Abstract
1α,25(OH)2-vitamin D3 (1,25D) is considered a bone anabolic hormone. 1,25D actions leading to bone formation involve gene transactivation, on one hand, and modulation of cytoplasmic signaling, on the other. In both cases, a functional vitamin D receptor (VDR) appears to be required. Here we study 1,25D-stimulated calcium signaling that initiates at the cell membrane and leads to exocytosis of bone materials and increased osteoblast survival. We found that rapid 1,25D-induction of exocytosis couples to cytoplasmic calcium increase in osteoblastic ROS 17/2.8 cells. In addition, we found that elevation of cytoplasmic calcium concentration is involved in 1,25D anti-apoptotic effects via Akt activation in ROS 17/2.8 cells and non-osteoblastic CV-1 cells. In both cases, 1,25D-stimulated elevation of intracellular calcium is due in part to activation of L-type Ca2+ channels. We conclude that 1,25D bone anabolic effects that involve increased intracellular Ca2+ concentration in osteoblasts can be explained at two levels. At the single-cell level, 1,25D promotes Ca2+-dependent exocytotic activities. At the tissue level, 1,25D protects osteoblasts from apoptosis via a Ca2+-dependent Akt pathway. Our studies contribute to the understanding of the molecular basis of bone diseases characterized by decreased bone formation and mineralization.
Keywords: Akt, apoptosis, L-Ca channels, Rapid actions, Vitamin D3
1. Introduction
The bone anabolic hormone 1α,25(OH)2 vitamin D3 (1,25D) increases bone matrix formation by binding to the nuclear vitamin D receptor (VDR), which is abundantly expressed in osteoblasts 1,2. In addition, 1,25D exerts rapid, membrane-initiated actions that do not involve gene transactivation, and involve modulation of signal transduction pathways 3. Non-genomic actions that lead to bone formation include exocytosis of bone materials and regulation of cell survival 4. While genomic mechanisms have been extensively studied, the signaling involved in membrane-initiated 1,25D actions remains only partially understood.
Here we study 1,25D membrane-initiated calcium signaling in relation with bone anabolic functions in osteoblasts. Rapid 1,25D-promoted elevation of cytoplasmic Ca2+ concentration in osteoblasts has been known for several decades 5,6. However, its physiological significance remains obscure. We recently demonstrated, for the first time, that nanomolar concentrations of 1,25D promote exocytosis in osteosarcoma ROS 17/2.8 cells within 1-3 min 7,8. Calcium-dependent regulated exocytosis has been described in several cell systems in response to different stimuli 9. It has been shown that 1,25D rapidly potentiates L-type Ca2+ channel activities at low depolarizing potentials in the same cell line 10. Therefore, we investigated the hypothesis that 1,25D modulation of Ca2+ channel activities couples to exocytosis in bone cells, and thus contributes to bone formation.
Changes in intracellular Ca2+ concentration have been implicated in cell death and survival. It has been recently shown that 1,25D exerts anti-apoptotic effects in osteoblasts 11,12. To investigate the link between 1,25D-promoted changes in intracellular Ca2+, regulation of apoptosis and bone anabolic functions, we studied the involvement of L-type Ca2+ channels in cell death and survival in osteoblasts and non-osteoblastic cells expressing different levels of VDR.
Our study contributes to the understanding of the physiological significance of rapid 1,25D actions that involve changes in intracellular calcium in osteoblasts and their signaling pathway. More specifically, our results provide new insights into mechanisms of bone anabolic properties in response to this steroid hormone.
2. Materials and methods
Chemicals
1,25D (Biomol Research Laboratories Inc, Plymouth, PA) was stored as a stock solution in ethanol at –20° C, in the dark. Nifedipine, S(-) Bay K8644, ionomycin, etoposide, and staurosporine (STSP) were purchased from Sigma (St. Louis, MO).
