Abstract
Staphylococci, common orthopedic pathogens, form antibiotic-resistant biofilms. Polymethylmethacrylate (PMMA) beads loaded with the quorum-sensing inhibitor RNAIII-inhibiting peptide (RIP) were implanted in rats and shown to prevent methicillin-resistant Staphylococcus aureus infection. RIP release was bimodal, typical of previously-tested antibiotics. These results suggest that RIP-PMMA warrants further evaluation for management of orthopedic infections caused by staphylococci.
Bacterial biofilms are important in the pathogenesis of many diseases, including osteomyelitis and prosthetic joint infections (8). Staphylococci are common pathogens in orthopedic infections (2, 10). The quorum-sensing inhibitor RNAIII-inhibiting peptide (RIP) inhibits staphylococcal-biofilm formation by inhibiting the phosphorylation of its target protein, TRAP, leading to suppression of virulence factors produced by staphylococci (4-7).
Polymethylmethacrylate (PMMA) is used in orthopedic surgery as a drug delivery system wherein antimicrobial agents are loaded into PMMA to deliver drugs to tissues with limited blood flow and to deliver high local antimicrobial concentrations (9).
The aims of this study were to determine the in vitro compatibility of RIP with PMMA, to measure the release of RIP from PMMA beads in a continuous-flow chamber, and to determine whether RIP-coated PMMA beads prevent Staphylococcus aureus biofilm formation in an experimental animal model.
In vitro experiments.
Vancomycin hydrochloride was purchased from USP, Novaplus, Harrisburg, PA. The amide form of RIP (YSPWTNF-NH2) was synthesized by Neosystem (Strasbourg, France). Beads were prepared by adding RIP or vancomycin powder to Surgical Simplex P radioopaque bone cement (Stryker Orthopedics, Mahwah, NJ) containing powder (3 g PMMA, 15 g methyl methacrylate-styrene-copolymer, 2 g barium sulfate, USP) and liquid (9.75 ml methyl methacrylate, 0.25 ml N, N-dimethyl-para-toluidine, 75 ± 15 ppm hydroquinone).
Beads containing 7.5%, 15%, and 22.5% (weight/weight) of drug were prepared by mixing 150, 200, or 340 mg RIP powder with 1,850, 1,132, or 1,170 mg of cement powder, respectively. We then added 0.925, 0.626, or 0.585 ml of methyl methacrylate liquid monomer, respectively, and spread the mixture into a mold to form beads, as previously described (3). We used a previously described continuous flow chamber (13) to test release of RIP from PMMA beads. Samples were collected for 48 h. Soluble material released from the PMMA beads at different time points (1 to 48 h) from three different 7.5% RIP-loaded beads (run A, B, and C) and from pulverized PMMA beads was applied to a reverse-phase C18 column (Hypersil GOLD; Thermo Electron Corp.) at a flow rate of 1 ml/min in mobile phase containing 0.1% trifluoroacetic acid in water. RIP was eluted using a gradient of acetonitrile from 0 to 70% for 15 min; RIP was eluted with 54% acetonitrile. The concentration of RIP released from the PMMA beads at different time points was compared to the elution profile (peak height) of 5 μg RIP under the same conditions.
In vivo experiments.
Methicillin-resistant S. aureus ATCC 43300 was studied. Adult male Wistar rats (weight range, 200 to 300 g) were assigned to six groups: those receiving bland PMMA beads (8 rats), 7.5% RIP-loaded PMMA beads (5 rats), 7.5% vancomycin-loaded PMMA beads (6 rats), 15% RIP-loaded PMMA beads (10 rats), 22.5% RIP-loaded PMMA beads (10 rats), or 15% RIP plus 7.5% vancomycin-loaded PMMA beads (7 rats). For each rat, a PMMA bead was aseptically implanted in a subcutaneous pocket. The pocket was closed, and 1 ml of saline containing 2 × 107 CFU S. aureus was inoculated around the bead surface. The beads were explanted 7 days following implantation, soaked in 2 ml of sterile saline solution for 5 min (to remove nonadherent organisms), placed in tubes containing 10 ml of phosphate-buffered saline solution, and sonicated for 5 min (to remove adherent bacteria from the surface). Aliquots (0.1 ml) were serially diluted in 9.9 ml of 10 mM of sodium HEPES buffer (pH 7.2) (Sigma-Aldrich, Milan, Italy). Viable bacteria were quantitated by plating 0.1 ml of each dilution on blood agar plates. The plates were incubated at 37°C for 48 h. The organisms were quantified by counting the number of CFU per plate. The limit of detection for this method was 10 CFU/ml.
