Abstract
We compared the efficacy of a novel rifamycin derivative, ABI-0043, with that of rifampin, alone and in combination with levofloxacin, against methicillin-susceptible Staphylococcus aureus ATCC 29213 in a guinea pig tissue-cage infection model. The MIC, logarithmic-growth-phase minimal bactericidal concentration, and stationary-growth-phase minimal bactericidal concentration of ABI-0043 were 0.001, 0.008, and 0.25 μg/ml, respectively; the corresponding concentrations of rifampin were 0.016, 0.8, and 3.6 μg/ml, respectively. After a single intraperitoneal dose of 12.5 mg/kg of body weight, the peak concentration in cage fluid was 1.13 μg/ml of ABI-0043 and 0.98 μg/ml of rifampin. Five days after completion of treatment, levofloxacin administered alone (5 mg/kg/12 h) resulted in bacterial counts in cage fluid that were similar to those for untreated controls (>8.0 log10 CFU/ml), whereas rifampin and ABI-0043 administered alone (12.5 mg/kg/12 h) decreased the mean titers of bacteria ± standard deviations to 1.43 ± 0.28 log10 and 1.57 ± 0.53 log10 CFU/ml, respectively, in cage fluid. In combination with levofloxacin, both rifamycins cleared bacteria from the cage fluid. The cure rates of cage-associated infections with rifampin and ABI-0043 administered alone were 46% and 58%, respectively, and increased to 88% and 92% in combination with levofloxacin. Emergence of rifamycin resistance was observed in 42% of cages after ABI-0043 therapy and in 38% of cages after rifampin therapy; no emergence of resistance occurred with combination treatment with levofloxacin. In conclusion, ABI-0043 had cure rates comparable to that of rifampin. ABI-0043 in combination with a quinolone has the potential for treatment of implant-associated infections caused by susceptible strains of S. aureus, potentially without drug-drug interactions.
Implanted devices are increasingly used in modern medicine to improve function (e.g., internal fixation, neurosurgical shunts, prosthetic heart valves), to alleviate pain (e.g., prosthetic joints), or for aesthetic reasons (e.g., breast implants) (6). Despite the use of perioperative antimicrobial prophylaxis, infection of the implant can occur, since less than 100 CFU of microorganisms can cause an infection when a foreign body is present (37). If infection occurs, it causes high morbidity and substantial cost, especially in orthopedic surgery (23). Since bacteria grow and persist as biofilms on the implant surface, they are difficult to eradicate (25). Consequently, until recently, standard treatment approaches to these infections in orthopedic surgery included removal or replacement of all foreign-body material (4). Since this surgical concept is invasive, may cause considerable tissue damage, and requires prolonged hospital stay, it has been challenged (26, 28, 36).
Successful chemotherapeutic treatment of device-associated infection requires an antimicrobial agent that acts against surface-adhering microorganisms in the stationary growth phase (1, 5, 29, 31, 34). Rifampin fulfills this requirement for staphylococci, based on experimental animal models (21, 29, 34) and on clinical studies (9, 30, 38). However, rifampin has several drawbacks, including important drug interactions due to liver enzyme induction, poor clinical tolerance (nausea, fever, myalgia), and rapid emergence of resistance (20, 32). Therefore, a novel rifamycin derivative that overcomes these shortcomings will be of interest.
Rifalazil, the most advanced of a new generation of rifamycins, the benzoxazinorifamycins, has the benefit of a long terminal half-life, a large volume of distribution, good tissue penetration, and lack of significant interactions with liver microsomal enzymes (cytochrome P450 system) (19). In a human clinical trial, rifalazil has shown efficacy in the treatment of sexually transmitted disease caused by Chlamydia trachomatis (22). Rifalazil is currently undergoing clinical testing for peripheral arterial disease, based on the hypothesis that this condition is associated with infection in the vasculature caused by Chlamydia pneumoniae (19). Derivative compounds, new chemical entities (NCEs) such as ABI-0043, ABI-0369, and ABI-0699, are of particular interest in the treatment of serious gram-positive infections. These compounds have even more potent activity in vitro than rifampin or rifalazil and retain some activity against rifampin-resistant strains (13). They are also effective at lower doses than rifampin or rifalazil in murine models of infection (17). Like rifalazil, the NCEs do not interact with P450 CYP 3A4 (19), whereas the induction of P450 enzymes by rifampin has been shown to be responsible for a number of drug-drug interactions (16, 32).
