Abstract
Hematological disturbances that develop during linezolid treatment are a major concern when linezolid is administered for prolonged periods of time. The aim of this study was to evaluate the influences of pyridoxine, rifampin, and renal function on hematological adverse events. From January 2002 to April 2006, 52 patients received a long-term course of linezolid. Blood cell counts were monitored weekly. Thrombocytopenia was defined as a decrease to <75% of the baseline platelet count, and anemia was defined when the hemoglobin concentration decreased by ≥2 g/liter from the baseline value. Twenty-four patients received linezolid alone, and 28 patients received linezolid plus 200 mg of pyridoxine. The Kaplan-Meier survival method, followed by the log-rank test, was used to estimate the cumulative probability of adverse events, and Cox regression analysis was performed to evaluate the independent predictors of toxicity. The baseline characteristics of the patients in both groups were similar. The cumulative probability of thrombocytopenia and anemia in patients who received pyridoxine was not different from that in patients who did not receive it. Hematological adverse events were less frequent in patients taking rifampin and were more frequent in patients with renal failure. However; the Cox regression analysis showed that rifampin was the only independent predictor associated with a lower risk of thrombocytopenia (hazard ratio, 0.37; 95% confidence interval, 0.14 to 0.98; P = 0.045). In conclusion, pyridoxine did not prevent linezolid-related hematological adverse events, and the coadministration of rifampin was associated with a lower risk of thrombocytopenia.
Linezolid belongs to a family of antimicrobials (oxazolidinones) that inhibit bacterial protein synthesis by preventing the fusion of 30S and 50S ribosomal subunits (14). Linezolid has shown excellent efficacy against gram-positive cocci, including Staphylococcus aureus, coagulase-negative staphylococci, enterococci, and streptococci, with MICs ranging from 0.5 to 4 μg/ml (12). Furthermore, linezolid has a 100% oral bioavailability and reaches high concentrations in different tissues (skin, synovial fluid, bone, cerebrospinal fluid, lung, and eye). Therefore, it seems to be a good alternative for the treatment of orthopedic implant infections, ventricle-peritoneal shunts, and other infections related to foreign bodies where gram-positive cocci are the main pathogens and where prolonged courses of antimicrobial therapy are needed. However, a major concern with this antibiotic is its safety profile, especially when it is administered for more than 4 weeks (10).
The most important adverse events are hematological disturbances, especially thrombocytopenia and anemia (1, 4, 11, 17, 19, 22), but the underlying mechanisms that explain this toxicity are still unknown. Spellberg et al. described that the administration of pyridoxine (vitamin B6) was able to revert linezolid-related thrombocytopenia and anemia in two patients (24). Although there is no clear mechanism to explain this effect, it is reasonable to evaluate the potential preventive effect of pyridoxine on hematological disturbances in patients receiving prolonged courses of linezolid. In the present study, we evaluated the influence of pyridoxine on the frequency of hematological disturbances in two consecutive cohorts of patients (those who received pyridoxine and those who did not receive pyridoxine) who had similar baseline characteristics and who received prolonged courses of linezolid, as well as the influences of other clinically relevant variables, such as renal function and the coadministration of rifampin.
