Abstract
Bone Morphogenetic Protein (Bmp) signaling is critical for the development and patterning of the mouse pituitary from the initial induction of Rathke's pouch to cell specification in the anterior lobe. We examined the regulation of Bmp signaling during pituitary development by analyzing null embryos for noggin, a Bmp 2 and 4 antagonist. Noggin is expressed in the ventral diencephalon during Rathke's pouch induction, in the underlying cartilage plate during cell specification, and in the adult anterior pituitary gland. Noggin null embryos have a variable pituitary phenotype, which ranges from a rostrally displaced Rathke's pouch to induction of secondary pituitary tissue. While cell specification in the anterior pituitary appears normal, patterning in the ventral diencephalon is disrupted; Bmp4 activity is expanded resulting in Fibroblast growth factor 10 repression and in a rostral shift in the boundary between the Bmp4 and Sonic hedgehog expression domains. The expanded domain of Bmp4 activity also results in additional invaginations of oral ectoderm and can shift the position of Rathke's pouch or create secondary pituitary tissue. This work demonstrates the importance of attenuating the activity of Bmp signaling during pituitary induction in order to maintain the proper balance of signaling factors necessary for pituitary organogenesis.
Keywords: Ventral diencephalon, Rathke's pouch, dorsal-ventral patterning, ectopic pituitary gland
Introduction
Signaling molecules such as Bone Morphogenetic Protein (BMP), Fibroblast Growth Factor (FGF), Hedgehog, Notch, and Wnt are instrumental for the development of a diverse array of mammalian organs including the pituitary gland. In most organs there is evidence that multiple signaling pathways are operative and interact positively and negatively to direct organogenesis. For instance, Wnt signaling negatively regulates branching morphogenesis in the lung by inhibiting the activity of Bmp and Fgf, which promote branching (Dean et al., 2005). In the third pharyngeal arch, Sonic Hedgehog (Shh) and Bmp antagonize each other during the development of the parathyroid and thymus (Moore-Scott and Manley, 2005). In the pituitary, Bmp and Fgf signaling are thought to antagonize each other during pituitary cell specification (Ericson et al., 1998; Treier et al., 1998). Wnt, Shh, and Notch signaling are also important for pituitary development and can affect organ shape (Cha et al., 2004), proliferation (Treier et al., 2001), and cell specification (Raetzman et al., 2004; Treier et al., 1998; Treier et al., 2001). The challenge now is to determine how these various signaling pathways interact with each other during organogenesis in general, and also within the developing pituitary.
Chick/quail fate maps originally localized the origin of the pituitary to the midline of the anterior neural ridge in a region adjacent to the future ventral diencephalon (Couly and Le Douarin, 1988). More recent work has also identified homologous regions in zebrafish anterior neural ridge that harbor pituitary precursors (Dutta et al., 2005). More lateral regions in the anterior neural ridge give rise to the sensory placodes of the eyes and ears. Recent work in the chicken has shown that the precise integration of Wnt, Bmp, and Fgf signaling is necessary for specifying this pre-placodal region that arises from the anterior neural ridge (Litsiou et al., 2005). The specification of the pre-placodal region requires the attenuation of Bmp and Wnt signals. The Bmp antagonist Neuroblastoma, suppressor of tumorigenicity 1 (Nbl1 also known as DAN) and the Bmp and Wnt antagonist Cerberus are thought to mediate this attenuation (Litsiou et al., 2005), highlighting the roles that secreted antagonists play in modulating the activity of signaling factors.
After the specification of the pre-placodal region, BMPs continue to play important roles in the development and function of the pituitary. In mice, the pituitary is comprised of anterior, intermediate, and posterior lobes. The anterior and intermediate lobes arise from Rathke's pouch, an invagination of the oral ectoderm. The posterior lobe arises from an evagination of neural ectoderm from the ventral diencephalon, known as the infundibulum. The infundibulum induces the formation of Rathke's pouch from the oral ectoderm at embryonic day 9.0 (E9.0) (Gleiberman et al., 1999). Bmp4 is expressed in the ventral diencephalon at the contact point between the neural and oral ectoderm (Ericson et al., 1998; Treier et al., 1998), suggesting that Bmp4 could induce the formation of Rathke's pouch. In fact, Bmp4 homozygous null embryos fail to form Rathke's pouch, demonstrating the requirement of Bmp4 for this induction (Takuma et al., 1998).
Once Rathke's pouch is induced, it continues to proliferate and expand. Eventually it will separate from the oral ectoderm by E11.5. Bmp2 is expressed in the ventral portion of Rathke's pouch as well as in the adjacent ventral mesenchyme at E10.5, just before the pouch separates from the oral ectoderm (Ericson et al., 1998; Treier et al., 1998). Whether or not Bmp2 maintains this ventral expression pattern in Rathke's pouch (Ericson et al., 1998) or expands to encompass all of Rathke's pouch by E12.0 is unclear (Treier et al., 1998). The continued expression of Bmp4 in the infundibulum is also unclear, with one report showing Bmp4 expression declining by E11.5 (Ericson et al., 1998) and another showing Bmp4 expression being maintained at E13.5 (Treier et al., 1998). Ectopic expression of noggin, an extracellular inhibitor of both Bmp 2 and 4, within Rathke's pouch and the oral ectoderm prevents the expansion of the pouch (Treier et al., 1998). Therefore, BMP signaling is required for the proliferation and expansion of Rathke's pouch, regardless of the precise expression of Bmps in and around the pituitary.
Fgf8 is also expressed in the infundibulum beginning at E9.5 and continues through to E14.5 (Ericson et al., 1998; Treier et al., 1998). Mouse embryos homozygous null for Titf1 (also known as T/ebp and Nkx2.1) lose Fgf8 expression specifically in the ventral diencephalon, which results in a rudimentary Rathke's pouch (Takuma et al., 1998). In addition, mouse embryos homozygous null for Fgfr2, a candidate receptor for Fgf8 in the pituitary, also develop only a rudimentary Rathke's pouch (De Moerlooze et al., 2000). Both of these genetic defects result in an increase in apoptosis within the pituitary primordium, suggesting that Fgf signaling is a survival factor for Rathke's pouch (De Moerlooze et al., 2000; Takuma et al., 1998). Ectopic expression of Fgf8 in the anterior lobe of the pituitary dramatically increases the size of the anterior lobe, further supporting a role for Fgf signaling in proliferation of pituitary cells (Treier et al., 1998).
Once Rathke's pouch is established, undifferentiated pituitary cells proliferate around the lumen, with the area of most active proliferation located at the dorsal aspect of the pouch, presumably in response to signaling from the ventral diencephalon (Ikeda and Yoshimoto, 1991). Beginning at E11.5 some undifferentiated cells become post-mitotic, migrate ventrally and rostrally away from the lumen into the anterior lobe, and begin to express differentiation markers that signify their cell type specification (Ward et al., 2005). Bmp and Fgf signaling also influence the cell specification process. Expression of a dominant-negative Bmp receptor throughout the anterior lobe causes a loss of the Pit1 lineage of cell types, including somatotropes that secrete growth hormone (GH), lactotropes that secrete prolactin (Prl), and thyrotropes that secrete thyroid stimulating hormone (TSH) (Treier et al., 1998). Corticotropes, which secrete adrenocorticotropic hormone (ACTH), and gonadotropes, which secrete follicle stimulating hormone (FSH) and luteinizing hormone (LH), are unaffected. Ectopic expression of Bmp4 throughout the anterior lobe prevents the terminal differentiation of all cell types of the anterior lobe except corticotropes. This occurs despite the fact that cell type specific transcription factors are still expressed, indicating that attenuation of the Bmp signal is probably necessary to allow for terminal differentiation. Ectopic expression of Fgf8 also prevents the differentiation of all cell types except corticotropes and melanotropes, which secrete melanocyte stimulating hormone (MSH) from the intermediate lobe. Fgf8 inhibits the expression of cell type specific transcription factors including Pit1, Isl1, and Gata2, indicating that it disrupts differentiation at an earlier stage than ectopic Bmp expression. These experiments suggest that a ventral to dorsal gradient of Bmp2 in the anterior lobe interacts with a dorsal to ventral gradient of Fgf8 from the infundibulum to drive cell specification in the anterior lobe of the pituitary.