Cell Culture
Rat osteosarcoma ROS 17/2.8 cells (kindly provided by A.W. Norman, University of California-Riverside) were cultured in Ham’s F-12 medium (Sigma) containing 5% fetal bovine serum (FBS, Sigma) and 5% Serum Plus (JRH Biosciences, Woodland, CA), with the addition of L-glutamine and antibiotics, and 1.1 mM CaCl2, at 37° C in a humidified 5% CO2 atmosphere, as described previously 13. CV-1 cells (kindly provided by A.W. Norman, University of California-Riverside) were cultured in DMEM medium (Sigma) supplemented with 10% FBS and antibiotics. Neuroblastoma-glioma hybridoma NG108-15 cells (a kind gift from M.E. Adams, University of California-Riverside) were cultured in DMEM medium supplemented with 1% HAT (hypoxanthine, aminopterin, and thymidine, Sigma), 10% FBS, L-glutamine and antibiotics.
Typically, cells were used 4-6 days after passage, at about 80% confluence. Culture medium was replaced by serum-free medium 24 h before treatment with 1,25D and/or the specified reagent. For apoptosis assays, cells were pretreated with 1,25D and/or specific reagent, or 0.01% ethanol for vehicle control, for an additional hour, and followed by 8 h exposure to 100 μM etoposide or 100 nM staurosporine (STSP) to induce apoptosis. For calcium measurements, 10 nM 1,25D or stimulating agent was added to the cells during the course of the experiments by means of automated injection.
Flow cytometry
Following apoptosis induction, cells were washed in ice-cold PBS buffer, trypsinized, centrifuged and resuspended in PBS buffer supplemented with 1% FBS. Cells were then fixed with methanol at 4°C, overnight, and stained with 0.2mg/ml μM propidium iodide (PI) at 37°C for 2 h. Percentage of apoptotic cells (M1 fraction) was measured with a FACScan flow cytometer (Becton Dickinson, Becton Drive Franklin Lakes, NJ).
Calcium measurements
Changes in intracellular Ca2+ concentration were measured with the calcium sensitive dye Fluo-4 (Fluo-4 NW calcium assay kit, Molecular Probes) in a multi detection microplate reader (Synergy HT, Bio-TECK Instruments, Winooski, VT) as described elsewhere 14. Briefly, ROS 17/2.8 cells were seeded in black walled, clear- bottomed 96 well plates (Costar, Corning Inc.) at a density of 15,000 cells/well. Cells were cultured for 3 days in Ham’s F-12 medium as described before, and transferred to serum free medium 24 hr before use. Cells were washed twice with Hank’s Hepes buffer and loaded with 100 μl of Fluo-4 NW dye mix according to the manufacturer’s protocol. The samples were excited at 494 nm and fluorescence emission was measured at 516 nm. Recordings were typically taken from each well, in a kinetic mode, for approximately 3 min.
Immunocytochemistry
Cells were cultured on cover slips in 35 mm Petri-dishes for 48 hours, fixed with 3.7% (v/v) formaldehyde (Sigma) at room temperature for 20 min, permeabilized with ice-cold ethanol for 5 min, and incubated with 5% goat serum at room temperature for 1 hour to reduce background staining. Cells were incubated with a primary antibody against VDR (C-20, Santa Cruz Biotechnology, Delaware, CA) at 4°C overnight, and a Cy3 anti-rabbit secondary antibody (Sigma Immunochemicals) (1:500 dilution) for 2 hours at room temperature. Nuclei were stained with 300 nM 4’-6-diamino-2-phenylindole dihydrochloride (DAPI, Molecular Probes). Immunostaining was visualized in an inverted Olympus IX50 fluorescence microscope. Images were taken with the Spot Pursuit digital camera (Diagnostic Instruments Inc. Sterling Heights, MI).
Western Blot analysis
Equal volumes of cell lysates were loaded and separated on 7.5-10 % SDS-PAGE gels, and transferred to PVDF membranes. After blockade with 5 % non-fat milk in TBST buffer, membranes were incubated with anti phospho-Akt (ser 473) primary antibody (Cell Signaling Technology, Cummings Center, Beverly, MA), or an anti-VDR antibody (C-20, Santa Cruz Biotechnology, Delaware, CA). Primary antibodies were detected with a horseradish peroxidase-conjugated secondary antibody (Pierce, Meridian Rd., Rockford, IL) and enhanced chemiluminescence. Blot density was digitalized and analyzed by using Un-Scan-It gel software (Silk Scientific Inc., Orem, UT).
Electrophysiology
Whole-cell capacitance, a measure for exocytosis, was recorded with the patch-clamp technique on single osteoblasts essentially as described before 7.