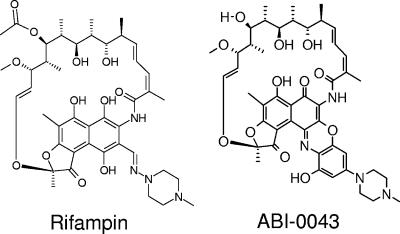
In this work, one representative NCE, ABI-0043 (Fig. 1), was tested against Staphylococcus aureus in vitro. Subsequently, the potential of ABI-0043 was investigated alone and in combination with levofloxacin for the treatment of implant-associated infections caused by a methicillin-susceptible S. aureus in guinea pigs.
FIG. 1.
Chemical structure of rifampin and its derivative compound ABI-0043.
(Part of the results were presented at the 45th Interscience Conference of Antimicrobial Agents and Chemotherapy, Washington, DC, 16 to 19 December 2005 [LB-6].)
MATERIALS AND METHODS
Study microorganism.
S. aureus ATCC 29213, which is susceptible to methicillin, levofloxacin, and rifampin, was used in this study. The strain was stored at −70°C using a cryovial bead preservation system (Microbank; Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada). One cryoculture bead was incubated in 1 ml of brain heart infusion broth for 7 h at 37°C without shaking. Cultures were then diluted 1:100 in 5 ml of brain heart infusion broth and incubated overnight at 37°C without shaking. Subsequently, bacteria were centrifuged at 4,000 × g for 10 min, and the pellet was washed twice and resuspended in pyrogen-free 0.9% NaCl before use.
Antimicrobial agents.
Levofloxacin intravenous solution (5 mg/ml; Aventis Pharma AG, Zurich, Switzerland) and rifampin in powder form (Medika AG, Aesch, Switzerland) were purchased from the manufacturer. ABI-0043 was provided by ActivBiotics, Inc. (Lexington, MA) in powder form. ABI-0043 powder was dissolved in 100% dimethyl sulfoxide (DMSO; Merck KGaA, Darmstadt, Germany) using bath sonication at 40 kHz ± 5 kHz for 20 min to prepare a stock solution of 50 mg/ml. One volume of stock solution was added to four volumes of diluted liquid fill (18) (375 g Etocas 35NF, 4.4 g pluronic acid F68, 50.8 g polyethylene glycol 400, and 10.8 ml water; 15 volumes were mixed with 33 volumes of water), resulting in a dosing solution containing ABI-0043 in 20% DMSO. The 20% DMSO diluent alone showed no antibacterial effect on S. aureus.
Antimicrobial susceptibility testing.
All in vitro susceptibility tests were performed in triplicate with a standard inoculum of 5 × 105 CFU of S. aureus/ml adjusted from an overnight culture and are reported as median values. The MIC was determined in cation-adjusted Mueller-Hinton broth (MHB) by the macrotube dilution method according to Clinical Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards) guidelines (15). The MIC was the lowest drug concentration that resulted in no detectable growth by visual inspection. During the preparation of serial dilutions, pipette tips were changed after each dilution to ensure minimal carryover of antimicrobial agents. The minimal bactericidal concentration for the logarithmic growth phase (MBClog) was defined as the antimicrobial concentration that reduced the initial bacterial concentration after incubation in cation-adjusted MHB for 24 h by >99.9% (i.e., 3 log10 CFU/ml), as described in the Manual of Clinical Microbiology (14). In addition, the MBC was determined also for the stationary growth phase (MBCstat), as previously described (34). For this purpose, the overnight culture of S. aureus was centrifuged (2,000 × g for 10 min) and resuspended in 0.01 M phosphate-buffered saline (pH 7.4). In this medium, bacterial counts remained within ±15% of the original inoculum in the antimicrobial-free culture for >36 h (data not shown).
Animal model.
A foreign-body infection model in guinea pigs was used, as previously described (2, 21, 33, 35, 37). In brief, four sterile polytetrafluorethylene (Teflon) cages (32 mm by 10 mm), perforated by 130 regularly spaced holes with a 1-mm diameter (Angst-Pfister AG, Zurich, Switzerland), were subcutaneously implanted in flanks of male albino guinea pigs (Charles River; weight, 640 to 800 g) under aseptic conditions. Animals were anesthetized with an intramuscular injection of ketamine (20 mg/kg of body weight) and xylazine (4 mg/kg). Two weeks after surgery, after complete healing of the wounds, the sterility of the cages was verified by culture of aspirated cage fluid. For pharmacokinetic studies, noninfected animals were used. For treatment evaluation, cages were infected by percutaneous inoculation of 200 μl of overnight culture containing 2 × 104 CFU S. aureus. Established infection was confirmed 24 h later by culture of aspirated cage fluid. Guinea pigs were kept under specific pathogen-free conditions in the Animal House of the Department of Research, University Hospital, Basel, Switzerland, and animal experimentation guidelines were followed according to the regulations of Swiss veterinary law. The study protocol was approved by the Institutional Animal Care and Use Committee.