This gradient model for pituitary cell specification is supported by in vitro assays. Explants of infundibulum or beads soaked in Fgf2 promote the expression of Lhx3 and ACTH, dorsal markers in the anterior lobe for corticotropes, and inhibit the expression of Isl1 and αGSU, ventral markers in the anterior lobe for gonadotropes and thyrotropes (Ericson et al., 1998). In addition, COS cells expressing Bmp2 promote and expand Isl1 in expression Rathke's pouch explants, while inhibiting the expression of ACTH.
The model for interacting Bmp and Fgf gradients in the pituitary has been popular for some time, but there is little data for how those gradients are generated or regulated. A key component of morphogenetic gradients is the presence of extracellular proteins that inhibit the activity of the morphogen (Mizutani et al., 2005). There are at least seven known extracellular inhibitors of Bmps in vertebrates (Balemans and Van Hul, 2002). At present there is little information on the expression of either Bmp or Fgf antagonists in or around the pituitary. Only the expression of chordin, an antagonist of Bmp signaling, has been described. It is expressed in the mesenchyme caudal to Rathke's pouch, but its role in regulating Bmp signaling within Rathke's pouch is unknown (Treier et al., 1998).
We hypothesized that Bmp antagonists play an important role in regulating Bmp signaling during Rathke's pouch induction and anterior lobe cell specification. To test this idea we examined the expression pattern of other known Bmp antagonists in and around the pituitary including Nbl1, follistatin-like 1 (Fstl1), and noggin. Noggin is expressed in the ventral diencephalon in a domain overlapping that of Bmp4 at E9.5, and then in the underlying cartilage plate by E13.5. Its expression pattern suggests that it could potentially attenuate Bmp4 activity, influencing formation of Rathke's pouch and later reduce Bmp2 signaling from the ventral mesenchyme during cell specification. We report that noggin−/− embryos have pituitary defects that range from a lack of a morphological Rathke's pouch to the formation of secondary pituitary tissue. We show here that noggin attenuates the Bmp4 signal emanating from the ventral diencephalon during the induction and early patterning of Rathke's pouch, but it does not play a critical role in cell specification in the anterior lobe. The effects of noggin deficiency on pituitary development are likely to be a consequence of the imbalance in signaling factors that occurs in the ventral diencephalon as a result of increased Bmp signaling. We present a model for the integration of signaling pathways necessary to establish patterns of gene expression in the ventral diencephalon that are critical for normal pituitary development.
Materials and Methods
Mice
Noggin+/− mice on a C57BL/6 background (Nogtm1Amc), kindly provided by R. Harland (McMahon et al., 1998), were crossed to 129×1/SvJ mice (Jackson Laboratories) to create a hybrid line with enhanced breeding characteristics. All data included here were generated using this hybrid line, except where indicated. The hybrid line was later crossed to outbred CD-1 mice (Charles River Laboratories). The same range of phenotypes was observed in the both the hybrid line and in the outbred line.
Bmpr1a+/− mice (Bmpr1atm1Bhr), kindly provided by Y. Mishina (Mishina et al., 1995), were crossed to αGSU-cre mice (Tg(Cga-cre)Sac3) (Cushman et al., 2000) to create Bmpr1a+/−; αGSU-cre studs. These studs were crossed to Bmpr1afx/fx (Bmpr1atm2.1Bhr) (Mishina et al., 2002) females to generate the conditionally deleted Bmpr1a null embryos: Bmpr1afx/−; αGSU-cre.
Histology and gene expression detection
Noggin−/− embryos generated from heterozygous intercrosses were fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS), pH 7.2 overnight at 4 C. After washing, dehydrating, and embedding in paraffin, embryos were sectioned in a sagittal orientation at 6 [.proportional]m (Hogan et al., 1994). Sections were stained with hematoxylin and eosin (Hogan et al., 1994).
β-galatosidase activity from the noggin null allele was detected with X-gal staining in whole mount (Hogan et al., 1994). Noggin+/− embryos were also frozen in a dry ice/ethanol bath, and then embedded in O.C.T. Compound (Electron Microscopy Services). Frozen sections were cut at 16 μm, stained with X-gal, and counterstained with Neutral Red (Hogan et al., 1994).
RNA in situ hybridization was preformed as described previously (Cushman et al., 2001). Fstl1, Id3, and Six6 cDNAs were isolated from an embryonic pituitary cDNA library (Carninci et al., 2003) and used to generate antisense probes. Bmp2 (Lyons et al., 1989), Bmp4 (Jones et al., 1991), Nbl1 (Stanley et al., 1998), and Fgf10 (Bellusci et al., 1997) in situ hybridizations were reported previously.
Immunohistochemistry, Cell Proliferation, Cell Death
Immunohistochemistry was performed after boiling 10 min in 0.1 M citrate, pH 6.0 to unmask epitopes and incubation for 20 min at room temperature in 3% H2O2:Methanol 1:1 to inactivate endogenous peroxidases. Antibodies used were LHX3 and ISL1 (Developmental Studies Hybridoma Bank), SHH (R&D Systems), TLE3 (Chemicon), PITX1 (kind gift of J. Drouin) and αGSU, POMC, LH, TSH, and GH (National Hormone and Pituitary Program). Species appropriate biotinylated secondary antibodies were used in conjunction with streptavidin conjugated horseradish peroxidase (Vectastain MOM for mouse, and Vector Elite for rabbit and human (Vector Laboratories)). Fluorescein color reaction was performed with a tyramide signal amplification system (PerkinElmer LifeSciences). Sections were then counterstained with 4,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Molecular Probes).
Cell proliferation was assayed as described previously (Ward et al., 2005) by incorporation of 100 mg BrdU per g of body weight injected into pregnant mice 2 hours prior to harvesting embryos. BrdU was detected by immunohistochemistry using a rat antibody (Harlan Sera) as above.
Cell death was assayed using an In situ Cell Detection Kit POD (Roche).
Results
Noggin expression suggests a role in regulation of Bmp signaling
We re-examined the expression patterns for Bmp2 and 4, and compared them to the expression of a panel of Bmp antagonists that might regulate the activity of these morphogenetic proteins. We found that Bmp2 is expressed throughout Rathke's pouch at E12.5 and not just in the mesenchyme ventral to Rathke's pouch (Fig 1 A, (Ericson et al., 1998; Treier et al., 1998). Bmp4 expression continues in the infundibulum after E11.5 until at least E14.5 (Fig 1 B). Three antagonists, Fstl1, Nbl1 (Dan), and noggin had spatial-temporal expression patterns that imply a possible role for these proteins during pituitary development. Nbl1 begins its expression in the mesenchyme caudal to Rathke's pouch at E12.5 and increases in intensity by E14.5, expanding to encompass all of the mesenchyme surrounding the pituitary (Fig 1 C, D). Fstl1 begins its expression in the infundibulum at E12.5 (Fig 1 E). Fstl1 is also expressed in the mesenchyme surrounding the pituitary at E14.5, and it is strongly expressed in the infundibulum and intermediate lobe (Fig 1 F).
Fig. 1.
Pituitary expression patterns of Bmps and Bmp antagonists. (A-F) RNA in situ hybridization on wild type embryos. (A) Bmp2 at E12.5. (B) Bmp4 at E14.5 (C) Nbl1 at E12.5 (D) Nbl1 at E14.5 (E) Fstl1 at E12.5 (F) Fstl1 at E14.5 (G-L) Nog +/− embryos stained with with X-gal for β-galactodsidase activity. (G) An E9.5 embryo where the pharyngeal arches were removed to visualize the oral ectoderm. The arrow indicates expression in the ventral diencephalon. (H - J) Midsagittal sections counterstained with neural red. (H) An E9.5 embryo where the arrow indicates expression in the ventral diencephalon, while the arrowhead indicates Rathke's pouch, which lacks expression. (I) An E10.5 embryo with Nog expression in the ventral diencephalon. Inset shows Bmp4 expression in the same region of the ventral diencephalon. (J) An E12.5 embryo where the arrow indicates expression in the forming cartilage plate. (K) An E17.5 embryo where strong expression is seen in the cartilage plate, but no expression in the anterior pituitary (arrowhead). (L) Pituitary from an adult mouse. Strong expression is seen in the anterior lobe (a) compared to no expression in the posterior lobe (p). Scale bars equal 400 μm
Noggin is expressed in the ventral diencephalon at E9.5 and 10.5 (Fig 1 G, H, I) and then in the cartilage plate underlying the pituitary beginning at E12.5 and continuing through embryonic development (Fig 1 J, K). No expression was detected in Rathke's pouch at E9.5 or the pituitary anterior lobe at E10.5, 12.5, or E17.5. At weaning, noggin expression is detected in the anterior lobe (data not shown) where it continues its expression into adulthood (Fig 1 L). The noggin expression in the ventral diencephalon is coincident with the expression of Bmp4 (Fig 1 I inset), suggesting that in the absence of noggin, excess Bmp4 could emanate from the ventral diencephalon. Bmp2 is expressed in Rathke's pouch and the adjacent ventral mesenchyme beginning at E10.5 (Ericson et al., 1998; Treier et al., 1998). The expression of noggin in the cartilage plate adjacent to this ventral expression of Bmp2 could attenuate this signal.