MATERIALS AND METHODS
From January 2002 to April 2006, patients who received a long-term course (≥3 weeks) of linezolid (Zyvoxoid; Pfizer) were identified and monitored in a tertiary-care University Hospital in Barcelona, Spain. The protocol included weekly blood cell count, serum creatinine level, and serum glucose level determinations. From December 2004 to April 2006, the oral administration of pyridoxine (Godabion; Merck Pharma Quimica) at a dosage of 200 mg once daily was added to the linezolid treatment. A total of 52 patients were included in the protocol. Linezolid was administered alone to the first 24 patients, and pyridoxine was added to regimen and was administered to the remaining 28 patients. The clinical variables gathered were, age, sex, type of infection, etiologic microorganism, comorbidity, baseline serum creatinine level, and estimated baseline glomerular filtration rate (GFR), the length of linezolid treatment (days), whether rifampin was coadministered, the baseline level of platelet count (109 platelets/liter), and the baseline hemoglobin concentration (g/liter). GFR was obtained by using the formula of Cockcroft and Gault: GFR (ml/min) = [(140 − age in years) × weight (kg)]/[72 (or 85 for women) × serum creatinine level (mg/dl)]. Thrombocytopenia was defined as a decrease in the platelet count to <75% of the baseline value, and anemia was defined as a hemoglobin concentration that decreased by ≥2 g/liter from the baseline value without another plausible explanation. No other medications with potential hematological toxicity were administered to any patient. The ethical committee of the hospital approved the administration of pyridoxine, and the patients signed an informed consent.
Statistical analysis.
Continuous variables were expressed as the mean and standard deviation (SD), and the median and interquartile range (IQR) were compared by using an unpaired Student's t test. Categorical variables were compared by using Fisher's exact test or the chi-square test, when necessary. The cumulative probability of thrombocytopenia and anemia was estimated by univariate analysis by the Kaplan-Meier survival method, followed by the log-rank test. Furthermore, a stepwise forward Cox regression analysis was performed to evaluate independent predictors of hematological toxicity. Variables with a P value less than 0.15 in the univariate analysis were entered into the multivariate analysis, and variables achieving P values <0.05 in the final model were considered significant. The hazard ratio with the 95% confidence interval was calculated for each significant variable. The statistical analysis was performed by using the SPSS 12.0 package (SPSS, Chicago, IL).
RESULTS
The baseline characteristics of the patients are shown in Table 1. There were no significant differences in the baseline parameters between patients who received pyridoxine and patients who did not receive pyridoxine.
TABLE 1.
Characteristics of patients at the baselinea
| Characteristic | Non-vitamin B6-treated patients (n = 24) | Vitamin B6-treated patients (n = 28) |
|---|---|---|
| Age (yr) | 64.7 (19.2) | 66 (18.2) |
| Sex (% males) | 58.3 | 67.8 |
| Type of infection (no. [%] of patients) | ||
| Prosthetic joint infection | 18 (75) | 17 (60.7) |
| Osteomyelitis | 2 (8.3) | 3 (10.7) |
| Others | 4 (16.7) | 8 (28.5) |
| Pathogen (no. [%] of patients) | ||
| MRbStaphylococcus epidermidis | 15 (62.5) | 14 (50) |
| MR S. aureus | 4 (16.7) | 5 (17.8) |
| Enterococcus spp. | 3 (10.7) | |
| Others | 2 (8.3) | 3 (10.7) |
| Unknown | 3 (12.5) | 3 (10.7) |
| Underlying disease (no. [%] of patients) | ||
| Diabetes mellitus | 3 (12.5) | 6 (21.4) |
| Rheumatoid arthritis | 2 (8.3) | 1 (3.5) |
| Chronic renal failure | 2 (7.1) | |
| Solid neoplasm | 1 (4.1) | 4 (14.2) |
| Chronic obstructive pulmonary disease | 1 (4.1) | |
| Mean (SD)/median (IQR) length of linezolid treatment (days) | 55.5 (28.4)/54.5 (29.7-77.7) | 50.8 (29.9)/44.5 (26.5-74) |
| No. of patients with >56 days linezolid treatment | 7 | 10 |
| No. (%) of patients with coadministration of rifampin | 7 (29.1) | 10 (35.7) |
| Mean (SD)/median (IQR) serum creatinine (mg/dl) | 1.1 (0.2)/1 (0-9-1.2) | 1 (1)/0.95 (0.8-1.3) |
| Mean (SD)/median (IQR) GFRc (ml/min) | 70.4 (27.2)/63.5 (46.7-83.5) | 70.7 (31.8)/73.5 (46-89) |
| No. (%) of patients with GFR (ml/min) of: | ||
| >50 | 19 (67.8) | 17 (70.8) |
| 30-50 | 7 (25) | 7 (29.2) |
| <30 | 2 (7.2) | 0 |
| Mean (SD) platelet count (109 platelets/liter) | 303 (113) | 331 (130) |
| Mean (SD) hemoglobin count (g/dl) | 11.4 (1.5) | 11.1 (1.5) |
P values for all characteristics were not significantly different between the two groups.
