Abstract
Ciprofloxacin, gatifloxacin, and levofloxacin were evaluated for their abilities to prevent mortality in hamsters infected with a lethal inoculum of Leptospira interrogans serovar Portlandvere. Each agent produced a statistically significant survival advantage compared to no treatment and demonstrated survival similar to that seen with doxycycline therapy.
The ideal treatment for acute leptospirosis has not been defined (6). The fluoroquinolones have in vitro activity against Leptospira spp. and thus have potential as therapeutic agents (8, 9, 13). Despite this, evaluation of their in vivo efficacies has been limited and has yielded conflicting results (11, 14). In this study, the efficacies of ciprofloxacin, gatifloxacin, and levofloxacin in preventing mortality in a hamster model of acute lethal leptospirosis were investigated.
Female Golden Syrian hamsters (Harlan-Sprague-Dawley, Indianapolis, IN) were infected by intraperitoneal injection of 105 Leptospira interrogans serovar Portlandvere organisms as previously described (7). In each experiment, groups of 10 hamsters received various doses of a study medication (ciprofloxacin, gatifloxacin, or levofloxacin) via intraperitoneal injection once daily for 5 days, starting on the second day after infection. The doses of the study agents used were 5, 25, and 50 mg/kg of body weight/day. The dose of 50 mg/kg/day was chosen as it approximated the human equivalent doses delivered by 500 mg of levofloxacin, 400 mg of gatifloxacin, and 400 mg of ciprofloxacin based on body surface area (5). Five- and 25-mg/kg/day doses were chosen to determine if efficacy would be seen with lower doses. In order to ensure reproducibility within the model and to verify the lethality of the inoculum, each study medication group was compared with a group of hamsters that received 5 days of doxycycline (5 mg/kg/day) and with an untreated control group. The experiments involving levofloxacin and ciprofloxacin were performed twice. Because of the development of diarrhea in animals treated with gatifloxacin, only a single experiment was performed with this drug. Kaplan-Meier plots were constructed for each study medication, and survival differences between study groups were compared by the log rank test. P values of ≤0.05 were considered significant. All experimentation was approved by our Institutional Animal Care and Use Committee.
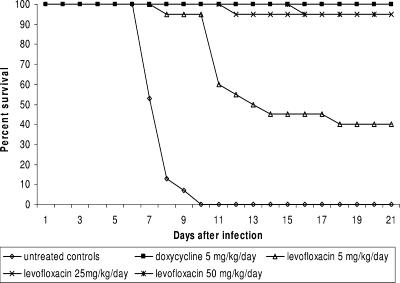
All untreated animals died by the eleventh day after infection. All but one (49/50) of the animals treated with doxycycline survived to the end of the study. Ninety-five percent of animals treated with levofloxacin at doses of 25 and 50 mg/kg/day survived to study the completion of the study, whereas the 5-mg/kg/day dose produced 40% survival (Fig. 1). Treatment with any dose of levofloxacin for 5 days significantly improved survival compared to no treatment (P < 0.01). At doses at or above 25 mg/kg/day, survival with levofloxacin therapy was not statistically different from that with doxycycline therapy.
FIG. 1.
Survival of hamsters with acute leptospirosis treated with levofloxacin.
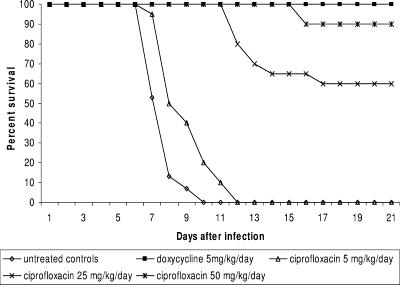
Ciprofloxacin therapy resulted in survival rates of 90% when given at 50 mg/kg/day and 60% when given at 25 mg/kg/day. All animals treated with 5 mg/kg/day of ciprofloxacin died prior to the end of the study (Fig. 2). All doses produced either significantly improved survival (P < 0.01) or, in the case of the lowest dose, a significant delay in mortality (P = 0.001) compared to no treatment. However, only the survival produced by treatment with 50 mg/kg/day was not statistically different from survival with doxycycline.