Antimicrobial treatment.
Twenty-four hours after cage infection (day 1), antimicrobial treatment was initiated. Animals were randomized into eight treatment groups: control (saline), levofloxacin at 5 mg/kg, rifampin at 12.5 mg/kg (each dose with or without levofloxacin at 5 mg/kg), and ABI-0043 at 3 mg/kg and 12.5 mg/kg (each dose with or without levofloxacin at 5 mg/kg). The volume of each dose was adjusted to a total volume of 5 ml by adding saline.
Each antibiotic was administered intraperitoneally every 12 h for 4 days (total, 8 doses). Doses and administration intervals for rifampin were chosen to achieve serum concentrations mimicking those in humans, based on previous experience (21, 29, 34). Quantitative cultures of aspirated cage fluid were performed immediately before the initiation of antimicrobial treatment (day 1), before the last antimicrobial dose during the treatment (day 4), and 5 days after completion of treatment (day 9). The animals were then anesthetized with fentanyl-droperidol, and cages were removed under aseptic conditions. Each cage was placed in 10 ml Trypticase soy broth (TSB), vortexed for 30 s, and incubated at 37°C. After 24 h, 50 μl of this broth was plated on 5% sheep blood agar plates (Becton Dickinson, Heidelberg, Germany) to determine the presence of bacteria. Guinea pigs dosed with 20% DMSO prepared with the diluted liquid fill but without ABI-0043 showed no visible symptoms of illness, including loss of weight, or hematological or liver biopsy irregularities over the full course of the study.
Each antimicrobial regimen was evaluated for at least 12 tissue cages (i.e., 3 animals with 4 cages each) by determining (i) the mean log CFU count during the treatment before the last antimicrobial dose (day 4) or 5 days after completion of treatment (day 9) compared to the bacterial counts 24 h after infection, immediately before initiation of treatment (day 1), and (ii) the cure rate, i.e., the fraction of cages in which the infection was eradicated, defined as the absence of growth of S. aureus in TSB containing the explanted cages. Comparisons between categorical variables were performed using χ2 or Fisher's exact tests, as appropriate.
Determination of rifamycin-resistant strains.
Positive cultures from TSB containing the explanted cages (i.e., treatment failures) were screened for rifamycin resistance. For this purpose, multiple colonies of each morphologically distinct colony type were collected from an agar subculture. A standardized inoculum of 5 × 105 CFU S. aureus was plated on Mueller-Hinton agar plates containing 1 μg/ml ABI-0043 or rifampin. Plates were incubated at 37°C and screened for growth after 24 h.
Pharmacokinetic studies.
Samples of cage fluid, corresponding to interstitial fluid, were aspirated by percutaneous cage puncture from noninfected animals at various times for 12 h following intraperitoneal administration of a single dose of ABI-0043 or rifampin. In addition, samples were taken once daily on subsequent days, just prior to dosing of rifampin and ABI-0043 for determination of trough concentrations of antimicrobials. Aliquots of 150 μl of cage fluid were transferred to tubes containing 15 μl of filter-sterilized 1.5% EDTA (pH 7.4), mixed by hand to avoid clotting, and centrifuged (2,000 × g for 10 min); the supernatant was stored at −20°C until further analysis.
Concentrations of ABI-0043 in cage fluid were determined in triplicate by agar plate diffusion bioassay using Streptococcus pneumoniae ATCC 49619 as the indicator organism. S. pneumoniae was grown overnight in cation-adjusted MHB supplemented with 3% laked horse blood (Oxoid, Basingstoke, Hampshire, United Kingdom), diluted 1:50 in the same medium, and grown until turbid (i.e., for 6 to 7 h). MHB agar plus 5% defibrinated sheep blood was warmed to 37°C and was then added to large assay plates (30 by 30 cm). After hardening of the base agar, 1.5 ml of the S. pneumoniae culture was diluted in 10 ml MHB seed agar at 48°C, poured onto the assay plates, and allowed to solidify. Concentrations of rifampin were determined by a bioassay using Micrococcus luteus and those of levofloxacin were determined using Escherichia coli V6311/65, as previously described (2). The bioassay detection limit was 0.0005 μg/ml for ABI-0043, 0.001 μg/ml for rifampin, and 0.01 μg/ml for levofloxacin.