MR, methicillin resistant.
GFR was estimated by using the Cockroft-Gault formula (see text).
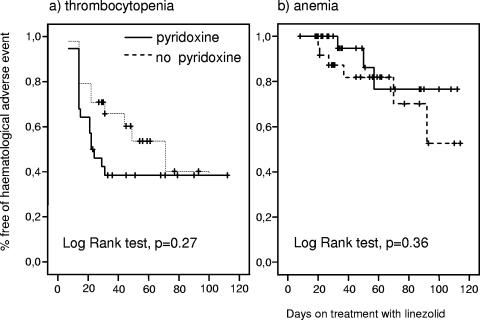
The survival curves demonstrated that the cumulative probability of thrombocytopenia and anemia was not different between patients who received pyridoxine and those who did not (Fig. 1a and b). Linezolid was stopped due to severe thrombocytopenia (<100 × 109 platelets/liter) in 7 of 52 (13.4%) patients: 3 of 24 (12.5%) patients who received linezolid alone and 4 of 28 (14.2%) patients who received pyridoxine (P = 0.58). Linezolid was stopped due to severe anemia (< 8 g/liter) in 4 of 52 (7.7%) patients: 3 of 24 (12.5%) patients who received linezolid alone and 1 of 28 (3.5%) patients who received pyridoxine (P = 0.24).
FIG. 1.
Cumulative probability of hematological adverse events in patients who received or did not receive pyridoxine.
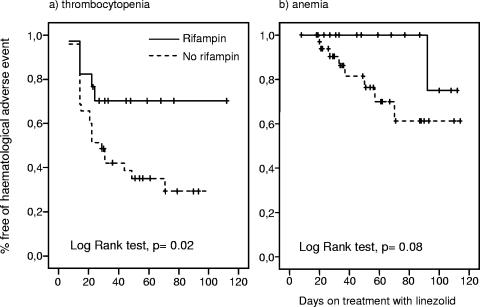
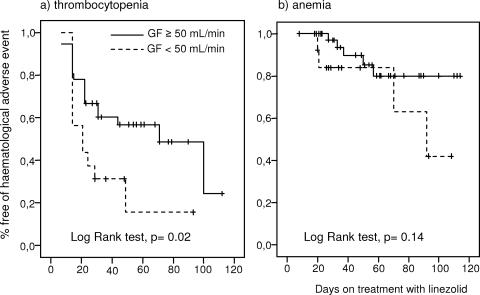
Rifampin was added to the linezolid regimen in 17 patients with an orthopedic implant infection from whom the implant was not removed. The age, length of linezolid treatment, basal serum creatinine level, GFR, platelet count, and hemoglobin concentration were not different between the patients who received rifampin and those who did not. The cumulative probability of thrombocytopenia was significantly lower in patients who received rifampin than in those that did not (P = 0.02) (Fig. 2a). Only 1 of 17 (5.8%) patients who received rifampin but 6 of 35 (17.1%) patients who did not receive rifampin had severe thrombocytopenia (<100 × 109 platelets/liter). There was a trend toward a lower cumulative probability of anemia in the rifampin group, but the difference did not reach statistical significance (P = 0.08) (Fig. 2b). The cumulative probability of hematological adverse events was analyzed according to the GFR. The risk of thrombocytopenia was significantly higher when the basal GFR was <50 ml/min (P = 0.02) (Fig. 3a). There was a trend toward a higher cumulative probability of anemia when the basal GFR was <50 ml/min; however, the difference was not statistically significant (P = 0.14) (Fig. 3b). The Cox regression model showed that the coadministration of rifampin was the only factor independently associated with a lower risk of thrombocytopenia (hazard ratio, 0.37; 95% confidence interval, 0.14 to 0.98; P = 0.045). No variable was independently associated with the development of anemia.
