Abstract
Purpose
To characterize the effects of visual form deprivation by diffuser in marmoset monkey eyes across a range of ages.
Methods
Twenty-four common marmosets were grouped by onset of deprivation (group 1: 0-39 days, n = 6; group 2: 40-99 days, n = 10; and group 3: 100-200 days, n = 8). Monocular form deprivation was induced with a white translucent diffuser worn for 28 to 88 days (mean durations: group 1, 32 days; group 2, 56 days; and group 3, 51 days). Refractive state, corneal curvature, and vitreous chamber depth were measured after cycloplegia. Both experimental and control eyes were measured multiple times before, during, and after the visual deprivation period.
Results
Marmosets in all age groups tested were susceptible to visual form deprivation myopia; however, the response to form deprivation was variable and included a majority with axial myopia (n = 15), several nonresponders (n = 4), a single late responder (axial myopia after the end of deprivation period), and several axial hyperopes (n = 4). For all animals that responded with axial myopia, the increase in vitreous chamber depth and myopia was inversely proportional to the age of onset of deprivation (ANOVA, P < 0.05). After the end of the period of deprivation, recovery from myopia by reduction of the axial growth rate was observed in three animals from group 1 and three animals from group 2.
Conclusions
Form deprivation by diffusers disrupted emmetropization in marmosets over a range of ages. The responses varied among individuals and with age, suggesting that the maturity of the eye may influence the response to visual signals responsible for form deprivation myopia and perhaps emmetropization. Recovery from diffuser-induced form deprivation myopia was apparent in some animals, in contrast to that reported for visual deprivation by lid-suturing, and appears more prevalent in the younger animals.
Experimental studies with animals of different species have established that vision plays an important role in the control of eye growth and the development of refractive state (e.g., Refs. 1-5). The discovery that visual form deprivation results in axial elongation and myopia was the first evidence, and subsequent studies with defocusing lenses have strongly supported this conjecture (for recent reviews, see Refs. 6,7). Since the original observations in tree shrews,8 macaques,9 and chicks,10 many investigators have used form deprivation myopia as the paradigm to examine the mechanisms of eye growth control.
In primates, form deprivation myopia has been produced by lid suture,9,11-13 translucent diffusers (also called occluders) mounted over the eye,14 or diffusing lenses, with various degrees of transmission.15 Although axial myopia is the most frequently reported result of form deprivation in primates, the susceptibility clearly differs between individuals in all studies, and responses other than myopia have occasionally been noted. Lid suture in primates produces axial hyperopia in some individuals,11,12,16 and, in other studies, myopia was induced in some animals after a brief period of form deprivation that initially resulted in hyperopia from a combination of corneal flattening and reduced axial length.13,15 Similar findings have also been reported in tree shrews,17 a species considered to be closely related to primates.
Most studies in which form deprivation has been used for experimental manipulation of eye growth in primates have been performed on neonates or immature subjects. In general, more myopia is produced faster in younger animals,6 but recent studies have shown that mature subjects are also susceptible to form deprivation myopia, although it is reduced in magnitude compared with that in neonatal primates.18,19 This finding leads to the questions of how the eye-growth response characteristics change with age and whether different mechanisms are involved at different ages. In this study, we examined the effects of form deprivation by using diffusers in marmoset monkeys across a range of ages of <200 days, which spans the phase of rapid ocular growth from young juvenile through prepubescent animals. Some of the results presented herein have been published in a preliminary report (Troilo D, et al. IOVS 2002;43:ARVO E-Abstract 186).
In addition to studying the effects of form deprivation as a function of age, we examined the possibility of recovery from experimentally induced myopia in marmosets. Recovery from form deprivation myopia may involve the same visual guidance mechanisms that result in emmetropization during normal development and in compensation for defocus produced by spectacle lenses, although other factors related to the size and shape of the eye may also be involved.20-22 Furthermore, recovery does not occur consistently in primates. Lid-suture-induced myopia in macaques12,23,24 and marmosets13 persists well after the end of the period of visual form deprivation. However, this appears to be related to the lid-suture manipulation. Form deprivation myopia produced with diffusers shows recovery after the deprivation is discontinued in tree shrews25 and macaques,24,26 and preliminary studies using form deprivation diffusers in marmosets reported recovery in some animals13 (Troilo D, et al. IOVS 2000;41:ARVO Abstract 691).
In this article we characterize the various responses and effects of age on form deprivation myopia in a New World primate, the common marmoset. Our results suggest that age and the maturity of the eye and visual system may alter visually guided eye growth. This study also provides a guide for studying form deprivation myopia in marmosets at different ages and will be useful for additional studies in this species examining the mechanisms of eye growth control, including lens-induced refractive changes in which the experimental manipulation of vision allows some degree of feedback control.
METHODS
Twenty-four common marmosets (Callithrix jacchus) were used in the study. All animals were bred and housed in family groups in our animal facility. Artificial lighting was provided by daylight-balanced fluorescent lamps (Durotest; Vita-Light, Philadelphia, PA) on a 12-hour light-dark diurnal cycle. Temperature was maintained at 75 ± 2°F with 45% ± 5% humidity. Food and water were provided ad libitum within the animal’s home cage. Food consisted of a formulated dry pellet (Mazuri New World Diet 5MA5; PMI Feeds, Richmond, IN) with regularly varied supplements of fresh fruit and protein. All animals in our facility are given regular access through a flexible 4-m-long tubular run to a remote activity cage containing large branches for climbing and a variety of toys for enrichment purposes. The home cages contained a nest box, perches, and branches for climbing. All animal care and work performed in this study conforms to U.S. Department of Agriculture standards and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Monocular form deprivation was induced in all subjects with full-field, white translucent hemispheric diffusers (also called occluders) identical with that used in several studies of myopia in chicks.1,10,27 The contralateral eye was left untreated and served as a control in all animals. The marmosets were randomly sorted into three groups by their age at onset of form deprivation: Group 1 (n = 6) began visual deprivation between 0 and 39 days of age (mean age at onset, 27 ± 10 days), for a mean duration of 32 ± 3 days. Group 2 (n = 10) began the deprivation between 40 and 99 days of age (mean age at onset, 58 ± 19 days), for a mean duration of 56 ± 14 days. Group 3 (n = 8) began the deprivation between 100 and 200 days (mean age at onset, 158 ± 32 days), for a mean duration of 51 ± 19 days. Figure 1 shows the axial lengths of eyes in 181 binocularly untreated marmosets as a function of age (both eyes plotted with some repeated measures at different ages) and illustrates the axial growth rates of cohorts in the three experimental groups during the experimental manipulations. The rates of growth in the untreated eyes in the corresponding groups were determined from linear regression (group 1 = 0.043 mm/d, r = 0.803; group 2 = 0.022 mm/d, r = 0.812; and group 3 = 0.007 mm/d, r = 0.678). According to the postnatal stages defined by Missler et al.,28 group 1 represented infancy in marmosets and spans a period of rapid postnatal ocular growth. Group 2 included juvenile marmosets, in which the rate of eye growth was appreciably slower. In group 3, eye growth was slowest; this age corresponds to adolescence in marmosets. Sexual (but not social) maturity occurs at ∼300 days in marmosets. Some eye growth remained for the animals in group 3. (For a study of deprivation effects in mature marmosets, see Ref. 18.)
