google Scholar]
- Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur J Neurosci. 2006;22:1751–1756. doi: 10.1111/j.1460-9568.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- Gemma C, Stellwagen H, Fister M, Coultrap SJ, Mesches MH, Browning MD, Bickford PC. Rosiglitazone improves contextual fear conditioning in age rats. Neuroreport. 2004;15:2255–2259. doi: 10.1097/00001756-200410050-00023. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, Bromidge F, Owens AP, Huscroft I, Myers J, Rupniak NM, Patel S, Whiting PJ, Huston PH, Fone KC, Biello SM, Kulagowski JJ, McAllister G. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacol. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, Antle MC, Silver R. The role of Period1 in non-photic resetting of the hamster circadian pacemaker in the suprachiasmatic nucleus. Neurosci Lett. 2004;362:87–90. doi: 10.1016/j.neulet.2004.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Lokshin M, Romero MT, Yan L, Venuti JM, Silver R. Calbindin influences response to photic input in suprachiasmatic nucleus. J Neurosci. 2003;23:8820–8826. doi: 10.1523/JNEUROSCI.23-26-08820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 Receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Yokota SI, Fuji K, Akiyama M, Moriya T, Okamura H, Shibata S. Nonphotic entrainment by 5-HT1A/7 receptor agonists accompanied by reduced Per 1 and Per 2 mRNA levels in the suprachiasmatic nucleus. J Neurosci. 2000;20:5867–5873. doi: 10.1523/JNEUROSCI.20-15-05867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ghosh P, van den Pol AN. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: A feedforward circuit that may enhance cognitive arousal. J Neurophysiol. 2006;95:1656–1668. doi: 10.1152/jn.00927.2005. [DOI] [PubMed] [Google Scholar]
- Iacopino AM, Christakos S. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proc Natl Acad Sci. 1990;87:4078–4082. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik D, Mrosovsky N. Intergeniculate leaflet lesions and behaviorally-induced shifts of circadian rhythms. Brain Res. 1994;651:174–182. doi: 10.1016/0006-8993(94)90695-5. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: Direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenceau A, Potier B, Battini R, Ferrari S, Dutar P, Billard JM. Glutamatergic synaptic responses and long-term potentiation are impaired in the CA1 hippocampal area of calbindin D28K-deficient mice. Synapse. 1999;33:172–180. doi: 10.1002/(SICI)1098-2396(19990901)33:3<172::AID-SYN2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059:179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Kohen R, Heidmann DE, Anthony J, White SS, Hamblin MW, Szot P. Changes in 5-HT7 serotonin receptor mRNA expression with aging in rat brain. Mol Brain Res. 2000;79:163–168. doi: 10.1016/s0169-328x(00)00103-0. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, LeSauter J, Silver R. Targeted microlesions reveal novel organization of the hamster suprachiasmatic nucleus. J Neurosci. 2004a;24:2449–2457. doi: 10.1523/JNEUROSCI.5323-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, LeSauter J, Silver R. Targeted microlesions reveal novel organization of the hamster suprachiasmatic nucleus. J Neurosci. 2004b;24:2449–2457. doi: 10.1523/JNEUROSCI.5323-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvachnina E, Liu G, Dityatev A, Renner U, Dumuis A, Richter DW, Dityateva G, Schachner M, Voyno-Yasenetskaya TA, Ponimaskin EG. 5-HT7 Receptor is coupled to Gα subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J Neurosci. 2005;25:7821–7830. doi: 10.1523/JNEUROSCI.1790-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Park SH, Song WC, Waterhouse BD. Retrograde study of hypocretin-1 (orexin-A) projections to subdivisions of the dorsal raphe nucleus in the rat. Brain Res. 2005;1059:35–45. doi: 10.1016/j.brainres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Kriegsfeld LJ, Hon J, Silver R. Calbindin-D28K cells selectively contact intra-SCN neurons. Neuroscience. 2002;111:575–585. doi: 10.1016/s0306-4522(01)00604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Silver R. Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity. J Neurosci. 1999;19:5574–5585. doi: 10.1523/JNEUROSCI.19-13-05574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 2006;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, Bickford PC. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol Aging. 2004;25:315–324. doi: 10.1016/S0197-4580(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Morin LP. Destruction of serotonergic neurons in the median raphe nucleus blocks circadian rhythm phase shifts to triazolam but not to novel wheel access. J Biol Rhythms. 1998;13:494–505. doi: 10.1177/074873098129000327. [DOI] [PubMed] [Google Scholar]