FIG. 2.
Cumulative probability of hematological adverse events in patients who received did or did not receive rifampin.
FIG. 3.
Cumulative probability of hematological adverse events according to GFR.
DISCUSSION
The activity of linezolid against a broad range of gram-positive cocci, its high oral bioavailability (100%), and the good results described in studies of linezolid treatment of bone infections (3, 20, 21) make linezolid an attractive oral alternative to glycopeptides for the treatment of infections that require prolonged antimicrobial therapy. However, thrombocytopenia and anemia are common adverse events when linezolid is administered for more than 3 weeks. The administration of 50 mg pyridoxine (vitamin B6) orally once a day to two patients who developed hematological disturbances was useful in reverting these adverse events (24). In a recent study, Plachouras et al. (18) administered 125 mg of pyridoxine to 24 patients who received linezolid for bone infections, and the rates of thrombocytopenia (<140 × 109 platelets/liter) and anemia (hematocrit, <30%) were 45.8% and 25%, respectively. These data suggest that pyridoxine does not prevent linezolid-related hematological adverse events, since the frequencies of hematological adverse events were similar to or even higher than those reported in patients who did not receive pyridoxine (19, 22). The study of Plachouras et al. (18) was a noncomparative study in which variables that could influence the rate of cytopenia, such as age, sex, comorbidity, the coadministration of other antibiotics, or renal function, were not controlled. In our study, the influence of pyridoxine on hematological adverse events was analyzed by comparing two consecutive cohorts with similar baseline characteristics and similar lengths of linezolid treatment. The cumulative probability of thrombocytopenia and anemia was similar in both groups; therefore, our findings support the lack of a protective role of pyridoxine.
Rifampin is the most active antibiotic against biofilm-forming microorganisms, but it should not be administered alone due to the high risk of selection of resistant mutants (2, 27). It is of note that the administration of rifampin was independently associated with a lower risk of thrombocytopenia. Although in vitro studies have demonstrated that linezolid is not metabolized by human cytochrome P450 (26), recently, Egle et al. (7) observed in eight healthy men a 35% decrease in the serum linezolid concentration after the administration of 600 mg of rifampin. They hypothesized that linezolid may be a substrate of P-glycoprotein, whose expression is rapidly induced by rifampin, as a consequence of which the intestinal secretion of linezolid may be increased. On the other hand, hematological adverse events were more frequent in patients with renal failure, as previously described by other authors (13, 25). Although linezolid does not require dosage adjustment in patients with renal failure, its area under the serum concentration-time curve (AUC) is higher in patients with renal failure than in patients with a normal renal function (5). These findings suggest that hematological adverse events could be related to the serum linezolid concentration.
The underlying mechanism of hematological adverse events is unknown. Our group has recently described that linezolid inhibits the mitochondrial ribosomes (9, 23), and this effect may be the cause of hematological alterations. Since the efficacy of linezolid (for the inhibition of bacterial ribosomes) is associated with the degree of exposure of the microorganism to the antibiotic, as measured by the AUC/MIC ratio (6), it is reasonable to assume that inhibition of the mitochondrial ribosome may also be associated with the degree of linezolid exposure. These hypotheses are supported by the relationship between the linezolid AUC and the development of thrombocytopenia (8).