FIGURE 1.
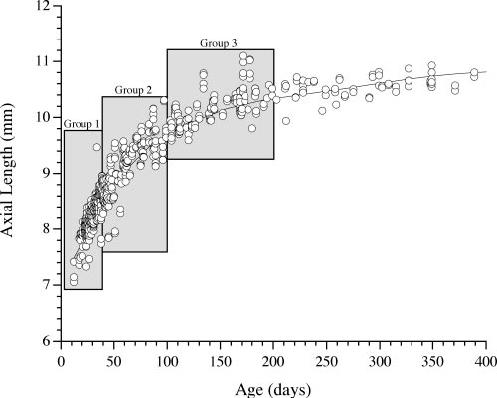
Axial length in untreated marmoset eyes as a function of age. A locally weighted curve fit with a 50% smoothing factor was used to illustrate the axial length growth curve. The three age groups corresponding to the ages of the experimental marmosets examined in this article are indicated for comparison. The average growth rate clearly diminished from group 1 through 3. The average rate of axial growth for untreated eyes in each group was estimated by linear regression.
Ocular measurements were performed on the experimental and control eyes of all marmosets before the onset of deprivation and several times during and after the deprivation period, to monitor the change in refractive error and the ocular components. All measures were performed 1 hour after 2 drops of 1% cyclopentolate was applied to achieve cycloplegia. Refractive state is given as the spherical equivalent averaged from retinoscopy and Hartinger refractometry. Refractions from one eye from each of a separate group of 41 randomly selected animals (mean age, 117 days; range, 23-331 days; range of refractive error: -9.6 to +10.6 D) were used to determine measurement repeatability. Using the statistical method to determine the reliability of repeated measures originally described by Bland and Altman29 and adopted for use in ocular measures by Zadnik et al.,30 the 95% confidence interval for agreement between repeated refractions was ±1.5 D (mean ± SD difference between repeated measures, -0.3 ± 0.8 D). Corneal curvature was averaged from three or more measures from an infrared videokeratometer.31 The 95% confidence interval for repeated measures was ±0.027 mm (mean ± SD difference between repeated measures, 0.0 ± 0.014 mm). The vitreous chamber depth of the eye was measured with high-frequency A-scan ultrasonography (for details, see Ref. 32). The 95% confidence interval was ±0.033 mm (mean ± SD difference between repeated measures, 0.0 ± 0.017 mm). Marmosets were anesthetized before refractometry and ultrasonography for tractability with a mixture of alphadolone acetate and alphaxalone (Saffan; Pittman-Moore, Harefield, UK).
RESULTS
Considering all the marmosets in this study together, regardless of age, form deprivation by diffusers produced significant myopia in the experimental eyes at the end of the deprivation period (experimental versus control, mean ± SD: -2.8 ± 4.8 D vs. -0.2 ± 2.3 D; paired t-test, P < 0.01). The length of the vitreous chamber of the experimental eyes was, on average, 0.114 ± 0.210 mm longer than in the control eyes (6.31 ± 0.63 mm vs. 6.19 ± 0.67 mm; paired t-test, P < 0.01). The interocular difference (experimental eye minus control eye) in refraction observed at the end of deprivation was well predicted by the interocular difference observed in vitreous chamber depth (r2 = 0.81, P < 0.01; Fig. 2). There were no significant differences between experimental and control eyes in the radius of corneal curvature (3.43 ± 0.15 mm vs. 3.45 ± 0.23 mm, paired t-test, P = 0.10) or choroid thickness (0.125 ± 0.026 mm vs. 0.127 ± 0.019 mm; paired t-test, P = 0.78).
FIGURE 2.
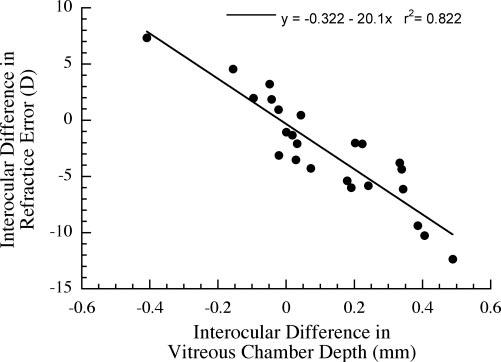
Correlation of interocular refractive difference (experimental minus control eye) with interocular difference in vitreous chamber depth. The change in vitreous chamber depth relative to the control eye was a strong predictor of the change in refractive state (ANOVA, P < 0.01).
Dividing the experimental animals into the three groups based on the age of onset of the deprivation revealed significantly different degrees of axial myopia induced by the diffusers. Figure 3 shows the mean interocular difference in refractive error and vitreous chamber depth at the end of deprivation. There was significantly greater axial myopia (ANOVA, P < 0.01) in group 1 (mean ± SD: refractions -8.25 ± 2.83 D; vitreous chamber depth 0.332 ± 0.124 mm) than in either group 2 (-0.4 ± 4.4 D, 0.048 ± 0.238 mm) or group 3 (-1.20 ± 02.2 D; 0.032 ± 0.080 mm). In group 1, corneal curvature was slightly, but significantly, steeper in the experimental eye at the end of deprivation (mean experimental minus control difference in corneal curvature, -0.077 ± 0.06; paired t-test, P < 0.05). This effect, which accounts for approximately 2.4 D of refractive change, was transient and was not observed with subsequent measures during the period after the end of deprivation. There were no significant changes in the average corneal curvature of the experimental eyes in the other age groups. Statistically significant changes in choroidal thickness in the experimental eyes relative to the control eye were not detected in any of the groups at the end of deprivation or in the period thereafter.