3. Results
We measured a rapid increase in cytoplasmic calcium in 4 days old osteoblastic ROS 17/2.8 cells immediately after the addition of 10 nM 1,25D in the presence of 1.3 mM external Ca2+ (Figure 1). This 1,25D-induced Ca2+ increase appears to be at least in part due to the activity of L-type Ca2+ channels present in the osteoblast plasma membrane, as reported before 5,10. We verified the presence of functional L-type Ca2+ channels in the plasma membrane of ROS 17/2.8 cells with the addition of 0.5 μM S(-) BayK 8644, as shown in Figure 1.
Figure 1.
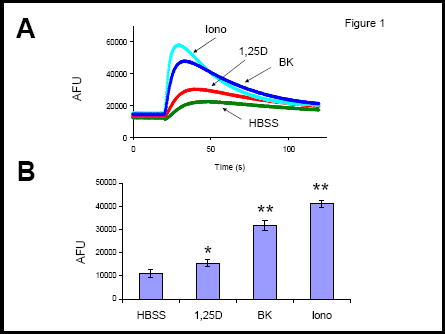
Calcium signals in ROS 17/2.8 cells. A:Changes of intracellular Ca2+ concentration as a function of time during the addition of saline (control, HBSS), 10 nM 1,25D, 0.5 μM S(-) Bay K8644, or 2 μM ionomycin. Traces correspond to arbitrary fluorescence units (AFU) measured in separate wells. Agents were delivered at the point of deflexion of the traces. B: Values obtained for the increase in AFUs calculated as the difference between the peak of maximum fluorescence and initial baseline value for each treatment. *, p<0.05; **, p<0.01; n=3. HBSS composition: 5.4 mM KCl, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 4.2 mM NaHCO3, 1.3 mM CaCl2, 0.5 mM MgCl2, 0.6 mM MgSO4, 137 mM NaCl, 5.6 mM glucose, pH 7.4.
We found that rapid 1,25D-induced elevation of intracellular Ca2+ develops in the same time frame as the stimulation of exocytosis by the hormone. We reported recently that nanomolar concentrations of 1,25D promote a rapid (sec-min) secretory response in osteoblasts and neuroblastoma-glioma cells8. We show here that the L-type Ca2+ channel antagonist nifedipine (2 μM) significantly reduced 1,25D-regulated exocytosis in ROS 17/2.8 osteoblasts, as measured with electrophysiology (Figure 2). In addition, 0.5 μM S(-)BayK 8644 caused partial stimulation of exocytosis. These results confirm the involvement of L-type Ca2+ channels in 1,25D-stimulated exocytosis in osteoblasts.
Figure 2.
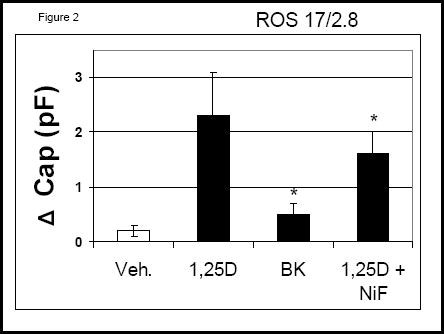
Changes in whole-cell capacitance measured on single ROS 17/2.8 cells after the addition of 10 nM 1,25D, 0.5 μM S(-) Bay K8644 (BK), and 2 μM nifedipine in combination with 10 nM 1,25D. *, p<0.05; n=5.
Anti-apoptotic effects of 1,25D in osteoblasts appear to involve the VDR and activation of the PI3K non-genomic pathway 11. In agreement with previous reports, we measured decreased levels of etoposide and staurosporine (STSP)-induced apoptosis in 1,25D-treated ROS 17/2.8 and non-osteoblastic CV-1 cells, which express significantly lower levels of VDR (Figures 3 and 4). We found that 10 nM 1,25D protects against STSP-induced apoptosis in both cell lines (see Figure 3). However, while the level of reduction of apoptosis in ROS 17/2.8 cells reached 68 ± 23 % of cells, we measured only 28 ± 10 % of 1,25D-induced reduction of apoptosis in CV-1 cells. Lower levels of 1,25D protection against apoptosis measured in CV-1 cells agree with lower VDR protein levels detected in this cell line (Figure 4).
Figure 3.
