Abstract
Rifampin is a key drug for tuberculosis (TB) treatment. The available data suggest that the currently applied 10-mg/kg of body weight dose of rifampin may be too low and that increasing the dose may shorten the treatment duration. A double-blind randomized phase II clinical trial was performed to investigate the effect of a higher dose of rifampin in terms of pharmacokinetics and tolerability. Fifty newly diagnosed adult Indonesian TB patients were randomized to receive a standard (450-mg, i.e., 10-mg/kg in Indonesian patients) or higher (600-mg) dose of rifampin in addition to other TB drugs. A full pharmacokinetic curve for rifampin, pyrazinamide, and ethambutol was recorded after 6 weeks of daily TB treatment. Tolerability was assessed during the 6-month treatment period. The geometric means of exposure to rifampin (area under the concentration-time curve from 0 to 24 h [AUC0-24]) were increased by 65% (P < 0.001) in the higher-dose group (79.7 mg·h/liter) compared to the standard-dose group (48.5 mg·h/liter). Maximum rifampin concentrations (Cmax) were 15.6 mg/liter versus 10.5 mg/liter (49% increase; P < 0.001). The percentage of patients for whom the rifampin Cmax was ≥8 mg/liter was 96% versus 79% (P = 0.094). The pharmacokinetics of pyrazinamide and ethambutol were similar in both groups. Mild (grade 1 or 2) hepatotoxicity was more common in the higher-dose group (46 versus 20%; P = 0.054), but no patient developed severe hepatotoxicity. Increasing the rifampin dose was associated with a more than dose-proportional increase in the mean AUC0-24 and Cmax of rifampin without affecting the incidence of serious adverse effects. Follow-up studies are warranted to assess whether high-dose rifampin may enable shortening of TB treatment.
Each year, 8 million persons develop active tuberculosis (TB), and 2 to 3 million people die from this infectious disease. The treatment of TB is complicated by the length and complexity of currently available drug regimens, which invite problems of nonadherence, inadequate response, and resistance development. Therefore, a long-term goal for TB control has been to shorten the duration of treatment. This may possibly be achieved by increasing the dose of the pivotal TB drug rifampin, considering that early bactericidal activity studies in TB patients (8) and recent work in the mouse model (4) suggest that the typical 10-mg/kg of body weight dose of rifampin is rather low. A 50% increase in the rifampin dose may reduce the duration of treatment by one-third (4). Apart from these studies, we and others have found low 2-hour (peak) plasma concentrations of rifampin in TB patients treated with 10 mg/kg rifampin daily (7, 17, 18), which also suggests that a higher dose of rifampin merits further study.
So far, only a few clinical data have been available with regard to the pharmacokinetics, tolerability, and effectiveness of drug regimens based on a higher dose of rifampin (6, 14). Hence, we performed a pilot study in Indonesian patients in which we compared a higher dose (600 mg) and the standard dose (450 mg, or 10 mg/kg, considering the body weight of Indonesian people) of rifampin. It appeared that 78% of patients in the higher-dose arm versus 48% of those in the standard-dose arm achieved a rifampin peak (2-hour) plasma concentration above a reference value of 8 mg/liter (16). As the pilot study evaluated only a single time point in the rifampin pharmacokinetic curve and was nonblinded, we decided to conduct a double-blind randomized clinical trial with intensive pharmacokinetic sampling to compare the steady-state pharmacokinetics and tolerabilities of a higher dose and the standard dose of rifampin.
MATERIALS AND METHODS
Subjects.
The study was conducted in an urban outpatient TB clinic in Bandung, Indonesia. The study subjects were patients with newly diagnosed, untreated pulmonary TB. The diagnosis of pulmonary TB was based on clinical symptoms and chest X-ray examination and confirmed by microscopic detection of acid-fast bacilli. Patients were excluded if they were below 18 years of age, had a body weight of less than 33 kg, were pregnant or lactating, had a history of liver or kidney disease or any other disease that might affect the pharmacokinetics of TB drugs, or had been treated for TB previously. Human immunodeficiency virus (HIV) status was assessed anonymously at the end of the study. Informed consent was obtained from all subjects, and the study was approved by the Independent Ethics Committee, Faculty of Medicine, University of Padjadjaran, Bandung, Indonesia, and by the Ethical Review Board Region Arnhem/Nijmegen, The Netherlands.