FIGURE 3.
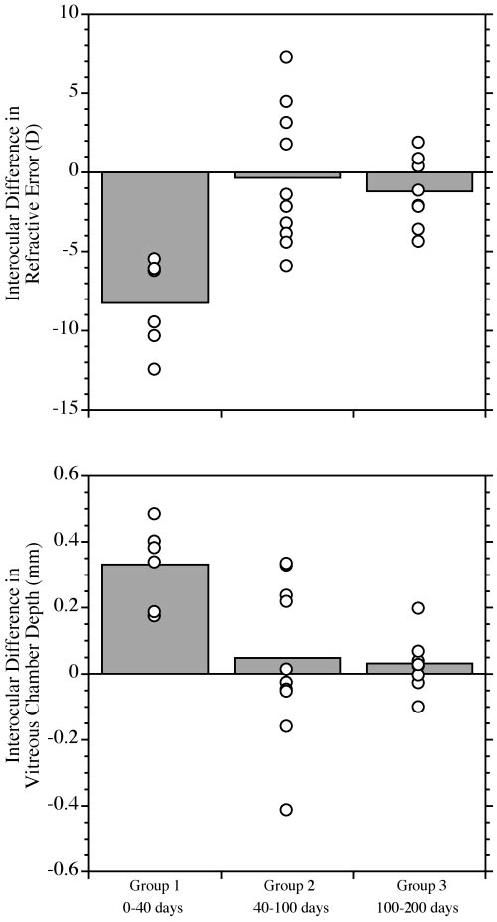
The average effect of form deprivation by diffuser, grouped by age of onset. The mean differences  between experimental and contralateral control eyes (experimental minus control; ○, individual data points for each group) at the end of the deprivation period. The differences are plotted for refractive error (top) and vitreous chamber depth (bottom). For refractive errors, individual data points below 0 indicate more myopia in the experimental eye. Points above 0 on the vitreous chamber plot indicate that the experimental eye was longer than the control eye. The differences in the interocular refractive error and vitreous chamber depth between groups were significant (ANOVA, P < 0.01). Group 1 (earliest age of deprivation onset: between 0 and 40 days of age) shows significant interocular differences indicative of form-deprivation-induced axial myopia, whereas the interocular differences in groups 2 and 3 do not, because of the variability in the individual responses.
between experimental and contralateral control eyes (experimental minus control; ○, individual data points for each group) at the end of the deprivation period. The differences are plotted for refractive error (top) and vitreous chamber depth (bottom). For refractive errors, individual data points below 0 indicate more myopia in the experimental eye. Points above 0 on the vitreous chamber plot indicate that the experimental eye was longer than the control eye. The differences in the interocular refractive error and vitreous chamber depth between groups were significant (ANOVA, P < 0.01). Group 1 (earliest age of deprivation onset: between 0 and 40 days of age) shows significant interocular differences indicative of form-deprivation-induced axial myopia, whereas the interocular differences in groups 2 and 3 do not, because of the variability in the individual responses.
The differences in the magnitude of the responses were due in large part to qualitatively different responses to form deprivation. Specifically, we found that not all marmosets responded to form deprivation with an increase in vitreous chamber depth and myopia. Measures taken after the end of the deprivation period showed several different responses that we categorized as either (1) axial elongation and myopia, (2) no response, (3) delayed-onset axial myopia (axial elongation and myopia were not apparent at the end of deprivation but were observed after the deprivation was discontinued), or (4) reduced axial growth and hyperopia (for examples, see Fig. 4). In Table 1 the different responses observed in each of the three groups are tallied. The response to form deprivation was qualitatively more variable in groups 2 and 3 than in group 1. Note that all animals in group 1 (in which form deprivation started before 39 days of age) responded with axial myopia. In groups 2 and 3, only half of the animals in each group responded with axial myopia. We quantified the different effects of form deprivation by sorting the individuals by response without regard to age. The mean and individual refractive errors and vitreous chamber depths are shown for each response category in Figure 5.
FIGURE 4.
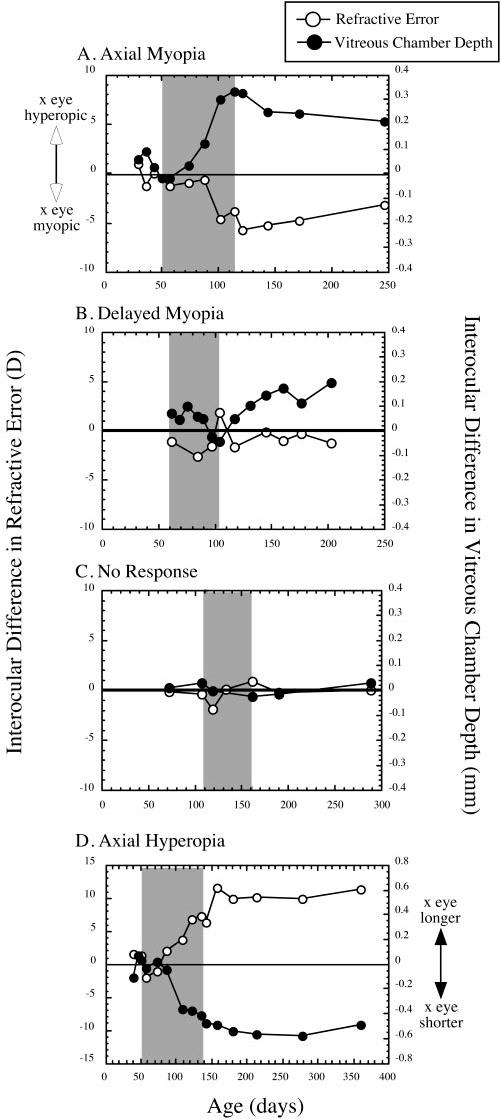
Examples of the different responses to form deprivation observed in this study. The interocular differences in refractive state and vitreous chamber depth (experimental minus control eye) are plotted against age. Shaded area: The period of deprivation by diffuser. (A) An example of axial myopia with an indication of some recovery after the end of deprivation. (B) An example of delayed myopia. There was no difference in vitreous chamber depth at the end of deprivation, but elongation of the eye and myopia occurred afterward. Note that some axial myopia was apparent during deprivation. The hyperopia displayed at the end of deprivation was associated with some corneal flattening. There was no indication of recovery from the vitreous chamber elongation once it began to elongate. (C) An example of no response. Reliable and corresponding interocular differences in refraction or vitreous chamber depth were not observed. (D) In an example of axial hyperopia, the experimental eye was shorter than the control eye at the end of deprivation and remained so, whereas the resultant hyperopia persisted for the duration of the follow-up measurement period.