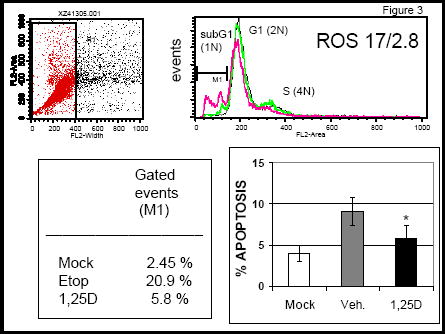
Apoptosis values measured in ROS 17/2.8 cells in the absence and presence of 10 nM 1,25D. Upper panels: Flow cytometry analysis of cell populations before induction of apoptosis (mock), after induction of apoptosis with 100 μM etoposide, and in the presence of 10 nM 1,25D. Apototic cells fall in the gated M1 region of the curve. Left lower panel: Percent of apoptosis measured in the absence (Veh.) and presence of 10 nM 1,25D. *, p<0.05, for the difference between treatments.
Figure 4.
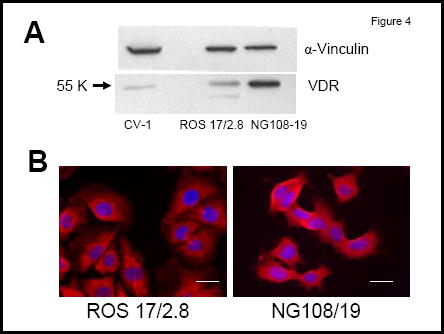
A: VDR protein levels as detected with Western blot analysis in CV-1, ROS 17/2.8, and NG108-15 cell lysates. B: Immunolocalization of VDR in ROS 17/2.8 and NG108/15 cells in the absence of 1,25D treatment. Cells were grown for 4 days in the presence of serum. Bars: 20 μm.
Sustained increase in intracellular calcium has been associated with apoptosis in a variety of cell systems 15. We found that prolonged treatment of ROS 17/2.8 cells with L-type Ca2+ channel modulators nifedipine (2 μM) and S(-) Bay K8644 (1 μM) in the absence of 1,25D, which lead to inactivation of the channels, mimics 1,25D protective effects against apoptosis, and increases ROS 17/2.8 cell survival (Figure 5A). Our results agree with previous observations that the potent L-type Ca2+ channel agonist S(-) Bay K8644 has inhibitory effects on the channel at concentrations as high as 1 μM used in this study 10. In addition, inhibitory effects of 1,25D on L-type Ca2+ channels have been described previously in ROS 17/2.8 cells for hormone concentrations of 10 nM and higher 10,13. Prolonged treatment of osteoblasts with 1,25D in combination with calcium channel modulators appears to have an overall decrease in intracellular calcium concentration that inhibits apoptosis16. We found that 1 μM of the potent L-type Ca2+ channel activator S(-) Bay K8644 did not modify 1,25D anti-apoptotic effects when used in combination with 10 nM 1,25D, and protected against STSP-induced apoptosis when applied alone to ROS 17/2.8 cells. From our results, we conclude that 1,25D anti-apoptotic effects in ROS 17/2.8 osteoblasts appear to involve reduced L-type Ca2+ channel activity associated with prolonged treatment with 1,25D.
Figure 5.
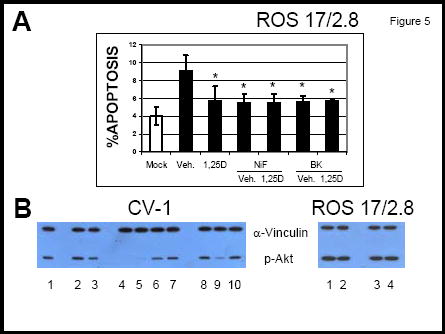
A: Percent of apoptotic cells measured after induction of apoptosis with 100 nM STSP. The reagents were added to the medium as described in the text. *, p<0.05, n= 3. B: Western blot analysis for the detection of p-Akt under the following conditions: (left panel) 1 and 8, vehicle (0.01% ethanol); 2, 10 nM 1,25D; 3, 100 nM 1,25D; 4, 10 nM 1,25D + 2 μM nifedipine; 5, 2 μM nifedipine; 6, 10 nM 1,25D + 2 μM thapsigargin; 7, 2 μM thapsigargin; 9, 10 nM 1,25D + 0.5 μM S(-) BayK 8644; 10, 0.5 μM S(-) BayK 8644; (right panel) 1, 0.5 μM Bay K8644; 2, 1 μM BayK 8644; 3, vehicle (0.01% ethanol); 4, 2 μM nifedipine.