FIG. 2.
Survival of hamsters with acute leptospirosis treated with ciprofloxacin.
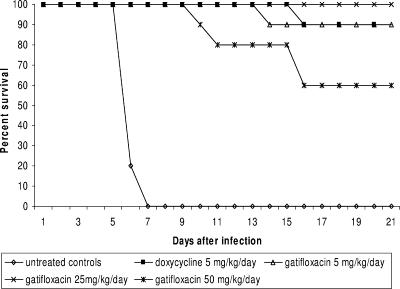
Gatifloxacin therapy at any dose improved survival compared to that for untreated animals (Fig. 3; P < 0.01). Survival rates with 5 and 25 mg/kg/day of gatifloxacin were 90 and 100%, respectively, whereas survival with 50 mg/kg/day was 60%. The decrease in survival seen in the high-dose gatifloxacin group appeared to be due to the development of antibiotic-associated diarrhea.
FIG. 3.
Survival of hamsters with acute leptospirosis treated with gatifloxacin.
In this study, levofloxacin, gatifloxacin, and ciprofloxacin were able to prevent mortality from acute leptospirosis in hamsters. When given at higher doses, each drug performed similarly to doxycycline, a well-accepted therapy for leptospirosis. This suggests that the fluoroquinolones may be effective therapy for cases of human leptospirosis.
At first glance, the fluoroquinolones seem like ideal therapy for leptospirosis. They are active against numerous Leptospira serovars in vitro, and their broad spectrum of activity allows coverage of other diseases that may be considered in differential diagnosis (8, 9, 13). Their long half-lives, good oral bioavailability, and relative lack of adverse effects allow convenient and easy-to-tolerate dosing regimens. Despite these advantages, their in vivo efficacies have not been adequately evaluated (3). To our knowledge, only two prior investigations have examined their use in animal models, and these gave conflicting results, likely because of methodological differences (11, 14).
Gatifloxacin performed well in this study, but treatment with high doses of this medication was associated with increased toxicity from diarrhea. Hamsters are very susceptible to antibiotic-associated diarrhea, and the wide antimicrobial spectrum of gatifloxacin (to include coverage of anaerobic bacteria) likely increased the risk of diarrhea in our animals.
Ciprofloxacin demonstrated a survival benefit smaller than those of the other fluoroquinolones, with only the highest dose producing survival similar to that for doxycycline. In vitro, ciprofloxacin is as active as levofloxacin, so this decrease in efficacy is not likely due to decreased activity of the antimicrobial itself (8, 9). Rather, the pharmacodynamics of ciprofloxacin are likely to blame. The pharmacodynamic index best predictive of fluoroquinolone efficacy is the ratio of the area under the concentration curve (AUC) relative to the MIC (1, 4, 10). The AUC for ciprofloxacin in hamsters is smaller than that for other fluoroquinolones in the dose range used in this experiment (2, 12). Thus, the low efficacy of ciprofloxacin compared to those of the other two agents is most likely related to relative underdosing.
This pharmacodynamic consideration highlights the main limitation of this study. While an attempt was made based on body surface area to use experimental doses that were roughly equivalent to the usual human doses of these medications, the doses used in this study likely produced AUCs for hamsters which are smaller than those obtained for humans, leading to relative underdosing for all study medications compared to doses for humans (2, 12). That aside, improved mortality was demonstrated in this model even with these relatively small AUCs. This suggests that these medications, when given at the usual human doses, are likely to be efficacious.
In this study, the fluoroquinolones were shown to be effective in reducing hamster mortality in a model of acute leptospirosis. Further research into their potential use against human leptospirosis is warranted.