Statistical analysis was performed using the SPSS 11.5 for Windows package (SPSS Inc.). Quantitative results for the in vivo experiments are presented as means ± standard deviations. Results were compared by nonparametric tests, using the Kruskal-Wallis and Mann-Whitney U tests for comparisons between groups.
Release profile of RIP.
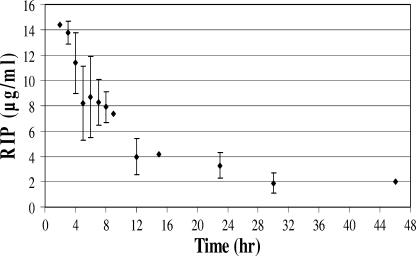
The continuous-flow system combined with high-performance liquid chromatography analysis of RIP was used to test the release profile of RIP from PMMA beads. As shown in Fig. 1, release profiles were bimodal, with an initial, rapid release of high concentrations, followed by a sustained, slow release.
FIG. 1.
In vitro RIP release from PMMA beads loaded with 7.5% RIP, as determined using reverse phase high-performance liquid chromatography analysis.
Prevention of S. aureus infection.
RIP with or without vancomycin-loaded PMMA beads was tested in vivo. As shown in Fig. 2, all active-treatment groups were more effective than the bland control (P ≤ 0.001). Among loaded beads, the combination of vancomycin plus RIP was more active than vancomycin alone (P ≤ 0.001). Beads containing 15% RIP showed more activity than beads containing 7.5% vancomycin or RIP (P ≤ 0.001). Beads containing 7.5% vancomycin showed more activity than those containing 7.5% RIP (P ≤ 0.004). Beads containing 22.5% RIP or 15% RIP plus 7.5% vancomycin yielded results below the limit of detection of the technique (<10 CFU) and showed more activity than any other combination studied (P ≤ 0.003).
FIG. 2.
Prevention of infection by S. aureus, using the rat model. PMMA beads with or without RIP or vancomycin were placed subcutaneously and were inoculated with methicillin-resistant S. aureus (MRSA). The beads were removed 7 days later and assayed for biofilm bacteria. Stars indicate no detectable CFU. The columns indicate mean CFU/ml. The error bars indicate standard deviations.
Staphylococci are common pathogens in orthopedic infections (8). The quorum-sensing inhibitor RIP has been previously shown to prevent staphylococcal biofilms on grafts in vivo (6, 7). However, to the best of our knowledge, RIP has not been previously tested in PMMA (11, 12). Our study shows in vitro compatibility of RIP with PMMA, release of RIP with PMMA, and activity in preventing S. aureus biofilm formation on PMMA beads in vivo with or without vancomycin.
Biofilm formation is advantageous for bacterial survival under harsh conditions. In the biofilm state, bacteria are relatively resistant to conventional levels of antimicrobial agents and to the host immune response (14). In addition, the emergence of resistance among staphylococci causing orthopedic infections (1) has led to the search for new strategies to prevent orthopedic infections. RIP inhibits the production of virulence factors necessary for the bacteria to survive within the host in the biofilm state (5). Use of RIP has been proposed as an alternative to or synergist with conventional antimicrobial agents for prevention of biofilm-associated infections (4, 7). The in vitro elution of RIP from PMMA followed a profile similar to that of traditional antimicrobials previously tested (3).
In conclusion, RIP elutes from PMMA and deserves further evaluation as an alternative to or an additional agent to use in conjunction with conventional antimicrobial agents in PMMA for prevention of orthopedic infections.
Acknowledgments
We acknowledge Mark S. Rouse for his technical assistance in preparing PMMA.
Paloma Anguita-Alonso was supported by a grant from Instituto de Salud Carlos III (BEFI 02/9374). This work was supported by grants from the Italian Ministry of Education, University and Research (PRIN 2003) and the Mayo Foundation.