Noggin is a more potent inhibitor of Bmp 2 and 4 than Nbl1 (Balemans and Van Hul, 2002). Nbl1 null mice also show no observable defects (Dionne et al., 2001). Fstl1 is assumed to inhibit Bmp and Activin based on its homology to follistatin (Balemans and Van Hul, 2002), but its biochemical interactions have not been demonstrated. Because noggin is a known, potent inhibitor of Bmp2 and 4 and because noggin null embryos have an observable embryonic phenotype, we chose to continue our analysis of noggin function in pituitary development.
Noggin deficient embryos exhibit pituitary dysmorphology
We examined noggin−/− embryos (McMahon et al., 1998) for a pituitary phenotype in order to determine if noggin regulates either the induction of Rathke's pouch by Bmp4, or cell specification by Bmp2. Noggin−/− embryos can have severe neural tube defects including exencephaly and spina bifida (McMahon et al., 1998). However, our observations suggest that contact between the ventral diencephalon and the oral ectoderm is typically maintained in noggin−/− embryos at E9.5 and 10.5 when Rathke's pouch induction occurs. Therefore, any defects in the pituitary resulting from a loss of noggin are probably not secondary to a broader forebrain defect.
We grouped noggin−/− embryos into an early pituitary development cohort (E11.5 - 14.5) and a late pituitary development cohort (E15.5 - 18.5). Noggin−/− mice have a variable pituitary phenotype (Fig 2 and Table 1) that includes an absence of a morphologically identifiable pituitary, an apparent secondary Rathke's pouch or anterior lobe, and rostral displacement of Rathke's pouch at both the early and late time points. The difference observed between the two time points is a decrease in the percentage of embryos with secondary pituitary tissue at the later stages.
Fig 2.
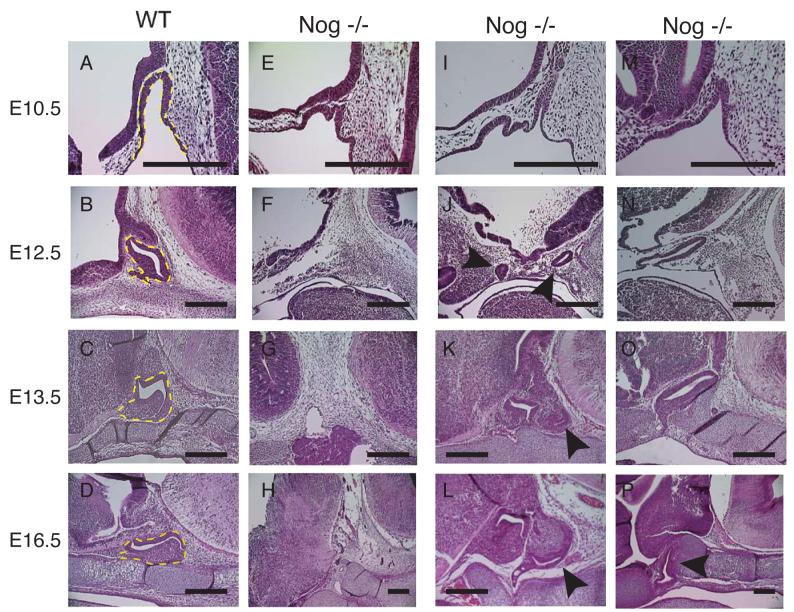
Phenotypic variability in nog−/− embryos. (A, B, C, D) Wild type embryos at E10.5, 12.5, 13.5, and 16.5, respectively. Yellow dotted line indicates Rathke's pouch and the anterior lobe. (E-P) Nog−/− embryos at (E, I, M) E10.5 (F, J, N) E12.5 (G, K, O) E13.5 (H) E15.5 (L & P) E16.5. A morphological pituitary is not visible in F, G, or H. Arrowheads in J, K, L indicate secondary pituitary tissue. Arrowhead in P indicates Rathke's pouch displaced more rostrally. Scale bars equal 400 μm.
Table 1.
Phenotypes observed in Nog−/− embryos at early and late stages of pituitary development.
| Developmental stage* | ||
|---|---|---|
| Phenotype | E11.5 – 14.5 | E15.5 – 18.5 |
| No Rathke's pouch | 3 (14%) | 3 (21%) |
| Rostral displacement | 10 (48%) | 8 (57%) |
| Secondary tissue | 6 (29%) | 2 (14%) |
| Normal location | 2 (10%) | 1 (7%) |
| Total number of embryos | 21 | 14 |
The number of embryos observed for each phenotype is listed with the percentage of the total included in parentheses.
At E10.5 Rathke's pouch is severely malformed (Fig 2 E, I, M). Multiple invaginations occur within the oral ectoderm (Fig 2 E, I), especially toward the rostral side of Rathke's pouch were it is in close proximity to the ventral diencephalon. When only one invagination is observed, Rathke's pouch appears enlarged and extends further rostrally compared to wild type embryos (Fig 2 M). Some individuals had no obvious pituitary tissue, and the cleft between the midbrain and hindbrain where the pituitary normally forms was filled with mesenchyme (Fig 2 F, G, H). In embryos that have duplications of Rathke's pouch the location of the two invaginations can be widely spaced (Fig 2J) or arise adjacent to each other (Fig 2K). By E16.5 the pituitary duplication can be very prominent (Fig 2L). In the most frequent phenotype, rostral displacement, Rathke's pouch is elongated and extends more rostrally than in wild type embryos (2 N). This elongated morphology can be maintained until later stages of development (Fig 2 O, P), including maintaining a connection with the oral ectoderm (Fig 2O). Eventually, the cartilage plate, which usually lies beneath the pituitary, can surround the mutant pituitary tissue, which appears to lack a developed anterior lobe (Fig 2P).
The ventral diencephalon of mutants has morphological abnormalities. The characteristic indention of the forming infundibulum at E10.5 (Fig 2A) is not observed in noggin−/− embryos (Fig 2 E, I, M). The characteristic thickening of the neuroepithelium in the ventral diencephalon just rostral to Rathke's pouch is (Fig 2 B) decreased or absent in noggin−/− embryos (Fig 2 F, J, N). A morphologically distinct infundibulum is frequently absent in noggin−/− embryos. Of the nine noggin−/− embryos examined between E12.5 and 16.5 that are presented in figure 2, only two (Fig 2 K, L) have an obvious infundibulum.
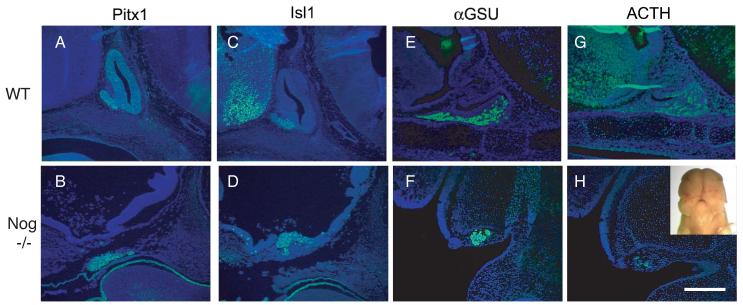
Despite the fact that 14% of noggin−/− embryos do not have an observable Rathke's pouch, two early markers of pituitary development, Pitx1 (Fig 3 A, B) and Isl1 (Fig 3 C,D), are clearly expressed at E12.5. This indicates that Rathke's pouch is induced, in terms of transcriptional activation of pituitary markers, but lacks the proper morphology. The domain of expression for these markers is also displaced rostrally, similar to the rostral displacement of pituitary tissue observed in the majority of mutant embryos. To determine if pituitary cell specification occurs in these embryos, an E15.5 embryo lacking a morphological pituitary was examined for the presence of the hormones αGSU (alpha subunit of the glycoprotein hormones TSH, LH, and FSH, also known as Cga) (Fig 3 E, F) and pro-opio-melanocortin (POMC) (Fig 3 G, H). POMC is proteolytically processed to produce ACTH in the anterior lobe and MSH and β-endorphin in the intermediate lobe. The embryo examined had a severe neural tube defect that altered its gross head morphology, splitting the embryo down the anterior midline (Fig 3 H inset). Both αGSU and POMC were expressed in a small domain located near the rostral end of the embryo, despite the severe disruptions in morphology, indicating that cell specification can occur in these embryos.