Study design.
This was a double-blind, randomized, two-arm, phase II clinical trial. Eligible patients were randomized to a standard dose (450 mg, corresponding to 10 mg/kg in Indonesian people) or a higher dose (600 mg) of rifampin in addition to other TB drugs. In accordance with the Indonesian National TB Program, TB treatment consisted of daily isoniazid (300 mg), rifampin (higher or standard dose), pyrazinamide (1,500 mg), and ethambutol (750 mg) for 2 months, followed by isoniazid (600 mg) and rifampin (higher or standard dose) three times weekly for 4 months (3). All patients received TB drugs from the same manufacturer formulated in separate tablets. The bioequivalence of the rifampin tablets and an international reference standard has been established (19).
Double blinding for the dose of rifampin was accomplished by inserting rifampin tablets of 450 mg and 600 mg into blank capsule shells of the same color and size. Five capsules were sealed in an aluminum blister. The encapsulation and sealing of tablets were performed by the manufacturer of the drugs. Information about the randomization of the participants was not available to anyone involved in the study until it was completed.
A full pharmacokinetic curve was recorded at steady state after 6 weeks of treatment. Patients were followed every other week during the intensive phase and once monthly thereafter for evaluation of possible adverse events, as well as microbiological tests (sputum microscopy and culture).
Based on available data from the previous pilot study (16), it was estimated that at least 24 participants were required in each arm to be able to demonstrate a change (two-sided test) of at least 40% in the peak plasma concentration of rifampin at a 5% significance level and with 80% statistical power. The study was not empowered to detect differences in bacteriological outcomes.
Pharmacokinetic assessment.
Patients refrained from the intake of any food or any drugs (other than the study medication) from 11 p.m. on the day preceding the pharmacokinetic assessment until 4 hours after the intake of study medication. They took all antituberculosis drugs with 230 ml of still water. Serial venous blood samples were collected just prior to and 1, 1.5, 2, 2.5, 3, 4, 6, and 12 h after witnessed drug intake. Plasma was immediately separated and frozen at −20°C, transferred to −80°C within 72 h, and transported on dry ice to The Netherlands for bioanalysis.
Bioanalysis.
The plasma concentrations of rifampin, desacetylrifampin, pyrazinamide, and ethambutol were assessed by validated high-performance liquid chromatographic (HPLC) methods. Concentrations of isoniazid were not assessed, as undue degradation of this unstable drug was anticipated during storage and transport.
Rifampin and desacetylrifampin were measured by protein precipitation, followed by HPLC with UV detection. One hundred microliters of acetonitrile and 10 μl ascorbic acid solution (20 mg/ml) were added to 200 μl of plasma sample. The mixture was vortexed for 20 s and centrifuged for 5 minutes. Then, 200 μl of 10 mM potassium dihydrogen phosphate buffer was added, and the mixture was vortexed and centrifuged again. One hundred microliters of the clear supernatant was injected into the HPLC system, which consisted of an Omnispher 5 C18 column (250 by 4.6 mm; Varian, Middelburg, The Netherlands) protected with a Chromguard RP guard column (10 by 3 mm; Varian, Middelburg, The Netherlands). The mobile phase consisted of 10 mM potassium dihydrogen phosphate (pH 4.5) and acetonitrile (62%-38% [vol/vol]). The flow rate was set at 1 ml/min, and the wavelength for UV detection was 334 nm. The accuracy for standard concentrations was between 99.8% and 100.4% for rifampin and between 102.4 and 103.9% for desacetylrifampin, depending on the concentration level. The intra- and interassay coefficients of variation were less than 4% over the ranges of 0.28 to 30 mg/liter and 0.15 to 3 mg/liter for rifampin and desacetylrifampin, respectively. The lower limits of quantitation were 0.28 and 0.15 mg/liter, respectively. Rifampin-containing plasma samples were stable (<5% loss) for at least 16 months at −20°C and −80°C.