This study was approved by the Animal Research Ethics Committee of the I.N.R.C.A. I.R.R.C.S., Università Politecnica delle Marche, Ancona, Italy.
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Anguita-Alonso, P., A. D. Hanssen, D. R. Osmon, A. Trampuz, J. M. Steckelberg, and R. Patel. 2005. High rate of aminoglycoside resistance among staphylococci causing prosthetic joint infection. Clin. Orthop. Relat. Res. 43943-47. [DOI] [PubMed] [Google Scholar]
- 2.Anguita-Alonso, P., A. D. Hanssen, and R. Patel. 2005. Prosthetic joint infection. Expert Rev. Anti. Infect. Ther. 3797-804. [DOI] [PubMed] [Google Scholar]
- 3.Anguita-Alonso, P., M. S. Rouse, K. E. Piper, D. J. Jacofsky, D. R. Osmon, and R. Patel. 2006. Comparative study of antimicrobial release kinetics from polymethylmethacrylate. Clin. Orthop. Relat. Res. 445239-244. [DOI] [PubMed] [Google Scholar]
- 4.Balaban, N., A. Giacometti, O. Cirioni, Y. Gov, R. Ghiselli, F. Mocchegiani, C. Viticchi, M. S. Del Prete, V. Saba, G. Scalise, and G. Dell'Acqua. 2003. Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J. Infect. Dis. 187625-630. [DOI] [PubMed] [Google Scholar]
- 5.Balaban, N., T. Goldkorn, Y. Gov, M. Hirshberg, N. Koyfman, H. R. Matthews, R. T. Nhan, B. Singh, and O. Uziel. 2001. Regulation of Staphylococcus aureus pathogenesis via target of RNAIII-activating protein (TRAP). J. Biol. Chem. 2762658-2667. [DOI] [PubMed] [Google Scholar]
- 6.Balaban, N., Y. Gov, A. Bitler, and J. R. Boelaert. 2003. Prevention of Staphylococcus aureus biofilm on dialysis catheters and adherence to human cells. Kidney Int. 63340-345. [DOI] [PubMed] [Google Scholar]
- 7.Balaban, N., P. Stoodley, C. A. Fux, S. Wilson, J. W. Costerton, and G. Dell'Acqua. 2005. Prevention of staphylococcal biofilm-associated infections by the quorum sensing inhibitor RIP. Clin. Orthop. Relat. Res. 43748-54. [DOI] [PubMed] [Google Scholar]
- 8.Costerton, J. W. 2005. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin. Orthop. Relat. Res. 4377-11. [DOI] [PubMed] [Google Scholar]
- 9.Henry, S. L., and K. P. Galloway. 1995. Local antibacterial therapy for the management of orthopaedic infections. Pharmacokinetic considerations. Clin. Pharmacokinet. 2936-45. [DOI] [PubMed] [Google Scholar]
- 10.Lew, D. P., and F. A. Waldvogel. 2004. Osteomyelitis. Lancet 364369-379. [DOI] [PubMed] [Google Scholar]
- 11.Ostermann, P. A., D. Seligson, and S. L. Henry. 1995. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J. Bone Jt. Surg. Br. Vol. 7793-97. [PubMed] [Google Scholar]
- 12.Ozaki, T., T. Yoshitaka, T. Kunisada, T. Dan'ura, N. Naito, and H. Inoue. 1998. Vancomycin-impregnated polymethylmethacrylate beads for methicillin-resistant Staphylococcus aureus (MRSA) infection: report of two cases. J. Orthop. Sci. 3163-168. [DOI] [PubMed] [Google Scholar]
- 13.Perry, A. C., M. S. Rouse, Y. Khaliq, K. E. Piper, A. D. Hanssen, D. R. Osmon, J. M. Steckelberg, and R. Patel. 2002. Antimicrobial release kinetics from polymethylmethacrylate in a novel continuous flow chamber. Clin. Orthop. Relat. Res. 40349-53. [DOI] [PubMed] [Google Scholar]
- 14.Trampuz, A., D. R. Osmon, A. D. Hanssen, J. M. Steckelberg, and R. Patel. 2003. Molecular and antibiofilm approaches to prosthetic joint infection. Clin. Orthop. Relat. Res. 41469-88. [DOI] [PubMed] [Google Scholar]