1,25D anti-apoptotic effects have been reported to occur via PI3K activation in osteoblasts 11. Here we measured levels of phosphorylated Akt (p-Akt), a downstream molecule in the PI3K pathway involved in cell survival 17. We found that 1,25D increases p-Akt levels in ROS 17/2.8 as well as CV-1 cells (Figure 5B), which correlates with decreased apoptosis measured in both cell systems in the presence of the hormone (see Figures 3 and 5). As shown in Figure 5A, we found that 1,25D-activation of Akt is sensitive to L-type Ca2+ channel modulators in CV-1 cells. 1,25D failed to induce Akt phosphorylation in the presence of nifedipine. On the other hand, 2 μM thapsigargin or 0.05 μM Bay K8644 with and without 10 nM 1,25D caused a significant increase in p-Akt levels. This suggests that rapid elevation of intracellular Ca2+ concentration from internal stores in combination with L-type Ca2+ channels may be involved in the initial steps of 1,25D signaling to CV-1 cell survival. In addition, we found a slight increase in p-Akt levels in ROS 17.2.8 cells in the presence of L-type Ca2+ channel activator Bay K8644 but not the calcium channel inhibitor nifedipine, as shown in Figure 5B. This indicates that different steps in the anti-apoptotic pathway have different sensitivities to Ca2+ ions that enter the cell via Ca2+ channels present in the plasma membrane.
4. Discussion
Rapid or sustained 1,25D-stimulated elevation of intracellular calcium concentration through L-type Ca2+ channels appear to be required for different physiological responses in osteoblasts. We showed that 1,25D-stimulated exocytosis, which develops within seconds to minutes, depends in part on the activity of L-type Ca2+ channels present in the plasma membrane. On the other hand, prolonged exposure (hours to days) to nanomolar concentrations of the hormone, as well as calcium channel inhibitors, appears to protect against apoptosis, showing that intracellular Ca2+ reduction leads to the activation of cell survival pathways. Initial steps in anti-apoptotic actions of 1,25D, however, show sensitivity to intracellular Ca2+ elevation, such as 1,25D-induction of p-Akt. Interestingly, 1,25D-induced Akt activation via intracellular calcium modulators was found to be more accentuated in CV-1 cells, despite their lower VDR levels. This emphasizes on the critical role that cytoplasmic Ca2+ increase via plasma membrane channels and internal stores in the proximity to the plasma membrane play in early (rapid) stages of cell survival signalling.
Our results confirm a direct participation of 1,25D-sensitive Ca2+ channels in exocytosis. We found that 1,25D promotes a rapid exocytotic response in undifferentiated neuroblastoma-glioma NG108-15 cells (data not shown), which express significantly higher levels of VDR as well as L-type Ca2+ channels. In comparison, 1,25D-induced exocytosis in NG108-15 cells was significantly higher than in ROS 17/2.8 cells.
By prolonging osteoblast survival via PI3K/Akt pathway, 1,25D contributes to bone anabolic functions. We conclude that membrane-initiated 1,25D bone anabolic effects in osteoblasts can be explained at least at two levels: at the single-cell level, by promoting exocytotic activities, and at the tissue level, by protecting osteoblasts, the bone-forming cells, from apoptosis. Our studies contribute to the understanding of the molecular basis of degenerative diseases characterized by reduced extracellular matrix secretion and high cell death, and the potential therapeutic effect of steroid hormones.