Fig. 3.
Pituitary tissue forms in embryos without a morphologically identifiable pituitary. (A-D) E12.5 mid-sagittal sections. (E-H) E15.5 mid-sagittal sections. (A, C, E, and G) Wild type embryos. (B, D, F, and H) Nog−/− embryos. (A, B) Pitx1 immunohistochemistry. (C, D) Isl1 immunohistochemistry. (E, F) αGSU immunohistochemistry. (G, H) ACTH immunohistochemistry. All sections counterstained with DAPI. Scale bar equals 400 [.proportional]m for all images.
We extended the cell specification and terminal differentiation analysis to both primary and secondary pituitary tissue at E17.5 and 18.5 using molecular markers of the anterior and intermediate lobes. Secondary pituitary tissue expresses Pitx1 at E17.5 confirming that this structure is specified as pituitary tissue (Fig 4 A, G). However, the hormones GH, LH, TSH, and POMC are not expressed in the secondary tissue (Fig 4 H, I, J, K). All of these hormones are detected in the primary anterior lobe of mutant embryos, indicating that cell specification is occurring in portions of the pituitary tissue in the absence of noggin. Given the variable phenotype of noggin mutant embryos, we examined additional representative mutant embryos at E18.5 (Fig 4 M – X). Noggin mutant embryos expressed all of the hormones examined, but at variable levels. We conclude that noggin is not required for cell specification, but it can affect the number of terminally differentiated cells produced. Prolactin expression was not detectable at these time points; therefore, we cannot rule out an affect of noggin on cell specification of lactotropes. At no time did we observe precocious expression of the hormones examined (data not shown). Occasionally, we observed a more pronounced effect on the expression of Tle3, a marker of the prospective intermediate lobe (Fig 4 F). In two out of five embryos examined at E16.5 to 18.5, Tle3 was absent from the intermediate lobe (Fig 4 R). The loss of Tle3 in the intermediate lobe may be responsible for the decrease in POMC expression that was also observed in these same embryos (Fig 4 Q).
Fig 4.
Cell specification detected in nog−/− pituitaries using immunohistochemistry for cell type specific hormones and transcription factors. All images are E17.5 or 18.5 mid-sagittal sections. (A - F) Wild type E18.5 embryos. (G - L) Nog−/− E17.5 embryos. (M – X) Nog−/− E18.5 embryos (A, G, M, and S) Pitx1 (B, H, N, and T) GH (C, I, O, and U) LH (D, J, P, and V) TSH (E, K, Q, and W) POMC (F, L, R, and X) Tle3. (G) 1 indicates primary pituitary, 2 indicates secondary pituitary tissue. (H, I, J, and K) Note the absence of terminal differentiation in the secondary pituitary. All images counterstained with DAPI. Scale bar equals 400 μm for all images.
Bmp Signaling is enhanced in the absence of noggin
The morphology of Rathke's pouch in noggin mutants at E10.5 suggests that multiple invaginations of the oral ectoderm may occur in response to increased BMP4 activity. To determine whether all of the invaginations had a Rathke's pouch identity, we carried out immunohistochemistry using the early transcription factor markers for Rathke's pouch, Pitx1, Isl1, and Lhx3. Both Pitx1 (Fig 5 A, B) and Isl1 (Fig 5 D, E) are expressed throughout the multiple invaginations. Lhx3, however, was not expressed at E10.5 in mutant embryos (Fig 5 G, H). Explant experiments have shown that Lhx3 is expressed in response to FGF signals from the infundibulum (Ericson et al., 1998); therefore, increased BMP4 activity in the ventral diencephalon may be antagonizing FGF signaling resulting in repression of Lhx3. In noggin−/− embryos at E16.5 Isl1 is still expressed in both the wild type and noggin−/− embryos (Fig 5 J, K). Expression of Lhx3 recovers at this time in the mutant embryos, suggesting that there are different requirements for initiation of Lhx3 transcription at different developmental times. This phenomena is also observed in Lhx4−/− mice (Raetzman et al., 2002).
Fig. 5.
Early markers of Rathke's pouch visualized by immunohistochemistry. (A-I) E10.5 mid-sagittal sections. (J-M) E16.5 mid-sagittal sections. (A, D, G, J, and L) Wild type embryos. (B, E, H, K, M) Nog−/− embryos. (C, F, and I) ?GSUcre: Bmpr1a fx/- embryos. (A-C) Pitx1 (B-F, J-K) Isl1 (G-I, L-M) Lhx3. Note the lack of expression of Lhx3 in H and lack of expression of Isl1 in F. All sections counterstained with DAPI. Scale bars in M equals 400 μm for all images except C, F, and I. Scale bar in I equals 400 μm for C, F, and I.
Bmpr1a is critical for Bmp signaling
Bmp4 null embryos fail to form Rathke's pouch, preventing an examination of Bmp signaling in Rathke's pouch following this initial induction (Takuma et al., 1998). In order to investigate the role of Bmp signaling in the early formation of Rathke's pouch, we used a conditional allele of Bmpr1a (Mishina et al., 2002) in combination with a cre recombinase driven by αGSU (Cushman et al., 2000), which begins expression in Rathke's pouch at E9.5 after the initial induction of the pouch. This αGSU-cre transgene is effective for deletion between loxP sites in the pituitary gland (Zhao et al., 2001); however, it exhibits ectopic cre activity in the heart and muscle (Cushman et al., 2000).
We analyzed Rathke's pouch patterning in αGSU-cre; Bmpr1a fx/- embryos at E10.5. The pouch is thin and under-developed compared to wild type littermates (Fig 5 C, F, I). Some commitment to pituitary organogenesis is evident by the expression of Pitx1 and Lhx3 (Fig 5 C, I); however, Isl1 expression is absent (Fig 5 F). Explant studies suggest that Bmp signaling induces Isl1 in the ventral aspect of the pituitary (Ericson et al., 1998). Therefore, Bmp induction of Isl1 in Rathke's pouch is dependent on Bmpr1a. Pituitary analysis at later time points is not possible because embryonic lethality occurs at E12.5. αGSU-cre; Bmpr1a fx/- embryos are smaller in size than wild type littermates and have heart defects, which is the probable cause of death (data not shown).
Loss of noggin alters patterns of cell death
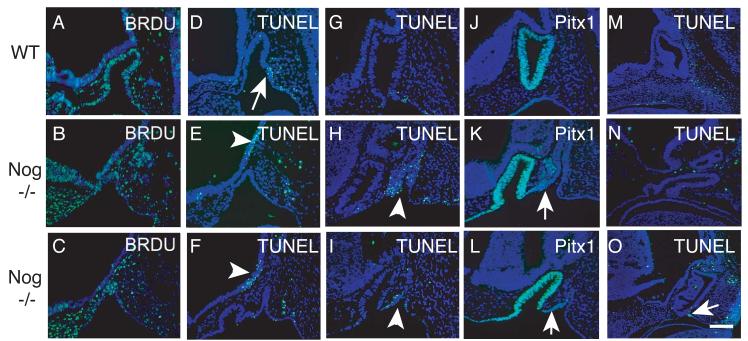
Bmp4 activity is necessary for the induction of Rathke's pouch and cell proliferation within the pouch (Takuma et al., 1998). An increased level of Bmp4 activity could cause an increase in proliferation. We injected pregnant mice with bromo-deoxyuridine (BrdU) 2 hours before harvesting E10.5 embryos in order to label actively dividing cells. BrdU incorporation was detected by immunohistochemistry. Proliferating cells in the ventral diencephalon are detected both rostral and caudal to the forming infundibulum in wild type embryos, but they are infrequently observed in the infundibulum itself at E10.5 (Fig 6 A). Proliferating cells are uniformly distributed throughout Rathke's pouch. We examined three noggin−/− embryos and observed variable results. Two embryos had an equal or greater percentage of proliferating cells in the ventral diencephalon relative to wild type (Fig 6B, Table 2). This trend was also true for cells in Rathke's pouch where considerable variability was also observed (Table 2). This variability in proliferation is not unexpected given the variable morphology and size of noggin−/− pituitaries.