TABLE 1.
Categories of Response to Form Deprivation in Marmosets Grouped by Age of Onset
| Response | Group 1 (n = 6) | Group 2 (n = 10) | Group 3 (n = 8) | Total (n = 24) |
|---|---|---|---|---|
| Axial myopes | 6 | 5 | 4 | 15 |
| Nonresponders | 0 | 1 | 3 | 4 |
| Delayed-onset myopes | 0 | 1 | 0 | 1 |
| Axial hyperopes | 0 | 3 | 1 | 4 |
| Recovery from myopia | 3 | 3 | 0 | 6 |
| No recovery data available | 2 | 1 | 0 | 3 |
The number of animals recovering from form deprivation myopia is also presented. Several animals were not followed up after the end of the deprivation, and recovery data were not available for them.
FIGURE 5.
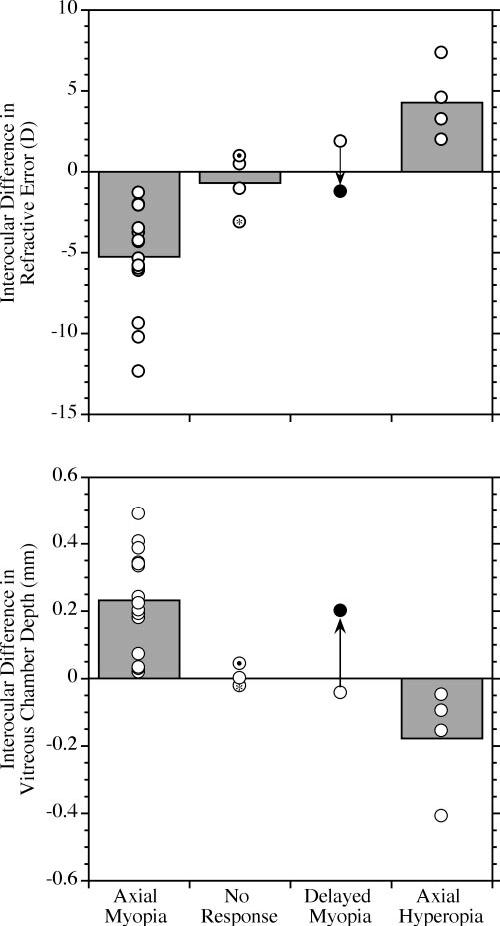
Categories of qualitatively different responses to form deprivation diffusers. The plot and symbols are as described in Figure 3. The four categories of response observed were: (1) Axial myopia: the myopia in the experimental eye was apparent and the eye was longer than the contralateral control eye at the end of deprivation. (2) No response: no clear change in refraction with an associated change in vitreous chamber depth in the experimental eye was observed at the end of deprivation or during the period afterward. In the subject marked with the asterisk there was myopia relative to the control eye but no difference in vitreous chamber depth. The myopia was due to a small amount of corneal steepening in the deprived eye equal to approximately 2.2 D (0.07-mm steeper base curve relative to the control eye). In the subject indicated by a dot within the circle, the vitreous chamber of the experimental eye was slightly longer than the control (43 μm), but there was no discernible refractive difference between the eyes. There was no obvious difference in corneal curvature in this subject. (3) Delayed myopia: axial myopia was not present at the end of deprivation, but developed in the period afterward. There was only one animal in the present study that exhibited this pattern (see Fig. 4B). (●) The increased depth of the vitreous chamber and myopia observed 103 days after the end of form deprivation. (4) Axial hyperopia: in four subjects at the end of deprivation, there was relative hyperopia in the experimental eye, and the vitreous chambers were shorter than in the control eyes.
Axial myopia relative to the control eye was the most frequently observed response (n = 15, mean interocular difference ± SD: vitreous chamber depth 0.232 ± 0.149 mm, refractive error -5.25 ± 3.24 D). In this group, we also observed slight, but significant, corneal steepening (interocular difference in corneal curvature, -0.044 ± 0.05; paired t-test, P < 0.01) that accounts for approximately 1.4 D of refractive change.
Four animals failed to respond in a clear way (defined as a change in vitreous chamber depth with a corresponding change in refractive error) and are referred to as nonresponders in Table 1. In these animals, the mean interocular difference in vitreous chamber depth (0.0 ± 0.03 mm), refractive error (-0.7 ± 1.83), and corneal curvature (-0.012 ± 0.04) were all within resolution limits.
There was one animal that showed a delayed increase in vitreous chamber depth and myopia after a transient increase in vitreous chamber depth and myopia during the period of deprivation (see Fig. 4B) and is referred to as a delayed-onset myope in Table 1. At the end of deprivation the vitreous chamber of the experimental eye was 43 μm shallower and the refraction 1.86 D more hyperopic than in the control. This hyperopia appears to be an underestimate, as the cornea of the experimental eye was also found to be 0.09 mm flatter (about 2.7 D more myopic) than the control eye at the end of deprivation. In the period after the end of deprivation, the corneal flattening appears to have largely resolved (mean interocular difference, 0.031). During that same period the vitreous chamber depth increased and refraction shifted toward myopia in the experimental eye. By 106 days after the end of deprivation, the experimental eye had become 0.20 mm longer than the control eye, and the refraction had shifted 3.1 D toward a relative myopia of -1.24 D.