Cage fluid was serially diluted two- and fourfold in serum; 10 μl of each cage fluid dilution was spotted onto an assay plate and incubated overnight. The diameters of zones of inhibition were measured with calipers. The mean intra- and interassay coefficients of variation were <5%. Results were calculated by exponential regression analysis (Microsoft Office Excel 2003; Microsoft Corporation, Redmond, WA). The concentration-time data were analyzed with WinNonlin (Pharsight Corp., Mountain View, CA).
At each time point, the mean concentration of two cages from three animals (n = 6) was used to calculate concentrations and error bars. The area under the concentration-time curve from time zero to 12 h (AUC0-12) was determined by the log-linear trapezoidal method.
RESULTS
Antimicrobial susceptibility.
Table 1 compares the in vitro susceptibilities of S. aureus to the pharmacokinetic parameters (peak concentration in cage fluid). For levofloxacin, the MBCstat was 125 times higher than the MBClog, whereas the rifamycins showed lower ratios between MBCstat and MBClog (4.5- to 31.3-fold). ABI-0043 had the lowest MIC, MBClog, and MBCstat values for S. aureus (0.001, 0.008, and 0.25 μg/ml, respectively), which are reflected by the highest ratios of peak/MIC and peak/MBCstat in cage fluid.
TABLE 1.
In vitro susceptibility of S. aureus and linked pharmacokinetic data
| Drug | MIC (μg/ml) | MBClog (μg/ml) | MBCstat (μg/ml) | MBCstat/MBClog ratio | Peaka/MIC ratio | Peaka/MBCstat ratio |
|---|---|---|---|---|---|---|
| Levofloxacin | 0.16 | 0.32 | 40 | 125 | 6.1 | 0.02 |
| Rifampin | 0.016 | 0.8 | 3.6 | 4.5 | 61.2 | 0.27 |
| ABI-0043 | 0.001 | 0.008 | 0.25 | 31.3 | 1130 | 4.52 |
Peak concentration after a single intraperitoneal dose of 5 mg/kg (for levofloxacin) or 12.5 mg/kg (for rifampin and ABI-0043) was used.
Pharmacokinetic studies.
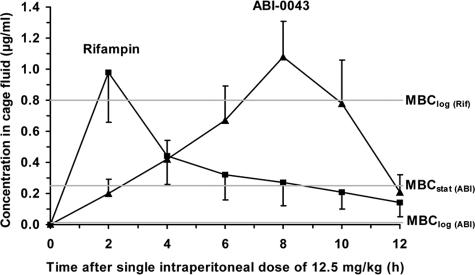
Table 2 shows the pharmacokinetics in cage fluid after single and multiple intraperitoneal administrations in noninfected animals. As shown in Fig. 2, the drug concentration of ABI-0043 in cage fluid from noninfected animals after a single dose of 12.5 mg/kg (day 1) was well above the MIC, the MBClog, and the MBCstat. Following this single dose, the AUC0-12 of ABI-0043 (6.53 μg·h/ml) was similar to that of rifampin (4.56 μg·h/ml). However, the peak concentration of rifampin was achieved after a single intraperitoneal dose at 2 h, whereas ABI-0043 had a longer time to peak concentration in serum (8 h). The terminal half-life of rifampin was calculated to be 5.8 h. The determination of the half-life of ABI-0043 from the single-dose data is difficult because of the longer time to peak concentration, yet it is considerably longer than that of rifampin, based on trough levels following multiple dosing. ABI-0043 continued to accumulate through the 4 days of dosing, after rifampin reached the steady state (Table 2).
TABLE 2.