Fig. 6.
Proliferation and cell death in noggin mutants. (A-B) Immunohistochemistry for BrdU incorporation. (A) E10.5 wild type mid-sagittal section. (B-C) E10.5 nog−/− mid-sagittal sections. (D-I) TUNEL analysis for cell death. (D) E10.5 wild type mid-sagial section. Arrow indicates cell death domain in Rathke's pouch that is absent in E. (E-F) E10.5 nog−/− mid-sagittal sections. Arrowhead indicates cell death domain in ventral diencephalon that is absent in D. (G) E11.5 wild type mid-sagittal section. (H-I) E11.5 nog−/− mid-sagittal sections. Arrowheads indicate cell death in secondary invagination. (J-L) Immunohistochemistry for Pitx1. (J) E11.5 wild type mid-sagittal section. (K-L) E11.5 nog−/− mid sagittal section. Arrows indicate lower levels of Pitx1 expression in the secondary invagination. (M) E12.5 wild type mid-sagittal section. (N-O) E12.5 nog−/− mid-sagittal sections from noggin outbred embryos. Arrow in O indicates cell death domain that is absent in N. Scale bar equals 400 μm for all images.
Table 2.
Quantitation of proliferating cells*
| Region | Wild type | Nog−/− | |||
|---|---|---|---|---|---|
| Ventral diencephalon | |||||
| BrdU positive cells | 18 | 14 | 27 | 8 | 23 |
| Total cells | 45 | 40 | 60 | 34 | 33 |
| Percent | 40 | 35 | 45 | 23 | 69 |
| Rathke's pouch | |||||
| BrdU | 80 | 57 | 14 | 36 | 29 |
| Total Cells | 111 | 77 | 51 | 57 | 53 |
| Percent | 72 | 74 | 27 | 63 | 55 |
Data are tabulated for two wild type and three noggin−/− embryos.
A small zone of cell death is normally located on the ventral side of Rathke's pouch and may be important for the separation of Rathke's pouch from the oral ectoderm (arrowhead in Fig 6 D). Because the noggin−/− embryos appear to have a delay in pinching off Rathke's pouch (Fig 2 J), we used TUNEL analysis to examine apoptosis and found that this domain is absent in noggin−/− embryos at E10.5, but already detectable in wild type littermates (Fig 6 E, F). Some noggin−/− embryos are able to undergo separation of Rathke's pouch from the oral ectoderm (Fig 2 K); therefore, we investigated whether there is a delay in apoptosis in noggin−/− embryos. At E11.5 wild type embryos still have apoptotic domains at the contact points between Rathke's pouch and the oral ectoderm (Fig 6 G). However, this apoptotic domain was not observed in E11.5 noggin−/− embryos (Fig 6 H, I). Apoptotic cells are observed in the secondary invaginations of oral ectoderm of Noggin−/− embryos (arrowheads Fig 6 H, I), where lower levels of Pitx1 expression are observed (Fig 6 K, L). This suggests that these secondary invaginations lack important survival factors, which could account for the decrease in secondary pituitary tissue in later stages of pituitary development. By E12.5 Rathke's pouch is fully separated from the oral ectoderm in wild type embryos and cell death is not detectable within Rathke's pouch (Fig 6 M). Cell death is absent in noggin−/− embryos that fail to undergo separation of Rathke's pouch (Fig 6 N). Apoptotic cells are evident in mildly affected embryos in a small domain at the contact point between the oral ectoderm and Rathke's pouch (Fig 6 O).
Apoptosis is not observed in the ventral diencephalon in wild type embryos (Fig 6C). Noggin−/− embryos do have a significant domain of cell death in the ventral diencephalon (arrowhead in Fig 6 D), suggesting that increased Bmp4 activity could promote apoptosis within this domain.
Loss of noggin expands Bmp activity in the ventral diencephalon
Because Noggin and Bmp4 interact extracellularly, no change in Bmp4 transcription in the ventral diencephalon of noggin mutants is expected. We confirmed this by examining three noggin−/− embryos for Bmp4 expression in the ventral diencephalon by in situ hybridization. There were no apparent differences in the signals observed in wild type and noggin−/− embryos, suggesting that Bmp4 transcription is not affected by the loss of noggin (data not shown).
We used two different techniques to determine whether Bmp signaling is increased in the absence of noggin. Id3 is an immediate response gene for Bmp signaling (Hollnagel et al., 1999), that had been uncharacterized in the developing pituitary gland. We determined that Id3 is normally expressed at low levels in the ventral diencephalon and Rathke's pouch at E10.5 (Fig 7 A). In noggin−/− embryos, Id3 is up regulated in the ventral diencephalon, indicating an increase in Bmp activity (Fig 7 B). We also assessed Bmp activity using immunohistochemical staining specific for the phosphorylated form of Smad1 (pSmad1), an intracellular signaling component of the Bmp pathway (Fig 7 C). Smad1 is phosphorylated in the forming infundibulum of wild type embryos where Bmp4 is expressed and in scattered cells within the ventral diencephalon domain rostral to the infundibulum (bracket Fig 7 C). Also, pSmad1 immunoreactivity is detected in the ventral half of Rathke's pouch, where expression of Bmp2 initiates, and immunoreactivity tapers off toward the dorsal side of the pouch (Fig 7 C). Noggin mutants exhibit increased pSmad1 staining at E10.5 in both the ventral diencephalon (bracket Fig 7 D) and in the dorsal domain of Rathke's pouch, confirming that noggin normally inhibits Bmp signaling (Fig 7 D).
Fig. 7.

Increased Bmp singaling in the ventral diencephalon disrupts ventral diencephalon patterning. All images are from E10.5 mid-sagittal sections. (A, C, E, G, and I) Wild type (B, D, F, H, and J) Nog−/− (A, B) Id3 in situ hydridization. Compare bracketed region in B to A. (C, D) Anti-phosphorylated Smad1 immunohistochemistry, counterstained with DAPI. Compare bracketed region in D to C. Boxed-in region in C and D shown at higher magnification in C' and D' (E, F) Fgf10 in situ hybridization. (G, H) Six6 in situ hybridization. (I, J) Shh immunohistochemistry, counterstained with DAPI. All paired in situ hybridizations were developed equally. Scale bar equals 400 [.proportional]m for all images.
To examine the effect of increased Bmp signaling on ventral diencephalon patterning, we performed in situ hybridization for Fgf10 and Six6, which mark adjacent domains within the ventral diencephalon (Fig 7 E, G). Six6 is expressed in the ventral diencephalon and within Rathke's pouch beginning at E10.5, where it is necessary for regulating proliferation within pituitary precursor cells (Jean et al., 1999; Li et al., 2002). Fgf10 is normally expressed in an overlapping pattern with Bmp4 and noggin. In the absence of noggin, Fgf10 expression is reduced and shifted rostrally (Fig 7 F). This could explain the lack of Lhx3 expression in noggin−/− embryos and the loss of secondary invaginations through apoptosis. Six6 marks a domain immediately rostral to the Bmp4 expression domain (Fig 7 G). In the absence of noggin, Six6 expression is also reduced and shifted rostrally (Fig 7 H). The wild type domain of Six6 expression in the ventral diencephalon is also marked by Shh (Fig 7 I). In noggin−/− embryos Shh is still expressed in this domain (Fig 7 J), although the domain appears to be decreased in size. Together this data suggests that the effective domain of Bmp activity has been expanded in the ventral diencephalon of the mutants. This expanded domain can, therefore, induce a broader region of oral ectoderm to become Rathke's pouch, producing the secondary pituitary tissue observed in some noggin−/− embryos. These results highlight the complex regulation of signaling molecules and their inhibitors that are necessary for normal pituitary organogenesis.