There were four animals that responded to form deprivation with reduced vitreous chamber depth (-0.178 ± 0.160 mm) and corresponding hyperopia (+4.27 ± 2.3 D) relative to the control eyes. There was no detectable change in the corneal curvature of the experimental eyes of these animals relative to their control eyes either at the end of deprivation or in the period after the end of deprivation.
For those animals that responded to form deprivation with myopia (n = 15), we examined the amount of axial elongation and myopia induced and found that they differed as a function of age (see Figs. 6, 7). The mean amount of axial elongation and myopia induced relative to the contralateral control eye at the end of deprivation was significantly greater (ANOVA, P < 0.01) in group 1 (0.332 ± 0.124 mm, -8.25 ± 2.83 D) compared with either group 2 (0.231 ± 0.130 mm, -3.5 ± 1.8 D) or group 3 (0.084 ± 0.082 mm, -3.0 ± 1.1 D). Normalizing the induced changes for treatment period did not reduce the differences between the experimental groups. Analysis of variance by group was still significant for the rate of change in vitreous chamber depth (P < 0.01) and rate of refractive change (P < 0.01). There were no significant changes in the corneal curvature or choroid thickness of the experimental eye relative to the control eye in any of the groups. For all animals that responded with myopia, the age of deprivation onset correlated significantly (P < 0.05) with the relative amount of myopia (r = 0.534) and vitreous chamber elongation (r = 0.619) observed at the end of deprivation. Linear regressions show that the amount of axial myopia induced is inversely proportional to the age of onset (Fig. 7).
FIGURE 6.
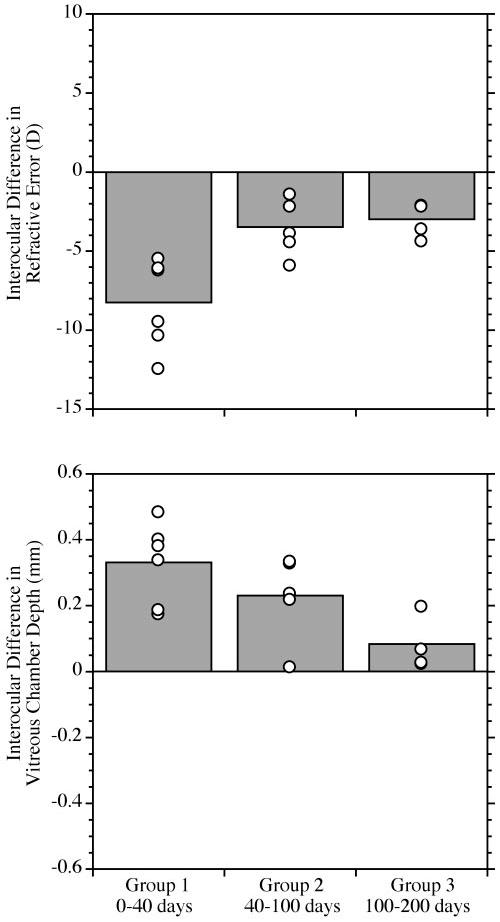
The effects of diffuser-induced form deprivation grouped by age of onset for those animals that responded with axial myopia. The plot and symbols are as described in Figure 3. There was a significant reduction in the experimentally induced increase in vitreous chamber depth and myopia with age (ANOVA, P < 0.01).
FIGURE 7.
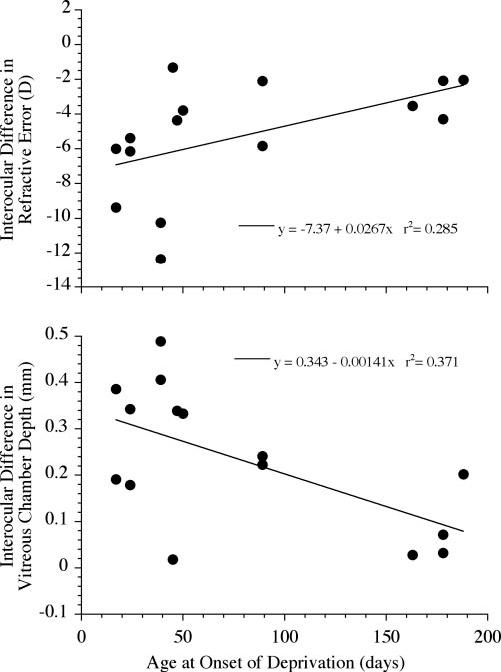
For those eyes with axial myopia, the intraocular difference (experimental eye minus control eye) in refractive error (top) or vitreous chamber depth (bottom) correlated significantly with the age of onset of the deprivation. Linear regressions show that the amount of myopia and increase in vitreous chamber depth were inversely proportional to the age at onset of deprivation (ANOVA, P < 0.05).
We examined the ability of the eyes to recover from form deprivation-induced axial myopia. In 12 of the animals that responded to diffuser-induced deprivation with axial myopia, we tracked refractions and vitreous chamber depth after the end of deprivation (data from three animals were unavailable for analysis of recovery, because they were killed for biochemistry at the end of deprivation). Of the 12, 6 animals recovered after the end of deprivation (see Table 1). Recovery was observed only in animals from groups 1 and 2 (3/4 animals in group 1 and 3/4 animals in group 2), but there was an insufficient number to determine statistically whether there was an age effect. The development and recovery from form deprivation myopia in the six animals examined are shown in Figure 8. When all six animals are considered together, the recovery in refractive state is clearly related to a change in vitreous chamber depth after the end of deprivation. In Figure 9, the change in refraction and vitreous chamber depth relative to control eyes during deprivation is compared with the change after deprivation ended. Refractive error shifted significantly toward myopia during deprivation and toward hyperopia after (-5.2 ± 2.4 vs. +2.8 ± 1.0 D; paired t-test, P < 0.01). Vitreous chamber growth shifts significantly from increasing depth during the period of deprivation to a reduction in depth during the period after (0.234 ± 0.125 mm vs. -0.120 ± 0.067 mm; paired t-test, P < 0.01). Changes in corneal curvature did not contribute to the recovery, nor were significant changes in choroidal thickness observed. Furthermore, in the six myopes that did not recover, the corneal curvature of the experimental eyes were not significantly different from the control eyes, ruling out the possibility that a change in corneal curvature was responsible for the persistence of the myopia in these animals.