Pharmacokinetics in cage fluid after intraperitoneal administration of antimicrobials into noninfected animalsa
| Dosing regimen | Antibiotic | Dose (mg/kg) | Cmax (μg/ml) | Cmin (μg/ml) |
|---|---|---|---|---|
| Single dose | Levofloxacin | 5 | 0.97 ± 0.20 | 0.01 ± 0.01 |
| Rifampin | 12.5 | 0.98 ± 0.32 | 0.14 ± 0.09 | |
| ABI-0043 | 3 | 0.11 ± 0.03 | 0.04 ± 0.01 | |
| ABI-0043 | 12.5 | 1.13 ± 0.23 | 0.14 ± 0.11 | |
| Multiple doseb | Rifampin | 12.5 | 0.61 ± 0.13 | 0.22 ± 0.09 |
| ABI-0043 | 12.5 | 0.82 ± 0.49 | 0.41 ± 0.09 |
Concentration values are means ± standard deviations from 12 cage fluid aspirates. The Cmin (trough concentration) was measured 12 h after dosing.
The indicated dose was administered every 12 h for 4 days; the pharmacokinetic values were determined on day 4.
FIG. 2.
Pharmacokinetics in cage fluid after a single intraperitoneal dose (12.5 mg/kg) of rifampin (▪) and ABI-0043 (▴). Values are means ± standard deviations (n = 6). Horizontal lines indicate the corresponding MBClog and MBCstat values for S. aureus with rifampin (Rif) and ABI-0043 (ABI).
Antimicrobial treatment studies.
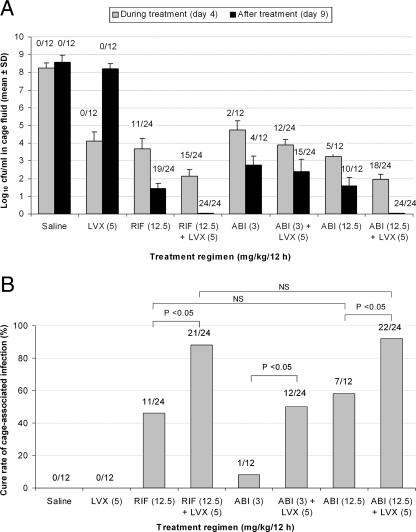
Cages were sterile prior to inoculation. The titer of bacteria 24 h after infection increased to ≈107 CFU/ml S. aureus in all cage fluid samples (data not shown). In the cage fluid of infected, untreated control animals, bacterial counts increased during 9 days after infection to >108 CFU/ml of cage fluid (Fig. 3A). No spontaneous cure of cage-associated infection occurred in 12 untreated cages through the entire 9 days of the study. Levofloxacin alone reduced the bacterial count during treatment, but there was a rebound in the bacterial titer to counts similar to those of untreated controls 5 days after treatment ended. Rifampin alone decreased the mean titer of bacteria after 4 days of treatment to 3.68 ± 0.59 log10 CFU/ml and further to 1.43 ± 0.28 log10 CFU/ml 5 days after completion of treatment, whereas in combination with levofloxacin, the reduction reached 2.14 ± 0.35 log10 CFU/ml before the last dose of treatment and 0 CFU/ml 5 days after completion of treatment. ABI-0043 at 12 mg/kg/12 h alone and with levofloxacin induced a comparable reduction of bacterial titers during treatment (3.24 ± 0.27 log10 CFU/ml and 1.95 ± 0.34 log10 CFU/ml, respectively) and after treatment (1.57 ± 0.53 log10 CFU/ml and 0 CFU/ml, respectively).
FIG. 3.
Treatment efficacy with different antimicrobial regimens. (A) Mean log10 CFU/ml S. aureus in aspirated cage fluid during (day 4) and after treatment (day 9). (B) Cure rates of cage-associated infection determined by the fraction of explanted cages without growth of S. aureus in TSB. In both panels, numbers above the bars represent the numbers of culture-negative cage specimens/total numbers of cage fluid specimens. SD, standard deviation; LVX, levofloxacin; RIF, rifampin; ABI, ABI-0043; NS, not significant.
With levofloxacin alone, no cage-associated infection was cured (Fig. 3B). Rifampin as a single treatment (12.5 mg/kg/12 h) showed a cure rate of 46% (11 of 24 cages), which was further improved to 88% (21 of 24 cages) by the addition of levofloxacin (P < 0.05). Similarly, exposure to ABI-0043 alone at 12.5 mg/kg/12 h resulted in a cure rate of 58% (7 of 12 cages), compared with 92% (22 of 24 cages) when it was used in combination with levofloxacin (P < 0.05). The low-dose therapy of ABI-0043 (3 mg/kg/12 h) had a cure rate of only 8% (1 of 12 cages), which increased to 50% (12 of 24 cages) when it was used in combination with levofloxacin (P < 0.05). The high-dose therapy with ABI-0043 (12.5 mg/kg/12 h) was comparable to that with rifampin (12.5 mg/kg/12 h) and superior to the low-dose therapy with ABI-0043 (3 mg/kg/12 h).