Total plasma concentrations of pyrazinamide were measured by solid-phase extraction, followed by HPLC-UV. Briefly, Waters Oasis HLB 1 ml (30 mg) extraction cartridges were washed sequentially with methanol and HPLC grade water. A half milliliter of plasma sample and 0.5 ml of water were drawn slowly onto the column and allowed to stand. The column was washed with water, and elution was performed with methanol. The eluate was dried, and after the addition of 10 mM phosphate buffer (pH 6.0)-methanol (95%-5% [vol/vol]), vortexing, and centrifugation, the clear supernatant was injected into the HPLC apparatus. Chromatographic analysis was performed on an Atlantis dC18 column (150 by 4.6 mm; Waters). The mobile phase consisted of 10 mM phosphate buffer (pH 6.0)-acetonitrile (99%-1% [vol/vol]) (fluid A) and acetonitrile (fluid B), in which the percentage of acetonitrile (fluid B) was changed linearly as follows: 0 min, 0%; 11.5 min, 0%; 12 min, 45%; 17 min, 45%; 17.5 min, 0%; and 25 min, 0%. The flow rate was set at 1.3 ml/min, and the wavelength for UV detection was 266 nm. The accuracy for standard concentrations was between 96.0% and 109.2%, intra- and interassay coefficients of variation were less than 10% over the range of 0.1 to 66.8 mg/liter, and the lower limit of quantitation was 0.1 mg/liter. Plasma samples with pyrazinamide were stable (<5% loss) for at least 3 months at −20°C and −80°C.
Ethambutol was quantitated by liquid-liquid extraction, followed by derivatization and HPLC-UV. Plasma samples (100 μl) were spiked with internal standard (N,N-diisopropylethylenediamine, 99%), alkalized, and extracted with chloroform. The chloroform layer was poured into other tubes containing derivatization reagent (0.01% phenylethylthyocyanate) and evaporated to dryness. Samples were resolved in acetonitrile-0.05 M phosphate buffer (40:60 [vol/vol]). After being vortexed and centrifuged, the supernatant was injected into the HPLC apparatus. Chromatographic analysis was performed on an Omnispher 5 C18 column (100 by 4.6 mm; Varian, Middelburg, The Netherlands). The mobile phase was a 0.05 M phosphate buffer (pH 5.6)-acetonitrile gradient in which the percentage of phosphate buffer was changed linearly as follows: 0 min, 65%; 21 min, 46%; 22 min, 65%; and 25 min, 65%. The flow rate was set at 1.5 ml/min, and the wavelength for UV detection was 215 nm. The accuracy was between 101% and 105%, intra- and interassay coefficients of variation were less than 4% over the range of 0.05 to 10 mg/liter, and the lower limit of quantitation was 0.05 mg/liter. Plasma samples with ethambutol were stable (<5% loss) for at least 8 months at −20°C and −80°C.
Pharmacokinetic analysis.
A noncompartmental analysis with WinNonLin version 4.1 (Pharsight Corp., Mountain View, CA) was performed to compute the pharmacokinetic parameters of rifampin, desacetylrifampin, pyrazinamide, and ethambutol.
The maximum concentration of drug in plasma was defined as the Cmax, and the time to this maximum concentration as the Tmax. The Cmax and Tmax were determined directly from the plasma concentration-time data. The value of the slope (log C versus time, −β/2.303, where β is the first-order elimination rate constant) was calculated by least-squares linear regression analysis. If the concentration at 12 h postdose (C12) was quantifiable, the concentration at 24 h (C24) was estimated using the equation C24 = C12 × e−β(t24 − t12). The area under the concentration-time curve from 0 to 24 h postdose (AUC0-24) was assessed using the linear-log trapezoidal rule from zero up to the last concentration. The half-life (t1/2) was calculated from the expression 0.693/β. The apparent clearance (CL/F, where F is the bioavailability) was calculated by dividing the dose by the AUC0-24, and the apparent volume of distribution (V/F) was obtained by dividing CL/F by β.
The relative exposure of the metabolite desacetylrifampin to rifampin was expressed as the ratio of the metabolite and the parent drug.
Tolerability.