FIGURE 8.
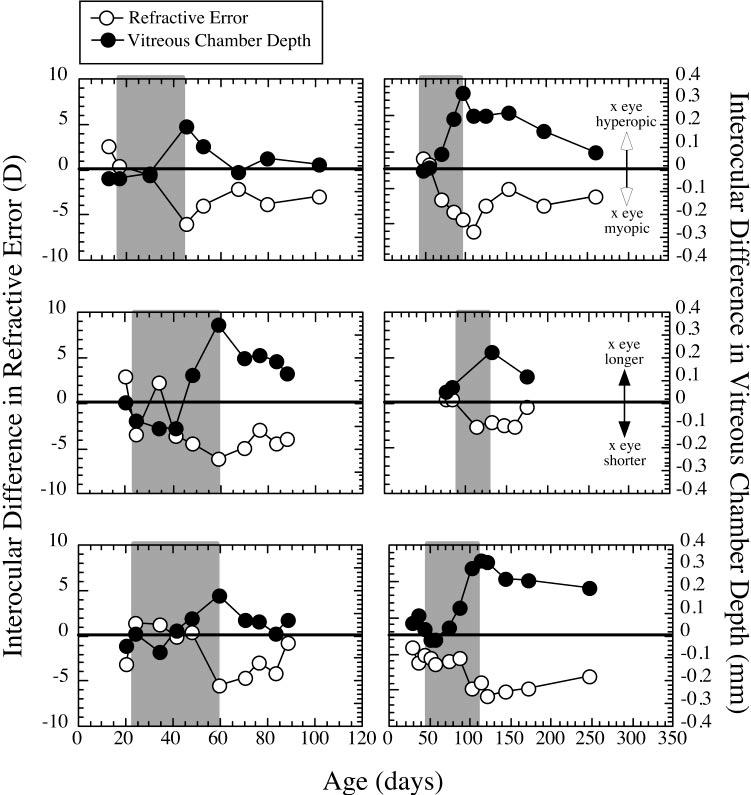
Six examples of various degrees of recovery from form deprivation myopia. The difference in refractive state and vitreous chamber depth between the experimental eye and the contralateral control eye (experimental minus control eye) are plotted against age. Shaded area: period of deprivation.
FIGURE 9.
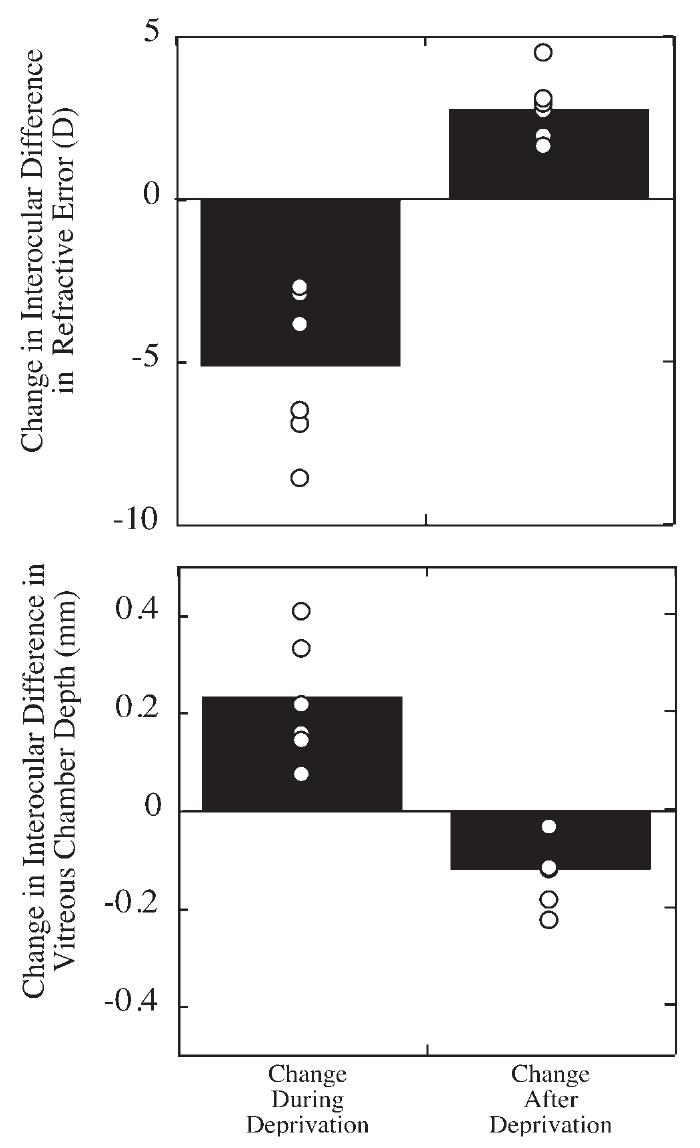
Relative change in refractive error and vitreous chamber depth in eyes undergoing form deprivation myopia followed by recovery after deprivation was discontinued. The relative change during deprivation or the period after the deprivation is shown for the interocular difference (experimental eye minus control eye, ∎ individual subjects, ○) for refractive state (top) and vitreous chamber depth (bottom). Points above 0 for refraction indicate experimental eyes becoming relatively more hyperopic than their control eyes. Points below 0 for vitreous chamber depth indicate experimental eyes with decreasing growth relative to control eyes.
DISCUSSION
Recent studies of experimental myopia have shown that mature animals in a variety of species are susceptible to experimental visual manipulations, but with a reduced response.18,19,33-35 The reduced responses compared with those in neonates may be due to structural limitations related to the larger size of more mature eyes, but possibly also to an agerelated reduction in the visually guided eye-growth controller’s sensitivity, gain, or both. The fact that mature eyes do respond, however, to experimental manipulations known to induce axial elongation and myopia in neonates suggests that visual experience can affect eye size and refractive state throughout life and may contribute to the development of late-onset axial myopia in humans.36,37 In this study, we examined the effects of visual form deprivation by diffusers on eye growth and refraction in marmosets at several ages corresponding to different rates of ocular growth. We report not only reduced form deprivation myopia in older animals with normally slower growth rates, but we found and characterize several other possible responses to form deprivation and suggest that their manifestations are also age dependent and related to the maturation of the eye and visual system.