Acknowledgments
Funded by a USPHS grant DK-071115-01 to LPZ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kraichely DM, MacDonald PN. Transcriptional activation through the vitamin D receptor in osteoblasts. Front Biosci. 1998;3:D821–D833. doi: 10.2741/a325. [DOI] [PubMed] [Google Scholar]
- 2.Boivin G, Mesguich P, Pike JW, Bouillon R, Meunier PJ, Haussler MR, Dubois PM, Morel G. Ultrastructural immunocytochemical localization of endogenous 1α,25-dihydroxyvitamin D 3 and its receptors in osteoblasts and osteocytes from neonatal mouse and rat calvaria. J Bone Miner Res. 1987;3:125–136. [PubMed] [Google Scholar]
- 3.Farach-Carson MC, Ridall AL. Dual 1,25-dihydroxyvitamin D3 signal response pathways in osteoblasts: Cross-talk between genomic and membrane-initiated pathways. Am J Kidney Dis. 1998;31(4):729–742. doi: 10.1053/ajkd.1998.v31.pm9531195. [DOI] [PubMed] [Google Scholar]
- 4.Zanello LP, Norman AW. Electrical responses to 1α,25(OH)2-vitamin D3 and their physiological significance in osteoblasts. Steroids. 2004;69:561–565. doi: 10.1016/j.steroids.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Lieberherr M. Effects of vitamin-D3 metabolites on cytosolic free calcium in confluent mouse osteoblasts. J Biol Chem. 1987;262:13168–13173. doi: 10.1515/9783110846713.769. [DOI] [PubMed] [Google Scholar]
- 6.Baran DT, Sorensen AM, Shalhoub V, Owen T, Oberdorf A, Stein G, Lian J. 1,25-Dihydroxyvitamin D3 rapidly increases cytosolic calcium in clonal rat osteosarcoma cells lacking the vitamin D receptor. J Bone Miner Res. 1991;6:1269–1275. doi: 10.1002/jbmr.5650061202. [DOI] [PubMed] [Google Scholar]
- 7.Zanello LP, Norman AW. Rapid modulation of osteoblast ion channel responses by 1α,25(OH)2-vitamin D3 requires the presence of a functional vitamin D nuclear receptor. Proc Natl Acad Sci U S A. 2102004;101:1589–1594. doi: 10.1073/pnas.0305802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanello LP, Norman AW. Steroid hormone-regulated exocytosis in osteoblasts. Biophysical Journal. 2004;86:171a. [Google Scholar]
- 9.Barclay JW, Morgan A, Burgoyne RD. Calcium-dependent regulation of exocytosis. Cell Calcium. 2005;38:343–353. doi: 10.1016/j.ceca.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Caffrey JM, Farach-Carson MC. Vitamin D3 metabolites modulate dihydropyridine-sensitive calcium currents in clonal rat osteosarcoma cells. J Biol Chem. 1989;264:20265–20274. [PubMed] [Google Scholar]
- 11.Vertino AM, Bula CM, Chen J-R, Almeida M, Han L, Bellido T, Kousteni S, Norman AW, Manolagas SC. Nongenotropic, anti-apoptotic signaling of 1alpha,25(OH)2-vitamin D3 and analogs through the ligand binding domain of the vitamin D receptor in osteoblasts and osteocytes. Mediation by Src, phosphatidylinositol 3-, and JNK kinases. J Biol Chem. 2005;280:14130–14137. doi: 10.1074/jbc.M410720200. [DOI] [PubMed] [Google Scholar]
- 12.Hansen CM, Hansen D, Holm PK, Binderup L. Vitamin D compounds exert anti-apoptotic effects in human osteosarcoma cells in vitro. J Steroid Biochem Mol Biol. 2001;77:1–11. doi: 10.1016/s0960-0760(01)00033-4. [DOI] [PubMed] [Google Scholar]
- 13.Zanello LP, Norman AW. Stimulation by 1a,25(OH)2-vitamin D3 of whole cell chloride currents in osteoblastic ROS 17/2.8 cells: A structure-function study. J Biol Chem. 1997;272(36):22617–22622. doi: 10.1074/jbc.272.36.22617. [DOI] [PubMed] [Google Scholar]
- 14.Orriss Isabel R, Knight Gillian E, Ranasinghe Sam, Burnstock Geoffrey, Arnett Timothy R. Osteoblast responses to nucleotides increase during differentiation. Bone. 2006 doi: 10.1016/j.bone.2006.02.063. Epub. [DOI] [PubMed] [Google Scholar]
- 15.Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 16.Ares MP, Porn-Ares MI, Thyberg J, Juntti-Berggren L, Berggren PO, Diczfalusy U, Kallin B, Bjorkhem I, Orrenius S, Nilsson J. Calcium channel blockers verapamil and nifedipine inhibit apoptosis induced by 25-hydroxycholesterol in human aortic smooth muscle cells. J Lipid Res. 1997;38:2049–2061. [PubMed] [Google Scholar]
- 17.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