McKee et al. (16) reported that the linezolid concentration that inhibits 50% of mitochondrial protein synthesis in rat and rabbit heart and liver mitochondria is between 3.37 and 5.26 μg/ml. By using the standard dosage of 600 mg/12 h, the trough serum concentration at steady state is 6 μg/ml (15) and the expected AUC at 24 h is 260 mg·h/liter. When the MICs of the etiologic agent are 1 and 2 μg/ml, the AUC/MIC ratios obtained with the standard regimen are 260 or 130, respectively. In both cases, the AUC/MIC ratio is >50 to 80, which is the target associated with the highest linezolid efficacy against gram-positive cocci (6). Therefore, in order to reduce the rates of adverse events, in those patients who require prolonged treatment (>21 days) with linezolid and when the MIC of the etiologic agent is ≤2 μg/ml, the linezolid concentration could be reduced to obtain an AUC/MIC ratio of about 100.
The present study has two major drawbacks. First, this was a nonrandomized study; however; the principle baseline characteristics that could influence hematological adverse events, such as age, sex, comorbidity, the use of other antibiotics, baseline hematological parameters, and renal function, were similar in both cohorts. Second, the low number of patients reduces the statistical power of the study.
In conclusion, our data showed that 200 mg of pyridoxine once daily does not prevent linezolid-related hematological adverse events. The coadministration of rifampin was associated with a lower rate of hematological adverse events, while renal failure was associated with a higher rate. Since these factors affect the serum linezolid concentration, these findings suggest that hematological toxicity is directly related to the degree of linezolid exposure. In the future, it will be necessary to evaluate whether adjustment of the concentration in serum to obtain the pharmacodynamic target would be a reasonable strategy to avoid adverse events in patients receiving prolonged courses of linezolid.
Acknowledgments
We thank Laura García and Marta Sala for their collaboration with the protocol.
We declare no conflict of interest in connection with this article.
Footnotes
Published ahead of print on 30 April 2007.
REFERENCES
- 1.Attassi, K., E. Hershberger, R. Alam, et al. 2002. Thrombocytopenia associated with linezolid therapy. Clin. Infect. Dis. 34695-698. [DOI] [PubMed] [Google Scholar]
- 2.Bahl, D., D. A. Miller, I. Leviton, et al. 1997. In vitro activities of ciprofloxacin and rifampin alone and in combination against growing and nongrowing strains of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 411293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassetti, M., F. Vitale, G. Melica, et al. 2005. Linezolid in the treatment of gram-positive prosthetic joint infections. J. Antimicrob. Chemother. 55387-390. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein, W. B., R. F. Trotta, J. T. Rector, et al. 2003. Mechanisms for linezolid-induced anemia and thrombocytopenia. Ann. Pharmacother. 37517-520. [DOI] [PubMed] [Google Scholar]
- 5.Brier, M. E., D. J. Stalker, G. R. Aronoff, et al. 2003. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob. Agents Chemother. 472775-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17479-501. [DOI] [PubMed] [Google Scholar]
- 7.Egle, H., R. Trittler, K. Kummerer, et al. 2005. Linezolid and rifampin: drug interaction contrary to expectations? Clin. Pharmacol. Ther. 77451-453. [DOI] [PubMed] [Google Scholar]
- 8.Forrest, A., C. R. Rayner, A. Meagher, et al. 2000. Pharmacostatistical modelling of hematologic effects of linezolid in seriously-ill patients. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr 283. American Society for Microbiology, Washington, DC