Emergence of rifamycin resistance.
After the 4-day therapy, emergence of resistance against ABI-0043 was observed for 8 of 12 (67%) cultures from explanted cages after low-dose ABI-0043 (3 mg/kg/12 h) and in 5 of 12 (42%) cage cultures after high-dose ABI-0043 (12.5 mg/kg/12 h). Rifampin-resistant strains were detected after rifampin therapy in 9 of 24 (38%) cage cultures. No emergence of resistance occurred during rifampin or ABI-0043 combination therapy with levofloxacin.
DISCUSSION
Implant-associated infections are characterized by persisting surface-adhering microorganisms and the lack of spontaneous cure (7). The biofilm mode of growth represents a basic mechanism by which microorganisms survive and persist in the presence of a foreign body (5). Depletion of metabolic substances and/or waste product accumulation within biofilms causes microbes to enter a slow- or nongrowing (stationary) state, in which microbes are up to 1,000 times more resistant to growth-dependent antimicrobial agents than are planktonic (free-living) bacteria (8, 24). Because of this, standard antimicrobial susceptibility tests do not predict the outcome in implant-associated infections (3, 29, 31, 34, 38). An animal model in which the eradication of device-associated bacteria can be monitored would allow the development of valuable concepts for antimicrobial therapy of such infections. The tissue-cage infection model in guinea pigs fulfills this requirement and was validated in clinical studies (11, 12, 27, 30, 38). In addition, the susceptibility of guinea pigs to cage infection closely simulates the human situation. The clinical use of rifampin in implant-associated infections has been based mainly on experimental studies using this model (2, 21, 29, 35). Therefore, in the present study, a novel rifamycin has been tested and compared with rifampin.
Rifampin has traditionally been used against mycobacteria. However, during the last 25 years, it has been increasingly used against staphylococci (20). Because of its efficacy against adherent and stationary-phase staphylococci, rifampin has become a standard combination drug in patients with device-associated infection (36). Since rifampin has several drawbacks, we tested ABI-0043, a representative of the most recent series of benzoxazinorifamycins that have more potent activity in vitro than rifampin and rifalazil against S. aureus. This new compound differs from rifampin in that it contains a four-ring, planar structure (Fig. 1), which may account for its greater lipophilicity, tissue penetration, and longer half-life (17, 18). An important distinguishing characteristic compared with rifampin is that ABI-0043 lacks significant interactions with liver microsomal enzymes (18). This is a significant advantage, especially in elderly patients with comorbidity requiring the use of many different drugs. In addition, ABI-0043 retains greater activity against strains highly resistant to rifampin, although emergence of resistance by single-step mutation occurs at a similar frequency as with rifampin (13), as we observed also in this study. It will be important to obtain data on human tolerance to fully evaluate NCEs, such as ABI-0043.
In this study, ABI-0043 was bactericidal for the logarithmic growing S. aureus at a concentration of 0.008 μg/ml. The MBCstat against S. aureus was below the peak and even trough levels of ABI-0043 in the cage fluid. Accordingly, ABI-0043 (12.5 mg/kg/12 h) eliminated staphylococcal infection in 58% of tissue cages when used as single agent and in 92% of cages when used in combination with levofloxacin. These cure rates for ABI-0043 were similar to those for rifampin, indicating its potency in device-associated infection. Related NCEs ABI-0369 and ABI-0699 have comparable MBCs against stationary cells (data not shown). The efficacy against staphylococcal foreign-body infection observed for ABI-0043 is expected to be exhibited also from these NCEs (34).
When used as single agent, the failure rate was high with rifampin as well as with ABI-0043. During the 4-day therapy, emergence of resistance was observed in 67% of cages after low-dose ABI-0043, in 42% of cages after high-dose ABI-0043, and in 38% of cages after rifampin therapy. Since S. aureus strains were grown in antibiotic-free medium before being plated on medium containing rifamycin, an unstable resistance could be lost. Nevertheless, the tendency to develop rifamycin resistance was considerably higher when rifamycin was administered alone than when it was used in the combination treatment with levofloxacin. In contrast, no emergence of resistance occurred during rifampin and ABI-0043 combination therapies with levofloxacin. Thus, both rifamycin compounds should be combined with another antimicrobial substance in this clinical setting. Quinolones are suitable combination agents due to their excellent bioavailability (≈100%), spectrum of activity, safety, and tolerability (38). Ciprofloxacin and ofloxacin have been used extensively in long-term therapy of bone infections and proved their efficacy against prosthetic joint infections in combination, particularly with rifampin (10, 30, 38). Newer quinolones have a better activity against gram-positive cocci than ciprofloxacin, but clinical data with levofloxacin, moxifloxacin, or gatifloxacin are still sparse.