Safety and tolerability were assessed by questioning patients actively (before the study and in weeks 2, 4, 6, and 8 in the intensive phase and weeks 12, 16, 20, and 26 in the continuation phase, a total of eight times after inclusion), guided by a list of nine possible adverse events that could occur during treatment with TB drugs. The patients were questioned by a field investigator, who was always a medical doctor. Serum glutamine pyruvate transferase was measured to evaluate hepatotoxicity in weeks 2, 4, 6, and 8 during the intensive phase, i.e., four times during the study. All possible adverse events were categorized according to an adverse-event grading system (http://ctep.cancer.gov). For elevations of serum glutamine pyruvate transferase, grade 1 was 1.25 to 2.5 times the upper limit of normal (ULN), grade 2 was 2.6 to 5 times the ULN, grade 3 was 5 to 20 times the ULN, and grade 4 was ≥20 times the ULN. Patients were withdrawn if they experienced grade 3 or 4 hepatotoxicity. After the reversal of hepatotoxicity, treatment was gradually resumed.
Bacteriological examinations and treatment outcome.
Microscopic examination of Ziehl-Neelsen-stained sputum slides was done for acid-fast bacilli, and Mycobacterium tuberculosis culture was performed on Ogawa 3% (an egg-based medium with 3% KH2PO4 buffer). Drug susceptibility testing for rifampin, isoniazid, ethambutol, and streptomycin was performed on cultured isolates, using an absolute-concentration method with supranational control.
After 6 months of TB treatment, a patient was cured (referring to an initially smear-positive patient who was smear negative in the last month of treatment and on at least one previous occasion), failed treatment (i.e., a smear-positive patient who remained smear positive at month 5 or later during treatment), completed treatment (a patient who completed treatment but did not meet the criteria for cure or failure because no sputum examination was possible during the last month of treatment, as the patient did not produce sputum), defaulted (treatment was interrupted for ≥2 consecutive months), or died (death from any cause during treatment) (20).
Statistical analysis.
Pharmacokinetic parameters were log transformed before statistical analysis. Differences in AUC0-24, Cmax, t1/2, CL/F, and V/F in the higher- versus standard-dose groups were tested with the independent-samples t test, and a geometric mean ratio plus 95% confidence interval was calculated for every comparison. Values for Tmax were not transformed and were compared using the Wilcoxon rank sum test. The Pearson chi-square test was used to compare the proportions of patients who reached a reference peak plasma concentration of 8 mg/liter for rifampin (13), as well as the incidence of adverse events as reported at least once in eight consecutive reporting times during the study.
Univariate analyses were performed in the higher-dose and standard-dose arms separately to assess the effects of gender, age, and body weight and the occurrence of nausea or vomiting on the AUC0-24 and Cmax of rifampin, pyrazinamide, and ethambutol. A multivariate linear regression analysis was performed to assess the variation in AUC0-24 and Cmax attributable to the presence of those variables that emerged from the univariate analyses.
All statistical evaluations were performed with SPSS for Windows version 12.0.1 (SPSS Inc., Chicago, IL). P values of less than 0.05 were considered statistically significant in all analyses.
RESULTS
Patients.
Fifty patients were included in the study. They presented with a history of cough (100%), shortness of breath (70%), fever (76%), night sweats (62%), and weight loss (84%). All patients showed chest X-ray abnormalities, and M. tuberculosis culture was positive in 92% of them. Fifty-two percent of the patients were male, the median age was 28 years (range, 18 to 55 years), and the mean body weight was 46.1 kg (range, 35.6 to 71.2 kg). One patient was HIV positive, and type 2 diabetes was found in four patients (8%). One patient used glibenclamide as a comedication, a drug which is not known to affect the pharmacokinetics of TB drugs. Twenty-five patients were allocated to each of the two study arms. Both at baseline (data not shown) and at the time of the pharmacokinetic assessment (Table 1), patient characteristics were similar in the two arms, except for the rifampin dose per kg.
TABLE 1.