Discussion
Ectopic expression of noggin in Rathke's pouch and the oral ectoderm prevents expansion and proliferation of the pouch (Treier et al., 1998). Lack of expression results in failure of Rathke's pouch induction (Takuma et al., 1998). Together these observations provide convincing proof that Bmp signaling is required for the formation of Rathke's pouch. In a corollary experiment, Bmp4 expression was driven in the anterior lobe by a transgene prior to cell type determination, and it prevented terminal differentiation (Treier et al., 1998). While these transgenic experiments provide the basis for the current model for Bmp signaling in pituitary gland development, many questions remain regarding the in vivo role for Bmps and their inhibitors.
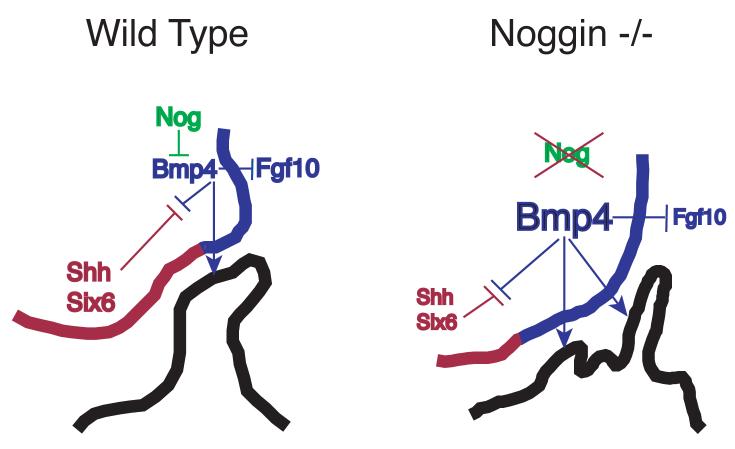
This report enhances our understanding of the endogenous roles of Bmp4 and noggin during pituitary development. We show that noggin is critical for modulating the inductive activity of Bmp4 in the formation of Rathke's pouch. Removing this inhibitor of Bmp signaling causes an expansion of the effective area of Bmp4 activity (Fig 8), which disrupts in the patterning of the ventral diencephalon. Noggin deficiency causes a rostral shift in the boundary between the Bmp4 expressing region (the upper domain) and the Shh expressing region (the lower domain) of the ventral diencephalon. Expanded Bmp signaling likely accounts for both the rostral shift in location of Rathke's pouch and the formation of multiple invaginations of oral ectoderm. The pituitary tissue produced by excessive Bmp activity is varied. The spectrum includes no morphologically discernable Rathke's pouch, a rostrally displaced Rathke's pouch, and formation of secondary pituitary tissue that is arrested in cell specification and likely lost through apoptosis.
Fig 8.
Model for how Noggin regulates Bmp4 during Rathke's pouch induction. In wild type embryos Noggin attenuates Bmp4 in the upper domain of the ventral diencephalon (blue). Bmp4 inhibits Fgf10, also in the upper domain, and establishes a boundary with the Shh and Six6 expressing lower domain (red). Bmp4 also induces Rathke's pouch from the oral ectorderm. In noggin−/− embryos, the attenuation of Bmp4 is lost, increasing the effective amount of Bmp4. An increased amount of Bmp4 inhibits Fgf10 within the upper domain, and shifts the boundary with the lower domain more rostrally. The increased amount of Bmp4 also causes multiple invaginations of the oral ectoderm.
Bmp and Bmp inhibitor expression suggest additional roles in pituitary development
The current models of pituitary development are built on expression data for Bmp 2 and 4 that are not consistent in the literature. In all reports Bmp4 is expressed in the ventral diencephalon and infundibulum; however, the timing of Bmp4 down regulation in the infundibulum is unclear. Our results indicate that Bmp4 is not down regulated at E11.0, but continues its expression at E14.5. The current model for anterior pituitary cell specification after E10.5 relies on an intrinsic program of signaling within Rathke's pouch that forms anterior lobe (Treier et al., 1998). However, both Fgf10 and Bmp4 are expressed in the infundibulum after E11.5 and could continue to influence pituitary development. While noggin−/− embryos can undergo anterior and intermediate lobe cell specification, the number of terminally differentiated cells is reduced or lost in a portion of the mutant embryos.
Bmp2 is expressed in the mesenchyme adjacent to Rathke's pouch at E10.5 – E12.5, but previous reports are not clear as to whether Bmp2 is expressed within Rathke's pouch. Our results indicate that Bmp2 is expressed throughout Rathke's pouch at E12.5, in addition to the expression in the adjacent ventral mesenchyme. The current model of cell specification describes a ventral to dorsal gradient of Bmp signaling that antagonizes a dorsal to ventral gradient of Fgf signaling. While such a gradient may exist at E10.5 when Bmp2 is restricted to the ventral portion of Rathke's pouch, it is difficult to imagine a gradient of Bmp2 activity at E12.5 when it is expressed throughout the pouch.
The presence of Bmp inhibitors could establish and maintain a ventral gradient of Bmp signaling within Rathke's pouch even as Bmp2 expands to the dorsal side. Prior to this report, chordin was the only Bmp antagonist known to be expressed near the pituitary (Treier et al., 1998). We show spatial and temporal expression of three different Bmp antagonists, which reveals the potential complexity of regulation of Bmp signaling. Nbl1 is expressed in the mesenchyme surrounding the entire pituitary, and Fstl1 is expressed in the mesenchyme, infundibulum, and intermediate lobe. The expression of Fstl1 suggests that it could regulate Bmp signaling within the anterior lobe, especially on the dorsal side. It will be interesting to assess the functional role of this and other Bmp antagonists within the developing pituitary.
Noggin deficiency causes severe morphological disruptions in pituitary development
The spatial and temporal expression pattern of noggin in the ventral diencephalon implies that noggin could regulate the induction of Rathke's pouch. Our analysis demonstrates that noggin is critical for regulating the size and location of Rathke's pouch. In the absence of noggin, there is an expanded domain of Bmp activity that results in a rostral shift in location of the oral ectoderm invagination and the production of secondary invaginations. Variable outcomes result from this variable field of induced pituitary tissue. The variability in phenotypes of noggin deficient mutants is not unique to the pituitary. Genetic background effects produce different phenotypes for noggin−/− embryos. Exencephaly occurs on the C57B/6J background, and not on 129X1/SvJ; however, exencephaly was a frequent phenotype on our hyrbrid background (data not shown) (McMahon et al., 1998). Background effects suggest that modifying genes influence Bmp signaling in the absence of noggin. Phenotypic variability has been described in other tissues when one or more inhibitors of Bmp are lost. Embryos of the complex genotype noggin+/− ;chordin−/− have a range of anterior defects from anencephaly to cleft palate (Anderson et al., 2002). Hesx1 deficiency also causes a range of pituitary and midline phenotypes. The most severely affected class of Hesx1−/− embryos lack both an infundibulum and Rathke's pouch, while the less severely affected have multiple branches of the lumen of Rathke' pouch (Dattani et al., 1998). Therefore, there are many precedents for the characteristic variability in pituitary development of noggin−/− embryos.
The expression of noggin in the cartilage plate underlying the pituitary does not appear to be important for pituitary cell specification. However, the expression of noggin in the cartilage is consistent with the role noggin plays in bone formation. In the absence of noggin the cartilage plate exhibits hypertrophy similar to that observed in the digits (Brunet et al., 1998).
The expression of noggin in the pituitary anterior lobe from weaning to adulthood is intriguing. A differential screen between prolactinomas and normal pituitary tissue showed that noggin is downregulated in the prolactinomas (Paez-Pereda et al., 2003). Further studies implicated upregulation of Bmp4 as a potential cause for prolactinoma formation (Paez-Pereda et al., 2003). Therefore, noggin may play a protective role against prolactinoma formation in adult pituitaries by inhibiting Bmp signaling. The embryonic lethality of noggin−/− embryos necessitates the development of a conditional allele of noggin to study noggin function in adult pituitaries and assess is potential contribution to this extremely common pituitary adenoma (Asa, 1998).