In general, the majority of marmosets in this study were found to be susceptible to form deprivation myopia produced by occlusion throughout the first 200 days of life (roughly equivalent to the time from birth to adolescence in human children), but the responses at the older ages are diminished and variable. Among the animals in our older groups that failed to respond to form deprivation with consistent axial myopia, in one animal myopia developed in the period after the end of deprivation, and in some hyperopia developed that was related to reduced vitreous chamber growth. Several others did not show any appreciable change in eye growth or refraction. All the refractive changes observed in all groups appear to be accounted for mainly by induced changes in ocular axial length, specifically vitreous chamber depth. Although corneal changes contributed to some of the observed refractive changes, as noted in the myopes and in some individual cases, the effects appear to be relatively minor.
Delayed myopia in response to form deprivation has been observed before in tree shrews,17 marmosets,13 and macaques,15 sometimes after an early transient response of axial hyperopia. In our subject with delayed-onset myopia, transient axial hyperopia with some corneal flattening was also observed. Of note, and unlike anything reported earlier, our animal initially exhibited vitreous chamber elongation and myopia during deprivation before presenting with axial hyperopia at the end of deprivation and subsequently returning to axial myopia. The cause of these changing responses in the relative growth and refraction of the experimental eye are unclear, but presumably they reflect an initial disruption of that subject’s eye-growth controller under the visually open-loop conditions of form deprivation. The axial myopia ultimately exhibited after the end of deprivation may be compensation for the hyperopia observed immediately after deprivation. The cause of this persistent myopia is also unclear, but it may be for the same reason that some primates fail to recover from form deprivation myopia13 and may reflect a reduction in the ability of some eyes to regulate growth rate after periods of abnormal visual experience.38
Another response to form deprivation by diffusers that has not been reported was the persistent axial hyperopia observed in several of the marmosets in the older age groups. Smith and Hung15 reported transient axial hyperopia in primates reared with diffusers, but these eyes quickly returned to control levels after deprivation ended. Reductions in axial length and hyperopia after lid suturing have been reported in cats39 and primates,11,40 but a mechanical effect on the cornea that contributed to the hyperopia was observed and may have influenced the axial growth of the eye in some way. Evidence that such a mechanical effect can influence the growth of the eye and refractive state comes from studies using contact lenses for optical defocus or form deprivation.41-43 In the present study, the animals exhibiting axial hyperopia showed no such corneal change, suggesting that their response to the diffusers is related instead to the visual processing of form deprivation. It is also possible that the ocular growth response observed in these animals was secondary to deprivation-induced changes in the central visual system. Central visual system influences on eye growth and refractive development have been suggested by findings in studies of the effects of induced amblyopia on refractive state.38,44
Although the ocular growth response to form deprivation provides hints as to the nature of the eye-growth controller, compensation for imposed refractive errors is a more direct means of studying visual feedback control. Recovery from form deprivation myopia in chicks1,3 and tree shrews25 has been suggested as evidence for the existence of feedback control of ocular growth. However, in earlier studies of lid-suture-induced form deprivation myopia in primates, recovery did not occur.13,18,23,45 Because compensation to lens-induced retinal defocus has been established in primates4,5 and recovery from lens-induced myopia shown,26 it seems likely that the lack of recovery is related to the severity of the deprivation or possibly the lid-suturing procedure itself. In this study, and a recent study in macaques,24 recovery from diffuser-induced form deprivation myopia was in fact observed. In the present study, some degree of recovery from myopia was observed in age groups 1 and 2, although not in all animals. We cannot at present explain why some animals failed to recover, but we rule out an optical effect from mechanically changed corneas and speculate that this apparent loss of growth regulation is because of deprivation-induced changes in the visual system. These data generally support the existence of visual regulation of eye growth across a range of ages, but also emphasize the intersubject variability in response to visual manipulations.
This study supports the hypothesis that the normal developmental growth of the eye and maturation of the visual system affect the response to form deprivation and perhaps the visual control of emmetropization. Considering separately the animals that responded to form deprivation with axial myopia shows that the amount of vitreous chamber elongation and myopia observed is inversely proportional to the age of onset of the deprivation. We speculate that this may be because, as the eye grows larger, the ability to respond to visual signals that elicit growth in young animals diminishes because the default growth rate that is unmasked by the open-loop condition of form deprivation is reduced. The qualitatively different responses to form deprivation exhibited by some more mature animals suggest that after the rapid eye growth of infancy has slowed, the disruptive effect of form deprivation, although still present, causes different responses, depending on the state of the default growth rate under open-loop conditions.
At this time we can still only speculate why individual subjects differ in their responses to form deprivation or their ability to recover from form deprivation myopia, but it seems likely that the answer involves a complex interaction of factors related to the timing and nature of the visual conditions experienced, the response characteristics of the retina and visual cortex, the maturity of the eye and visual system, and ultimately the individual’s genotype. Continued examination of these different responses may uncover subtleties in the underlying mechanisms that account for differences in the expression of ocular phenotypes and will be useful for understanding the differences in the refractive states of children.
Acknowledgments
The authors thank Heidi Denman and Kristen Totonelly for their expert animal care, and Elise Harb, John Potter, and Frank Thorn for their comments.
Supported by National Eye Institute Grant EY11228. Submitted for publication December 5, 2004; revised February 6, 2005; accepted February 9, 2005.