Patient characteristics at the time of the pharmacokinetic assessment
| Characteristic | Value at rifampin dose (mg):
|
|
|---|---|---|
| 600 | 450 | |
| n | 23 | 24 |
| No. (%) male | 12 (52) | 13 (54) |
| Age (yr) [median (range)] | 27 (18-55) | 34 (19-55) |
| Wt (kg) [mean (SD)] | 47.3 (6.9) | 48.4 (8.1) |
| Body mass index (kg/m2) [mean (SD)] | 18.4 (2.6) | 18.8 (2.7) |
| No. with diabetes mellitus (%) | 1/23 (4) | 2/24 (8) |
| HIV positive (%) | 0 | 1 (4) |
| Rifampin dose (mg/kg) [mean (SD)] | 12.9 (1.7) | 9.5 (1.4) |
Pharmacokinetic data were available for 47 patients (23 of whom were in the higher-dose arm), tolerability data were available for 49 patients (24 in the higher-dose arm), and 47 patients were available for an evaluation of treatment response (23 in the higher-dose arm).
Pharmacokinetics of TB drugs.
All pharmacokinetic assessments occurred as planned without any events (e.g., vomiting) that might affect the pharmacokinetic profiles that were recorded.
Marked interindividual variability in AUC0-24 and Cmax values for rifampin was observed in both the higher-dose and standard-dose arms (Table 2).
TABLE 2.
Steady-state pharmacokinetics of rifampin and desacetylrifampina
| Parameter | Value at rifampin dose (mg):
|
600-mg/450-mg ratio (geometric mean + 95% CIb) | P value | |
|---|---|---|---|---|
| 600 (n = 23) | 450 (n = 24) | |||
| Rifampin | ||||
| AUC0-24 (mg·h/liter) | 79.7 (38.7-138.1) | 48.5 (26.7-72.8) | 1.65 (1.38-1.96) | <0.001c |
| Cmax (mg/liter) | 15.6 (5.1-26.6) | 10.5 (6.2-16.6) | 1.49 (1.22-1.81) | <0.001c |
| Cmax >8 mg/liter [no. (%)] | 22/23 (96) | 19/24 (79) | 0.090d | |
| Tmax (h) [median (range)] | 1 (1-6) | 2 (1-4) | 0.428e | |
| t1/2 (h) | 2.2 (1.3-6.3) | 1.9 (1.5-5.2) | 1.13 (0.95-1.35) | 0.176c |
| CL/F (liter/h) | 7.5 (4.3-15.5) | 9.2 (6.1-16.8) | 0.82 (0.69-0.97) | 0.021c |
| V/F (liters) | 23.3 (12.3-96.1) | 25.3 (12.9-55.7) | 0.92 (0.72-1.18) | 0.502c |
| Desacetylrifampin | ||||
| AUC0-24 (mg·h/liter) | 13.2 (7.2-25.6) | 6.4 (2.3-13.3) | 2.07 (1.61-2.65) | <0.001c |
| Cmax (mg/liter) | 2.2 (0.6-3.9) | 1.2 (0.5-2.6) | 1.83 (1.40-2.41) | <0.001c |
| Tmax (h) [median (range)] | 4 (1.5-6) | 4 (1.5-4) | 0.586e | |
| t1/2 (h) | 2.5 (1.7-9.9) | 3.2 (2.0-18.9) | 0.80 (0.63-1.03) | 0.085c |
| Desacetyrifampin/rifampin ratio | ||||
| AUC0-24 | 0.17 (0.10-0.25) | 0.13 (0.08-0.20) | 1.26 (1.09-1.45) | 0.002c |
| Cmax | 0.14 (0.07-0.22) | 0.11 (0.05-0.19) | 1.23 (1.03-1.48) | 0.024c |
After daily administration of a high (600-mg; 13-mg/kg) or standard (450-mg; 10-mg/kg) dose of rifampin (geometric mean plus range, unless stated otherwise).
CI, confidence interval.
Independent t test on log-transformed data.
Pearson chi-square test.
Wilcoxon rank sum test.
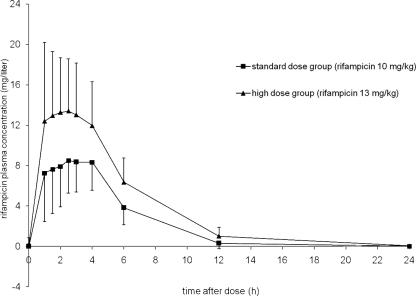
Exposure to rifampin (AUC0-24) was 65% (or 1.65-fold) higher in the higher-dose group, which reflects a more than dose-proportional increase of exposure upon increasing the dose (Table 2 and Fig. 1). Likewise, the rifampin Cmax was significantly higher in the higher-dose group (Table 2). The percentages of patients who reached a reference value of at least 8 mg/liter (13) were 96% in the higher-dose group and 79% in the standard-dose group (P = 0.094). At 2 hours postdose, 87% of patients in the higher-dose group and 58% in the standard-dose group had a concentration of at least 8 mg/liter (P = 0.01).