In conclusion, ABI-0043 had activity similar to that of rifampin against experimental device-associated infection, and its lack of serious drug-drug interactions make it a promising candidate for treatment of these infections.
Acknowledgments
This study was supported by an unrestricted educational grant from ActivBiotics, Inc., Lexington, MA, and by the Swiss National Science Foundation (3200B0-112547/1).
The authors thank Andrea Steinhuber and Reno Frei for critical reviews of the manuscript. We thank Zarko Rajacic, Mathias Schmaler, Daniela Baldoni, and Danica Nogarth for useful suggestions and laboratory assistance.
Footnotes
Published ahead of print on 14 May 2007.
REFERENCES
- 1.Anderl, J. N., J. Zahller, F. Roe, and P. S. Stewart. 2003. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 471251-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser, J., P. Vergeres, A. F. Widmer, and W. Zimmerli. 1995. In vivo verification of in vitro model of antibiotic treatment of device-related infection. Antimicrob. Agents Chemother. 391134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, C. M., W. W. Sistrunk, M. C. Duffy, A. D. Hanssen, J. M. Steckelberg, D. M. Ilstrup, and D. R. Osmon. 1997. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin. Infect. Dis. 24914-919. [DOI] [PubMed] [Google Scholar]
- 4.Brause, B. D. 2005. Infections with prostheses in bones and joints, p. 1332-1337. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. W.B. Saunders, Washington, DC.
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 2841318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Darouiche, R. O. 2001. Device-associated infections: a macroproblem that starts with microadherence. Clin. Infect. Dis. 331567-1572. [DOI] [PubMed] [Google Scholar]
- 7.Donlan, R. M. 2001. Biofilms and device-associated infections. Emerg. Infect. Dis. 7277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drancourt, M., A. Stein, J. N. Argenson, R. Roiron, P. Groulier, and D. Raoult. 1997. Oral treatment of Staphylococcus spp. infected orthopaedic implants with fusidic acid or ofloxacin in combination with rifampicin. J. Antimicrob. Chemother. 39235-240. [DOI] [PubMed] [Google Scholar]
- 10.Drancourt, M., A. Stein, J. N. Argenson, A. Zannier, G. Curvale, and D. Raoult. 1993. Oral rifampin plus ofloxacin for treatment of Staphylococcus-infected orthopedic implants. Antimicrob. Agents Chemother. 371214-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giulieri, S. G., P. Graber, P. E. Ochsner, and W. Zimmerli. 2004. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection 32222-228. [DOI] [PubMed] [Google Scholar]
- 12.Laffer, R. R., P. Graber, P. E. Ochsner, and W. Zimmerli. 2006. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin. Microbiol. Infect. 12433-439. [DOI] [PubMed] [Google Scholar]
- 13.Murphy, C. K., S. Mullin, M. S. Osburne, J. van Duzer, J. Siedlecki, X. Yu, K. Kerstein, M. Cynamon, and D. M. Rothstein. 2006. In vitro activity of novel rifamycins against rifamycin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 50827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken. 2003. Manual of clinical microbiology. American Society for Microbiology, Washington, DC.