Ventral diencephalon patterning is disrupted noggin deficiency
Noggin is not expressed in Rathke's pouch; therefore, the variability seen in Rathke's pouch of noggin deficient embryos is not due to a direct effect of noggin action in the pouch. Noggin deficiency has an indirect effect on the responsiveness of the oral ectoderm to inductive influence of the ventral diencephalon. Gene expression analyses reveal a boundary in the ventral diencephalon just rostral to the infundibulum that separates the inductive domain from the remaining ventral diencephalon. Shh and Six6 are expressed rostral to the boundary. Bmp4 and Fgf10 are expressed caudal and dorsal to the boundary, within the upper domain. Noggin deficient mice exhibit expansion of Bmp signaling in the ventral diencephalon, as evidenced by an expanded area of Id3 expression and phosphorylated Smad1. This alters the patterning of the ventral diencephalon such that the upper domain expands rostrally, compromising the adjacent lower domain by reducing the expression of Shh and Six6. This increased Bmp signaling also results in a decrease in expression of Fgf10 within the upper domain.
Based on these data, we propose a model where the ultimate effect of noggin deficiency on pituitary development is enlargement and rostral shift of the inductive upper domain. This effect likely causes additional oral ectoderm to be recruited to form pituitary tissue. The ability of this additional tissue to form a properly patterned pituitary appears to depend on its placement in relation to the other structures in the developing head. If the pituitary is induced more rostrally than normal, then the pituitary tissue may fail to develop a proper Rathke’s pouch or fail to progress to form a proper anterior lobe. If secondary pituitary tissue is induced more caudally, then access to survival factors, such as Fgf10, is restricted, leading to cell death, tissue loss, and failed cell specification. Terminal differentiation may not occur in residual secondary pituitary tissue due to restricted access to the ventral diencephalon and factors it produces.
Cell death domains are lost in Rathke's pouch and gained in the ventral diencephlaon
The embryonic pituitary has a low level of apoptotic activity during normal development. This low level of activity is generally confined to the point where Rathke's pouch will separate from the oral ectoderm (Charles et al., 2005). Genetic mutations that cause a loss or reduction of Rathke's pouch can show an increase in apoptosis within the pouch. Fgfr2 IIIb−/− (De Moerlooze et al., 2000), Lhx4−/−;Prop1−/− (Raetzman et al., 2002), Pitx2−/− (Charles et al., 2005), and Titf1−/− (Takuma et al., 1998) embryos all exhibit an increase in apoptosis that directly contributes to the loss or reduction of Rathke's pouch. Unlike these mice, noggin−/− mice show a decrease in apoptosis that could prevent the separation of Rathke's Pouch from the oral ectoderm. In fact, the closure of Rathke's pouch is often delayed in noggin mutants, resulting in prolonged connection with the oral ectoderm. However, when separation does occur, apoptosis is observed at the contact point between the pouch and the oral ectoderm. This result supports the idea that apoptosis is necessary for Rathke's pouch to separate from the oral ectoderm.
The presence of apoptotic cells within secondary invaginations of oral ectoderm of noggin−/− embryos at E11.5 suggests that these secondary invaginations lack access to a critical survival factor, perhaps Fgf10. The down regulation of Pitx1 expression within these secondary invaginations supports the idea that this tissue is losing its pituitary identity. The loss of these invaginations through apoptosis may occur because the invaginating tissue is not close enough to the ventral diencephalon to survive and continue to develop. The down regulation of Fgf10 in noggin mutants may enhance this effect by removing a key survival factor for Rathke's pouch.
While noggin deficiency causes reduced apoptosis in Rathke's pouch, a new region of apoptosis forms in the upper domain of the ventral diencephalon. This region does not encompass the entire upper domain, and we are unaware of any ventral diencephalon markers that might identify the cell types that are lost. Apoptosis may affect the evagination of the ventral diencephalon to form the infundibulum, as a well-formed infundibulum is rarely seen in noggin mutant embryos.
Gain or loss of Bmp signaling in Rathke's pouch disrupts pattern formation
Noggin−/− embryos exhibit reduced Lhx3 expression, probably as a result of the decrease in Fgf10 from the ventral diencephalon (Fig 5 H). Lhx3 expression appears to be Fgf-independent at later stages as Lhx3 is expressed by E16.5 in noggin−/− embryos (Fig 5 J). The recovery of Lhx3 expression later in development also occurs in Lhx4−/− embryos, and may be sufficient to drive cell specification at these later embryonic stages.
Isl1 expression in Rathke's pouch is regulated by Bmp signaling. Explant studies of Rathke's pouch demonstrated that Bmp2 increases Isl1 expression in this tissue (Ericson et al., 1998), while transgenic expression of Bmp4 in the anterior lobe of the pituitary expands the domain of Isl1 within the anterior lobe (Treier et al., 1998). Wild type and noggin−/− embryos express Isl1 throughout Rathke's pouch. However, noggin mutants occasionally express Isl1 ectopically in the mesenchyme adjacent to Rathke's pouch. These ectopic Isl1 cells could represent a secondary field of pituitary tissue, similar to the area expressing pituitary markers in noggin mutants without a morphological Rathke's pouch.
In contrast to the noggin−/− embryos, Isl1 expression is lost in Rathke's pouch of Bmpr1a fx-; αGSU-cre embryos. By E10.5, Isl1 is down-regulated in the dorsal domain of Rathke's pouch, despite the continued expression of Bmp4. Loss of Bmpr1a within the pouch, therefore, prevents the expression of Isl1, demonstrating that Bmpr1a is necessary for expression of this gene. Bmpr1b is also expressed in Rathke's pouch by E9.5 (Yoshikawa et al., 2000); however, it is not able to compensate for the loss of Bmpr1a. Perhaps Bmpr1b is necessary for stimulating additional arms of the Bmp signaling cascade, such as Map kinase signaling. The expansion of Isl1 expression in noggin mutant embryos and the reduction of Isl1 expression in Bmpr1a fx/-; αGSU-cre embryos provide important evidence in intact animals that confirm the transgenic and in vitro data that suggested regulation of Isl1 expression by Bmp signaling.
Noggin deficiency does not alter cell specification in the anterior lobe
Cell specification in the anterior lobe can occur in the absence of noggin, as some noggin−/− embryos develop the full range of cell types in the anterior lobe. Noggin is instrumental for the correct size and position of the pituitary. Once the size and location of the pituitary is determined; however, cell specification occurs independent of noggin expression. In contrast, pituitary tissue developing more rostrally or caudally than appropriate fails to differentiate fully, presumably because it is too distant from the signaling centers of the ventral diencephalon.
The specification of the intermediate lobe is variably affected by the loss of noggin. Tle3 and POMC expression is variably reduced in the absence of noggin. Bmp signaling represses transcription of POMC (Nudi et al., 2005). An increase in Bmp activity in the infundibulum may represses POMC and Tle3 as well. The role of Tle3 in pituitary gland development is not well established. Tle3 may act as a co-repressor with engrailed homology domain containing transcription factors to prevent the differentiation of proliferating cells around the lumen (Brinkmeier et al., 2003), or it may act positively to regulate the differentiation of melanotropes in the intermediate lobe.
Models of pituitary duplications
The pituitary duplication that we report for noggin mutant embryos has not been noted in other mouse models; however, there are other mutations that lead to an expansion of Rathke's Pouch. Tcf4−/− embryos have an enlarged anterior lobe, which appears to result from the recruitment of additional oral ectoderm into Rathke's pouch (Brinkmeier et al., 2003). Rx−/− embryos do not form a traditional pituitary, but instead a large field of oral ectoderm is transformed into pituitary hormone producing cells that line the roof of the mouth, suggesting that additional tissue has been recruited to form pituitary tissue (Kozhemyakina et al., 2006).
Pituitary duplication occurs very rarely in humans (Kollias et al., 1995). The anterior, intermediate, and posterior lobes and the pituitary stalk are all duplicated. Patients with duplicated pituitaries also exhibit additional midline defects including cleft palate and basilar artery duplication. The cause for the duplication is often attributed to a split at the rostral end of the notochord causing a local duplication of midline structures (Kollias et al., 1995). Supporting the idea that excess notochord tissue can induce pituitary duplication, ectopically placed notochord induces invagination of surface ectoderm in chickens, producing a structure that is morphologically similar to Rathke's pouch (Gleiberman et al., 1999). The human cases of pituitary duplication are morphologically different from the secondary anterior and intermediate lobes produced in the absence of noggin in mice. The human duplications are symmetric around the midline, whereas the mouse noggin−/− embryos are duplicated in the rostral to caudal dimension. In addition, the infundibulum is not duplicated in noggin−/− embryos, while it is consistently duplicated in the reported human cases. Thus, the human pituitary duplications and the noggin−/− mouse pituitary duplications are unlikely to arise from the same mechanism.