FIG. 1.
Mean steady-state plasma concentration-time profiles of rifampin in TB patients who received a high dose (600 mg; 13 mg/kg; n = 23) or a standard dose (450 mg; 10 mg/kg; n = 24) of rifampin, with standard deviations.
With regard to the metabolite desacetylrifampin, it appeared that absolute values for AUC0-24 and Cmax were approximately twofold higher in the higher-dose group than in the standard-dose group (Table 2). In addition, desacetylrifampin/rifampin ratios for AUC0-24 and Cmax were significantly higher in the higher-dose group.
The pharmacokinetics of pyrazinamide and ethambutol did not differ between the two study groups (Table 3). Of note, there were strong correlations between AUC0-24 values for rifampin, pyrazinamide, and ethambutol, and the same applied to Cmax values (data not shown).
TABLE 3.
Steady-state pharmacokinetics of pyrazinamide and ethambutola
| Parameter | Value at rifampin dose (mg):
|
600-mg/450-mg ratio (geometric mean + 95% CIb) | P value | |
|---|---|---|---|---|
| 600 (n = 23) | 450 (n = 24) | |||
| Pyrazinamide | ||||
| AUC0-24 (mg·h/liter) | 514.5 (266.4-775.3) | 472.8 (258.9-705.1) | 1.04 (0.93-1.27) | 0.269c |
| Cmax (mg/liter) | 46.0 (23.3-71.8) | 43.8 (19.2-62.1) | 1.05 (0.91-1.21) | 0.493c |
| Tmax (h) [median (range)] | 2.5 (2-6) | 2.75 (1-6) | 0.897d | |
| t1/2 (h) | 6.9 (4.1-11.7) | 6.6 (3.9-12.7) | 1.04 (0.89-1.22) | 0.593c |
| Ethambutol | ||||
| AUC0-24 (mg·h/liter) | 14.7 (10.0-23.4) | 14.4 (9.6-22.5) | 1.02 (0.88-1.17) | 0.830c |
| Cmax (mg/liter) | 2.3 (1.2-5.6) | 2.4 (1.1-4.9) | 0.98 (0.77-1.24) | 0.863c |
| Tmax (h) [median (range)] | 2.5 (1.5-4) | 2.5 (1.5-6) | 0.397d | |
| t1/2 (h) | 4.6 (1.8-6.8) | 4.2 (2.6-5.5) | 1.09 (0.95-1.24) | 0.217c |
After daily administration of 1,500 mg (30 mg/kg) and 750 mg (15 mg/kg) (geometric mean plus range, unless stated otherwise).
CI, confidence interval.
Independent t test on log-transformed data.
Wilcoxon rank sum test.
Tolerability and bacteriological examinations.
No significant differences were found between the high- and standard-dose groups in the incidence of nausea (33% versus 24%, respectively; P = 0.47), vomiting (21% versus 12%; P = 0.40), abdominal pain (4% versus 8%; P = 0.58), itching (50% versus 64%; P = 0.32), arthralgia (21% versus 28%; P = 0.57), hyperuricemia (21% versus 20%; P = 0.94), dizziness (13% versus 8%; P = 0.60), fever (13% versus 4%; P = 0.28), paresthesia (8% versus 8%; P = 0.97), and grade 3 hepatotoxicity (4% versus 12%; P = 0.32).
Grade 1 or 2 hepatotoxicity was more common in the higher-dose group (46% versus 20%; P = 0.054), but none of the patients developed serious hepatotoxicity and no action had to be taken. The grade 3 hepatotoxicity that developed in 4 of 49 patients (8%; 3 from the standard-dose arm and 1 from the higher-dose arm) was reversible in all patients. The majority of adverse events (99%) occurred in the first weeks of the intensive phase. No “flu-like syndrome” was reported during intermittent dosing of rifampin in the continuation phase.