- Molinari S, Battini R, Ferrari S, Possi L, Killcross AS, Robbins TW, Jouvenceau A, Billard JM, Dutar P, Lamour Y, Baker WA, Cox H, Emson PC. Deficits in memory and hippocampal long-term potentation in mice with reduced calbindin D28K expression. Proc Natl Acad Sci. 1996;93:8028–8033. doi: 10.1073/pnas.93.15.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, Jantos H. Effects of the 5-HT7 receptor antagonist SB-269970 microinjected into the dorsal raphe nucleus on REM sleep in the rat. Behav Brain Res. 2006;167:245–250. doi: 10.1016/j.bbr.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Mullins UL, Gianutsos G, Eison AS. Effects of antidepressants on 5-HT7 receptor regulation in the rat hypothalamus. Neuropsychopharmacology. 1999;21:352–367. doi: 10.1016/S0893-133X(99)00041-X. [DOI] [PubMed] [Google Scholar]
- Nagerl UV, Mody I, Jeub M, Lie AA, Elger CE, Beck H. Surviving granule cells of the sclerotic human hippocampus have reduced Ca2+ influx because of a loss of calbindin-D28k in temporal lobe epilepsy. J Neurosci. 2000;20:1831–1836. doi: 10.1523/JNEUROSCI.20-05-01831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier JF, Sexton TJ, Yracheta J, Diaz AM, Brownfield M. Localization of 5-HT7 receptors in rat brain by immunocytochemistry, in situ hybridization, and agonist stimulation of cFos expression. J Chem Neuroanat. 2001;21:63–73. doi: 10.1016/s0891-0618(00)00092-2. [DOI] [PubMed] [Google Scholar]
- Penev PD, Zee PC, Wallen EP, Turek FW. Aging alters the phase-resetting properties of a serotonin agonist on hamster circadian rhythmicity. Am J Physiol Regul Integr Comp Physiol. 1995;268:R293–R298. doi: 10.1152/ajpregu.1995.268.1.R293. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia G, Gonzalez-Espinosa C, Meneses A. An mRNA expression analysis of stimulation and blockade of 5-HT7 receptors during memory consolidation. Behav Brain Res. 2006;69:83–92. doi: 10.1016/j.bbr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia GS, Meneses A. Effects of the potential 5-HT7 receptor agonist AS 19 in an autoshaping learning task. Behav Brain Res. 2005;163:136–140. doi: 10.1016/j.bbr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Quintero JE, McMahon DG. Serotonin modulates glutamate responses in isolated suprachiasmatic nucleus neurons. J Neurophysiol. 1999;82:533–539. doi: 10.1152/jn.1999.82.2.533. [DOI] [PubMed] [Google Scholar]
- Rampon C, Peyron C, Gervasoni D, Pow DV, Luppi PH, Fort P. Origins of the glycinergic inputs to the rat locus coeruleus and dorsal raphe nuclei: a study combining retrograde tracing with glycine immunohistochemistry. Eur J Neurosci. 1999;11:1058–1066. doi: 10.1046/j.1460-9568.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, Hedlund PB. Mice lacking 5-HT7 receptors show specific impairments in contextual learning. Eur J Neurosci. 2004;19:1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- Schuhler S, Pitrosky B, Saboureau M, Lakhdar-Ghazal N, Pevet P. Role of the thalamic intergeniculate leaflet and its 5-HT afferences in the chronobiological properties of 8-OH-DPAT and triazolam in Syrian hamsters. Brain Res. 1999;849:16–24. doi: 10.1016/s0006-8993(99)01914-9. [DOI] [PubMed] [Google Scholar]
- Sleight AJ, Carolo C, Petit N, Zwingelstein C, Bourson A. Identification of 5-hydroxytryptamine7 receptor binding sites in rat hypothalamus: Sensitivity to chronic antidepressant treatment. Mol Pharmacol. 1995;47:99–103. [PubMed] [Google Scholar]
- Thomas DR, Melotto S, Massagrande M, Gribble AD, Jeffrey P, Stevens AJ, Deeks NJ, Eddershaw PJ, Fenwick SH, Riley G, Stean T, Scott CM, Hill MJ, Middlemiss DN, Hagan JJ, Price GW, Forbes IT. SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br J Pharmacol. 2003;139:705–714. doi: 10.1038/sj.bjp.0705290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K, Shibata S, Ueki S, Watanabe S. Effects of 5-HT1A receptor agonists on the circadian rhythm of wheel-running activity in hamsters. Eur J Pharmacol. 1992;214:79–84. doi: 10.1016/0014-2999(92)90099-p. [DOI] [PubMed] [Google Scholar]
- van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Yau JLW, Olsson T, Noble J, Seckl JR. Serotonin receptor subtype gene expression in the hippocampus of aged rats following chronic amitriptyline treatment. Mol Brain Res. 1999;70:282–287. doi: 10.1016/s0169-328x(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Ying SW, Rusak B. 5-HT7 receptors mediate serotonergic effects on light-sensitive suprachiasmatic nucleus neurons. Brain Res. 1997;755:246–254. doi: 10.1016/s0006-8993(97)00102-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kornhauser JM, Zee PC, Mayo KE, Takahashi JS, Turek FW. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, Fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70:951–961. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]