This paper highlights the role that noggin performs in regulating the inductive activity of Bmp4 on Rathke's pouch. In addition, it establishes the complicated interplay between the various signaling factors and their inhibitors that are necessary for proper pituitary development. Rathke's pouch is induced in response to Bmp signaling emanating from the ventral diencephalon. Disruption in Bmp regulation, therefore, results in a severe morphological disruption of Rathke's pouch.
Acknowledgments
We would like to thank Kate Barald, Jacques Drouin, Richard Harland, John Klingensmith, and Yuji Mishina for materials and reagents supplied in support of this paper. We thank Michelle Brinkmeier, Mary Anne Potok, Lori Raetzman, and Deneen Wellik who provided critical reading of manuscript. This work was supported by a Reproductive Sciences Training Grant (NIH T32 HD07048) to S. Davis and NIH grants R37HD30428 and R01HD34283 to S. Camper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–87. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- Asa SL. Tumors of the Pituitary Gland. Armed Forces Institute of Pathology; Washington, D.C.: 1998. [Google Scholar]
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–50. [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan B. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–78. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Cha KB, Gridley T, Stifani S, Meeldijk J, Clevers H, Camper SA. TCF and Groucho-related genes influence pituitary growth and development. Mol Endocrinol. 2003;17:2152–61. doi: 10.1210/me.2003-0225. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–7. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- Carninci P, Waki K, Shiraki T, Konno H, Shibata K, Itoh M, Aizawa K, Arakawa T, Ishii Y, Sasaki D, Bono H, Kondo S, Sugahara Y, Saito R, Osato N, Fukuda S, Sato K, Watahiki A, Hirozane-Kishikawa T, Nakamura M, Shibata Y, Yasunishi A, Kikuchi N, Yoshiki A, Kusakabe M, Gustincich S, Beisel K, Pavan W, Aidinis V, Nakagawara A, Held WA, Iwata H, Kono T, Nakauchi H, Lyons P, Wells C, Hume DA, Fagiolini M, Hensch TK, Brinkmeier M, Camper S, Hirota J, Mombaerts P, Muramatsu M, Okazaki Y, Kawai J, Hayashizaki Y. Targeting a complex transcriptome: the construction of the mouse full-length cDNA encyclopedia. Genome Res. 2003;13:1273–89. doi: 10.1101/gr.1119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. WNT5A signaling affects pituitary gland shape. Mech Dev. 2004;121:183–94. doi: 10.1016/j.mod.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol. 2005;19:1893–903. doi: 10.1210/me.2005-0052. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM. The fate map of the cephalic neural primordium at the presomititc to the 3-somite stage in the avian embryo. Development. 1988;103(Suppl):101–113. doi: 10.1242/dev.103.Supplement.101. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA. Cre-mediated recombination in the pituitary gland. genesis. 2000;28:167–174. doi: 10.1002/1526-968x(200011/12)28:3/4<167::aid-gene120>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Watkins-Chow DE, Brinkmeier ML, Raetzman LT, Radak AL, Lloyd RV, Camper SA. Persistent Prop1 expression delays gonadotrope differentiation and enhances pituitary tumor susceptibility. Human Molecular Genetics. 2001;10:1141–1153. doi: 10.1093/hmg/10.11.1141. [DOI] [PubMed] [Google Scholar]
- Dattani MT, Martinez-Barbera J-P, Thomas PQ, Brickman JM, Gupta R, Mårtensson I-L, Toresson H, Fox M, Wales JKH, Hindmarsh PC, Krauss S, Beddington RSP, Robinson ICAF. Mutations in the homeobox gene HESX1/Hesx1 associated with septooptic dysplasia in human and mouse. Nature Genetics. 1998;19:125–133. doi: 10.1038/477. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol. 2005;286:270–86. doi: 10.1016/j.ydbio.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Dionne MS, Skarnes WC, Harland RM. Mutation and analysis of Dan, the founding member of the Dan family of transforming growth factor beta antagonists. Mol Cell Biol. 2001;21:636–43. doi: 10.1128/MCB.21.2.636-643.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Dietrich JE, Aspock G, Burdine RD, Schier A, Westerfield M, Varga ZM. pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development. 2005;132:1579–90. doi: 10.1242/dev.01723. [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Fedtsova NG, Rosenfeld MG. Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Dev Biol. 1999;213:340–53. doi: 10.1006/dbio.1999.9386. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacey E. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press; Plainview, New York: 1994. [Google Scholar]
- Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–45. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Yoshimoto T. Developmental changes in proliferative activity of cells of the murine Rathke's pouch. Cell and Tissue Research. 1991;263:41–47. doi: 10.1007/BF00318398. [DOI] [PubMed] [Google Scholar]
- Jean D, Bernier G, Gruss P. Six6 (Optx2) is a novel murine Six3-related homeobox gene that demarcates the presumptive pituitary/hypothalamic axis and the ventral optic stalk. Mech Dev. 1999;84:31–40. doi: 10.1016/s0925-4773(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Jones CM, Lyons KM, Hogan BL. Involvement of Bone Morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development. 1991;111:531–42. doi: 10.1242/dev.111.2.531. [DOI] [PubMed] [Google Scholar]
- Kollias SS, Ball WS, Prenger EC. Review of the embryologic development of the pituitary gland and report of a case of hypophyseal duplication detected by MRI. Neuroradiology. 1995;37:3–12. doi: 10.1007/BF00588511. [DOI] [PubMed] [Google Scholar]
- Kozhemyakina EA, Harfe B, Mathers PH. Rx is required for posterior pituitary and craniofacial development. Development. 2006 submitted. [Google Scholar]
- Li X, Perissi V, Liu F, Rose D, Rosenfeld M. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297:1180–1183. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–62. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes Dev. 1989;3:1657–68. doi: 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–52. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–37. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Mizutani CM, Nie Q, Wan FY, Zhang YT, Vilmos P, Sousa-Neves R, Bier E, Marsh JL, Lander AD. Formation of the BMP activity gradient in the Drosophila embryo. Dev Cell. 2005;8:915–24. doi: 10.1016/j.devcel.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Scott BA, Manley NR. Differential expression of Sonic hedgehog along the anterior-posterior axis regulates patterning of pharyngeal pouch endoderm and pharyngeal endoderm-derived organs. Dev Biol. 2005;278:323–35. doi: 10.1016/j.ydbio.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Nudi M, Ouimette JF, Drouin J. Bone morphogenic protein (Smad)-mediated repression of proopiomelanocortin transcription by interference with Pitx/Tpit activity. Mol Endocrinol. 2005;19:31329–42. doi: 10.1210/me.2004-0425. [DOI] [PubMed] [Google Scholar]
- Paez-Pereda M, Giacomini D, Refojo D, Nagashima AC, Hopfner U, Grubler Y, Chervin A, Goldberg V, Goya R, Hentges ST, Low MJ, Holsboer F, Stalla GK, Arzt E. Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk. Proc Natl Acad Sci U S A. 2003;100:1034–9. doi: 10.1073/pnas.0237312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol. 2004;265:329–40. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129:4229–39. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- Stanley E, Biben C, Kotecha S, Fabri L, Tajbakhsh S, Wang CC, Hatzistavrou T, Roberts B, Drinkwater C, Lah M, Buckingham M, Hilton D, Nash A, Mohun T, Harvey RP. DAN is a secreted glycoprotein related to Xenopus cerberus. Mech Dev. 1998;77:173–84. doi: 10.1016/s0925-4773(98)00139-7. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BLM, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke's pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes & Development. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, O'Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang P-T, McMahon AP, Rosenfeld MG. Hedgehog signaling is required for pituitary gland development. Development. 2001;128:377–386. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in pituitary gland growth. Mol Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- Yoshikawa SI, Aota S, Shirayoshi Y, Okazaki K. The ActR-I activin receptor protein is expressed in notochord, lens placode and pituitary primordium cells in the mouse embryo. Mech Dev. 2000;91:439–44. doi: 10.1016/s0925-4773(99)00320-2. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Steroidogenic Factor 1 (SF-1) is Essential for Pituitary Gonadotrope Function. Development. 2001;128:147–154. doi: 10.1242/dev.128.2.147. [DOI] [PubMed] [Google Scholar]