Footnotes
Disclosure: D. Troilo, None; D.L. Nickla, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- 1.Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 2.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 3.Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–1250. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- 4.Hung L-F, Crawford MLJ, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 5.Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41:267–273. doi: 10.1016/s0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 6.Smith EL., III. Environmentally induced refractive errors in animals. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Butterworth Heinemann; Oxford, UK: 1998. pp. 57–90. [Google Scholar]
- 7.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- 9.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 10.Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 11.von Noorden GK, Crawford ML. Lid closure and refractive error in macaque monkeys. Nature. 1978;272:53–54. doi: 10.1038/272053a0. [DOI] [PubMed] [Google Scholar]
- 12.Smith EL, III, Harwerth RS, Crawford MLJ, von Noorden GK. Observations on the effects of form deprivation on the refractive status of the monkey. Invest Ophthalmol Vis Sci. 1987;28:1236–1245. [PubMed] [Google Scholar]
- 13.Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–1324. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- 14.Troilo D, Nickla DL. In: Thorn F, Troilo D, Gwiazda J, editors. Visual regulation of eye growth and refractive state in a new world primate; Myopia 2000: Proceedings of the VIII International Conference on Myopia; Boston, MA: Myopia 2000, Inc.. 2000.pp. 170–174. [Google Scholar]
- 15.Smith EL, III, Hung LF. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Res. 2000;40:371–381. doi: 10.1016/s0042-6989(99)00184-4. [DOI] [PubMed] [Google Scholar]
- 16.Harwerth RS, Smith EL, Boltz RL, Crawford MLJ, von Noorden GK. Behavioral studies on the effect of abnormal early visual experience in monkeys: spatial modulation sensitivity. Vision Res. 1983;23:1501–1510. doi: 10.1016/0042-6989(83)90162-1. [DOI] [PubMed] [Google Scholar]
- 17.McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Res. 1992;32:843–852. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- 18.Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in mature common marmosets (Callithrix jacchus) Invest Ophthalmol Vis Sci. 2000;41:2043–2049. [PubMed] [Google Scholar]
- 19.Smith EL, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optom Vision Sci. 1999;76:428–431. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 20.van Alphen GWHM. On emmetropia and ametropia. Ophthalmologica. 1961;142(Suppl):1–92. [PubMed] [Google Scholar]
- 21.Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31:717–734. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- 22.Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Res. 2000;40:3273–3282. doi: 10.1016/s0042-6989(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 23.Raviola E, Wiesel TN. An animal model of myopia. N Engl J Med. 1985;312:1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- 24.Qiao-Grider Y, Hung L-F, Kee C-S, Ramamirtham R, Smith EL., III. Recovery from form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3361–3372. doi: 10.1167/iovs.04-0080. [DOI] [PubMed] [Google Scholar]
- 25.Siegwart JT, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 26.Smith EL, III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 27.Wallman J, LeDoux C, Friedman MB. Simple devices for restricting the visual fields of birds. Behav Res Methods Instrum. 1978;10:401–403. [Google Scholar]
- 28.Missler M, Wolff JR, Rothe H, et al. Developmental biology of the common marmoset: proposal for a postnatal staging. J Med Primatol. 1992;21:285–298. [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 30.Zadnik K, Mutti DO, Adams AJ. The repeatability of measurement of the ocular components. Invest Ophthalmol Vis Sci. 1992;33:2325–2333. [PubMed] [Google Scholar]
- 31.Schaeffel F, Howland HC. Corneal accommodation in chick and pigeon. J Comp Physiol [A] 1987;160:375–384. doi: 10.1007/BF00613027. [DOI] [PubMed] [Google Scholar]
- 32.Troilo D, Nickla DL, Wildsoet CW. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41:1249–1258. [PubMed] [Google Scholar]
- 33.Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA. Induction of axial eye elongation and myopic refractive error shift in one-year-old chickens. Vision Res. 1998;38:1883–1888. doi: 10.1016/s0042-6989(97)00347-7. [DOI] [PubMed] [Google Scholar]
- 34.Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA, Laties AM. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp Eye Res. 1998;66:195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- 35.Wildsoet CF, Anchong R, Manasse J, Troilo D. Susceptibility to experimental myopia declines with age in the chick (abstract) Optometry Vision Sci. 1998;75(Suppl):265. [Google Scholar]
- 36.McBrien NA, Millodot M. A biometric investigation of late onset myopic eyes. Acta Opthalmol. 1987;65:461–468. doi: 10.1111/j.1755-3768.1987.tb07024.x. [DOI] [PubMed] [Google Scholar]
- 37.Bullimore MA, Gilmartin B, Royston JM. Steady-state accommodation and ocular biometry in late-onset myopia. Doc Ophthalmol. 1992;80:143–155. doi: 10.1007/BF00161240. [DOI] [PubMed] [Google Scholar]
- 38.Kiorpes L, Wallman J. Does experimentally-induced amblyopia cause hyperopia in monkeys. Vision Res. 1995;35:1289–1297. doi: 10.1016/0042-6989(94)00239-i. [DOI] [PubMed] [Google Scholar]
- 39.Gollender M, Thorn F, Erickson P. Development of axial ocular dimensions following eyelid suture in the cat. Vision Res. 1979;19:221–223. doi: 10.1016/0042-6989(79)90053-1. [DOI] [PubMed] [Google Scholar]
- 40.Repka MX, Tusa RJ. Refractive error and axial length in a primate model of strabismus and congenital nystagmus. Invest Ophthalmol Vis Sci. 1995;36:2672–2677. [PubMed] [Google Scholar]
- 41.Bradley DV, Fernandes A, Tigges M, Boothe RG. Diffuser contact lenses retard axial elongation in infant rhesus monkeys. Vision Res. 1996;36:509–514. doi: 10.1016/0042-6989(95)00279-0. [DOI] [PubMed] [Google Scholar]
- 42.Hung L-F, Smith EL., III. Extended-wear, soft, contact lenses produce hyperopia in young monkeys. Optom Vision Sci. 1996;73:579–584. doi: 10.1097/00006324-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Whatham AR, Judge SJ. Overnight lens removal avoids changes in refraction and eye growth produced by piano soft contact lenses in infant marmosets. Vision Res. 2001;41:257–265. doi: 10.1016/s0042-6989(00)00252-2. [DOI] [PubMed] [Google Scholar]
- 44.Smith EL, III, Hung L-F, Harwerth RS. Developmental visual system anomalies and the limits of emmetropization. Ophthalmic Physiol Opt. 1999;19:90–102. [PubMed] [Google Scholar]
- 45.Raviola E, Wiesel TN. Neural control of eye growth and experimental myopia in primates. In: Bock G, Widdows K, editors. Myopia and the Control of Eye Growth. Wiley; Chichester, UK: 1990. pp. 22–44. [DOI] [PubMed] [Google Scholar]