Acknowledgments
The views expressed herein are those of the authors and do not reflect the official policy or position of the Department of the Army, the Department of Defense, or the U.S. government.
None of the authors have conflicts of interest.
Footnotes
Published ahead of print on 30 April 2007.
REFERENCES
- 1.Andes, D., and W. A. Craig. 2002. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob. Agents Chemother. 461665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai, S., Y. Gohara, A. Akashi, K. Kuwano, M. Nishimoto, T. Yano, K. Oizumi, K. Takeda, and T. Yamaguchi. 1993. Effects of new quinolones on Mycoplasma pneumonia-infected hamsters. Antimicrob. Agents Chemother. 37287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bal, A. M. 2005. Use of ciprofloxacin for treating leptospirosis—need for clinical trials. J. Med. Microbiol. 54907. [DOI] [PubMed] [Google Scholar]
- 4.Bedos, J. P., E. Azoulay-Dupuis, P. Moine, M. Muffat-Joly, B. Veber, J. J. Pocidalo, and E. Vallee. 1998. Pharmacodynamic activities of ciprofloxacin and sparfloxacin in a murine pneumococcal pneumonia model: relevance for drug efficacy. J. Pharmacol. Exp. Ther. 28629-35. [PubMed] [Google Scholar]
- 5.Food and Drug Administration, U.S. Department of Health and Human Services. 2002. Guidance for industry and reviewers: estimating the safe starting dose in clinical trials for therapeutics in adult healthy volunteers. Draft document. http://www.fda.gov/cber/gdlns/dose.pdf.
- 6.Griffith, M. E., D. R. Hospenthal, and C. K. Murray. 2006. Antimicrobial therapy of leptospirosis. Curr. Opin. Infect. Dis. 19533-537. [DOI] [PubMed] [Google Scholar]
- 7.Moon, J. E., M. W. Ellis, M. E. Griffith, J. S. Hawley, R. G. Rivard, S. McCall, D. R. Hospenthal, and C. K. Murray. 2006. Efficacy of macrolides and telithromycin against leptospirosis in a hamster model. Antimicrob. Agents Chemother. 501989-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray, C. K., and D. R. Hospenthal. 2004. Broth microdilution susceptibility testing for Leptospira spp. Antimicrob. Agents Chemother. 481548-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray, C. K., and D. R. Hospenthal. 2006. Determination of susceptibilities of 26 Leptospira sp. serovars to 24 antimicrobial agents by a broth microdilution technique. Antimicrob. Agents Chemother. 484002-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scaglione, F., J. W. Mouton, R. Mattina, and F. Fraschini. 2003. Pharmacodynamics of levofloxacin and ciprofloxacin in a murine pneumonia model: peak concentration/MIC versus area under the curve/MIC ratios. Antimicrob. Agents Chemother. 472749-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shalit, I., A. Barnea, and A. Shahar. 1989. Efficacy of ciprofloxacin against Leptospira interrogans serogroup icterohaemorrhagiae. Antimicrob. Agents Chemother. 33788-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahata, M., M. Shimakura, R. Hori, K. Kizawa, Y. Todo, S. Minami, Y. Watanabe, and H. Narita. 2001. In vitro and in vivo efficacies of T-3811ME (BMS-284756) against Mycoplasma pneumonia. Antimicrob. Agents Chemother. 45312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takashima, I., M. Ngoma, and N. Hashimoto. 1993. Antimicrobial effects of a new carboxyquinolone drug, Q35, on five serogroups of Leptospira interrogans. Antimicrob. Agents Chemother. 37901-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truccolo, J., F. Charavay, F. Merien, and P. Perolat. 2002. Quantitative PCR assay to evaluate ampicillin, ofloxacin, and doxycycline for treatment of experimental leptospirosis. Antimicrob. Agents Chemother. 46848-853. [DOI] [PMC free article] [PubMed] [Google Scholar]