- 15.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. NCCLS document M7-A7. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 16.Niemi, M., J. T. Backman, M. F. Fromm, P. J. Neuvonen, and K. T. Kivisto. 2003. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin. Pharmacokinet. 42819-850. [DOI] [PubMed] [Google Scholar]
- 17.Rothstein, D. M., R. S. Farquhar, K. Sirokman, K. L. Sondergaard, C. Hazlett, A. A. Doye, J. K. Gwathmey, S. Mullin, J. van Duzer, and C. K. Murphy. 2006. Efficacy of novel rifamycin derivatives against rifamycin-sensitive and -resistant Staphylococcus aureus isolates in murine models of infection. Antimicrob. Agents Chemother. 503658-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothstein, D. M., A. D. Hartman, M. H. Cynamon, and B. I. Eisenstein. 2003. Development potential of rifalazil. Expert Opin. Investig. Drugs 12255-271. [DOI] [PubMed] [Google Scholar]
- 19.Rothstein, D. M., C. Shalish, C. K. Murphy, A. Sternlicht, and L. A. Campbell. 2006. Development potential of rifalazil and other benzoxazinorifamycins. Expert Opin. Investig. Drugs 15603-623. [DOI] [PubMed] [Google Scholar]
- 20.Sande, M. A. 1983. The use of rifampin in the treatment of nontuberculous infections: an overview. Rev. Infect. Dis. 5(Suppl. 3)S399-S401. [DOI] [PubMed] [Google Scholar]
- 21.Schwank, S., Z. Rajacic, W. Zimmerli, and J. Blaser. 1998. Impact of bacterial biofilm formation on in vitro and in vivo activities of antibiotics. Antimicrob. Agents Chemother. 42895-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamm, W. E., B. E. Batteiger, W. M. McCormack, P. A. Totten, A. Sternlicht, and N. M. Kivel for the Rifalazil Study Group. 8 February 2007, posting date. A randomized, double-blind study comparing single-dose rifalazil with single-dose azithromycin for the empirical treatment of nongonococcal urethritis in men. Sex. Transm. Dis. doi: 10.1097/01.olg.0000253348.44308.8c. [DOI] [PubMed]
- 23.Steckelberg, J. M., and D. R. Osmon. 2000. Prosthetic joint infection, p. 173-209. In A. L. Bisno and F. A. Waldvogel (ed.), Infections associated with indwelling medical devices. American Society for Microbiology, Washington, DC.
- 24.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358135-138. [DOI] [PubMed] [Google Scholar]
- 25.Trampuz, A., D. R. Osmon, A. D. Hanssen, J. M. Steckelberg, and R. Patel. 2003. Molecular and antibiofilm approaches to prosthetic joint infection. Clin. Orthop. Relat. Res. 41469-88. [DOI] [PubMed] [Google Scholar]
- 26.Trampuz, A., and A. F. Widmer. 2006. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 19349-356. [DOI] [PubMed] [Google Scholar]
- 27.Trebse, R., V. Pisot, and A. Trampuz. 2005. Treatment of infected retained implants. J. Bone Joint Surg. Br. 87B249-256. [DOI] [PubMed] [Google Scholar]
- 28.Widmer, A. F. 2001. New developments in diagnosis and treatment of infection in orthopedic implants. Clin. Infect. Dis. 33(Suppl. 2)S94-S106. [DOI] [PubMed] [Google Scholar]
- 29.Widmer, A. F., R. Frei, Z. Rajacic, and W. Zimmerli. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J. Infect. Dis. 16296-102. [DOI] [PubMed] [Google Scholar]
- 30.Widmer, A. F., A. Gaechter, P. E. Ochsner, and W. Zimmerli. 1992. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin. Infect. Dis. 141251-1253. [DOI] [PubMed] [Google Scholar]
- 31.Widmer, A. F., A. Wiestner, R. Frei, and W. Zimmerli. 1991. Killing of nongrowing and adherent Escherichia coli determines drug efficacy in device-related infections. Antimicrob. Agents Chemother. 35741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zavasky, D. M., and M. A. Sande. 1998. Reconsideration of rifampin: a unique drug for a unique infection. JAMA 2791575-1577. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerli, W. 1993. Experimental models in the investigation of device-related infections. J. Antimicrob. Chemother. 31(Suppl. D)97-102. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerli, W., R. Frei, A. F. Widmer, and Z. Rajacic. 1994. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J. Antimicrob. Chemother. 33959-967. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerli, W., P. D. Lew, and F. A. Waldvogel. 1984. Pathogenesis of foreign body infection: evidence for a local granulocyte defect. J. Clin. Investig. 731191-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmerli, W., A. Trampuz, and P. E. Ochsner. 2004. Prosthetic-joint infections. N. Engl. J. Med. 3511645-1654. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerli, W., F. A. Waldvogel, P. Vaudaux, and U. E. Nydegger. 1982. Pathogenesis of foreign body infection: description and characteristics of an animal model. J. Infect. Dis. 146487-497. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerli, W., A. F. Widmer, M. Blatter, R. Frei, P. E. Ochsner, et al. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA 2791537-1541. [DOI] [PubMed] [Google Scholar]