- 9.Garrabou, G., A. Soriano, S. Lopez, et al. 2007. Reversible inhibition of mitochondrial protein synthesis during linezolid-related hyperlactatemia. Antimicrob. Agents Chemother. 51962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerson, S. L., S. L. Kaplan, J. B. Bruss, et al. 2002. Hematologic effects of linezolid: summary of clinical experience. Antimicrob. Agents Chemother. 462723-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green, S. L., J. C. Maddox, and E. D. Huttenbach. 2001. Linezolid and reversible myelosuppression. JAMA 2851291. [DOI] [PubMed] [Google Scholar]
- 12.Jones, R. N., J. E. Ross, T. R. Fritsche, et al. 2006. Oxazolidinone susceptibility patterns in 2004: report from the Zyvox Annual Appraisal of Potency and Spectrum (ZAAPS) Program assessing isolates from 16 nations. J. Antimicrob. Chemother. 57279-287. [DOI] [PubMed] [Google Scholar]
- 13.Lin, Y. H., V. C. Wu, I. J. Tsai, et al. 2006. High frequency of linezolid-associated thrombocytopenia among patients with renal insufficiency. Int. J. Antimicrob. Agents 28345-351. [DOI] [PubMed] [Google Scholar]
- 14.Livermore, D. M. 2003. Linezolid in vitro: mechanism and antibacterial spectrum. J. Antimicrob. Chemother. 51(Suppl. 2)ii9-ii16. [DOI] [PubMed] [Google Scholar]
- 15.MacGowan, A. P. 2003. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with gram-positive infections. J. Antimicrob. Chemother. 51(Suppl. 2)ii17-ii25. [DOI] [PubMed] [Google Scholar]
- 16.McKee, E. E., M. Ferguson, A. T. Bentley, et al. 2006. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob. Agents Chemother. 502042-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monson, T., S. A. Schichman, and C. S. Zent. 2002. Linezolid-induced pure red blood cell aplasia. Clin. Infect. Dis. 35E29-E31. [DOI] [PubMed] [Google Scholar]
- 18.Plachouras, D., E. Giannitsioti, S. Athanassia, et al. 2006. No effect of pyridoxine on the incidence of myelosuppression during prolonged linezolid treatment. Clin. Infect. Dis. 43e89-e91. [DOI] [PubMed] [Google Scholar]
- 19.Rao, N., B. H. Ziran, M. M. Wagener, et al. 2004. Similar hematologic effects of long-term linezolid and vancomycin therapy in a prospective observational study of patients with orthopedic infections. Clin. Infect. Dis. 381058-1064. [DOI] [PubMed] [Google Scholar]
- 20.Rayner, C. R., L. M. Baddour, M. C. Birmingham, et al. 2004. Linezolid in the treatment of osteomyelitis: results of compassionate use experience. Infection 328-14. [DOI] [PubMed] [Google Scholar]
- 21.Razonable, R. R., D. R. Osmon, and J. M. Steckelberg. 2004. Linezolid therapy for orthopedic infections. Mayo Clin. Proc. 791137-1144. [DOI] [PubMed] [Google Scholar]
- 22.Senneville, E., L. Legout, M. Valette, et al. 2004. Risk factors for anaemia in patients on prolonged linezolid therapy for chronic osteomyelitis: a case-control study. J. Antimicrob. Chemother. 54798-802. [DOI] [PubMed] [Google Scholar]
- 23.Soriano, A., O. Miro, and J. Mensa. 2005. Mitochondrial toxicity associated with linezolid. N. Engl. J. Med. 3532305-2306. [DOI] [PubMed] [Google Scholar]
- 24.Spellberg, B., T. Yoo, and A. S. Bayer. 2004. Reversal of linezolid-associated cytopenias, but not peripheral neuropathy, by administration of vitamin B6. J. Antimicrob. Chemother. 54832-835. [DOI] [PubMed] [Google Scholar]
- 25.Wu, V. C., Y. T. Wang, C. Y. Wang, et al. 2006. High frequency of linezolid-associated thrombocytopenia and anemia among patients with end-stage renal disease. Clin. Infect. Dis. 4266-72. [DOI] [PubMed] [Google Scholar]
- 26.Wynalda, M. A., M. J. Hauer, and L. C. Wienkers. 2000. Oxidation of the novel oxazolidinone antibiotic linezolid in human liver microsomes. Drug Metab. Dispos. 281014-1017. [PubMed] [Google Scholar]
- 27.Zimmerli, W., A. F. Widmer, M. Blatter, et al. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA 2791537-1541. [DOI] [PubMed] [Google Scholar]