Among 47 patients available for an evaluation of treatment response, nobody died, 38 patients were cured (81%), 4 completed the treatment (9%), 3 (6%) showed bacteriological failure (1 received 600 mg rifampin), and 2 defaulted (4%; 1 from the higher-dose group). Drug susceptibility tests revealed isoniazid monoresistance in two patients (one from the higher-dose group), rifampin monoresistance in one (from the standard-dose group), and multidrug-resistant TB in one patient (from the standard-dose group). There was no significant difference in the cumulative culture conversion rate between the higher- and standard-dose groups, but it should be noted that the study was not empowered to detect a difference in this respect.
The low number of undesirable events precludes firm conclusions about relationships between pharmacokinetic data on the one hand and the occurrence of adverse effects or inadequate response on the other. Patients with rifampin Cmax values above an upper reference value of 24 mg/liter (13) did not report any serious adverse event, and patients with grade 3 and 4 hepatotoxicity did not show unduly high exposure to rifampin or pyrazinamide. Likewise, the few patients with bacteriological failure had rifampin Cmax values within the reference range.
Determinants of the pharmacokinetics of rifampin, pyrazinamide, and ethambutol.
In univariate analyses, both in the higher- and in the standard-dose groups, gender and age did not show a significant relationship with the AUC0-24 and Cmax of rifampin, pyrazinamide, and ethambutol. However, body weight correlated with the AUC0-24 of all three drugs in both study groups; for rifampin, the Pearson correlation coefficient was −0.371 (P = 0.081) in the higher-dose group and −0.445 (P = 0.029) in the standard-dose group. Patients in the separate study arms who had reported nausea or vomiting at least once did not have lower exposures to rifampin, pyrazinamide, or ethambutol than those patients who never reported nausea or vomiting. For example, in the higher-dose arm, the mean value for the AUC0-24 of rifampin was 80.6 h·mg/liter (geometric mean, 76.1 h·mg/liter) among patients who once experienced nausea or vomiting compared to 85.1 h·mg/liter (geometric mean, 81.7 h·mg/liter) among those who did not (P = 0.62; independent-samples t test on log-transformed data). Similarly, the occurrence of vomiting alone was not associated with large or significant decreases in exposure to the TB drugs in each of the study arms. Rifampin, pyrazinamide, and ethambutol Cmax values for three patients with diabetes mellitus (one in the higher-dose group and two in the standard-dose group) and one HIV-infected patient were within reference ranges.
Multivariate analysis in all study patients revealed that both the dose of rifampin and body weight were independent predictors of the AUC0-24 of rifampin, according to the formula ln AUC0-24 (in h·mg/liter) = 3.288 + [0.003 × rifampin dose (mg)] − [0.017 × body weight (kg)].
DISCUSSION
This phase II clinical study showed that an increase in the rifampin dose from 10 to 13 mg/kg daily results in a more than dose-proportional increase in the mean AUC0-24 (65% increase) and mean Cmax (49% increase) of rifampin without a significant increase in the incidence of serious adverse events. An increase in the AUC0-24 or Cmax of rifampin predicts an increase in effectiveness, as rifampin exhibits exposure-dependent (4) or concentration-dependent (12) activity against M. tuberculosis. Therefore, increasing the dose of rifampin appears to be effective (from a pharmacokinetic point of view) and feasible. This calls for follow-up phase II studies that evaluate an even higher (15- or 20-mg/kg) dose of rifampin. In vitro and murine data (4) and data from humans (5) indicate that the TB treatment duration could possibly be shortened to 4 months by using higher doses of rifampin. This should eventually be tested in larger numbers of patients within the context of a phase III trial.
The main problem with currently available TB treatment is its length and complexity. Strategies to shorten treatment include further optimization of the dosing of available TB drugs and the evaluation of new antituberculosis drugs (among which the quinolone moxifloxacin seems most promising) (2) or a combination of these (15). Based on available data (4, 8), increasing the dose of rifampin seemed a promising means to optimize the response to this drug. This intervention is particularly attractive when it is acknowledged that rifampin is widely available at low cost and that the properties of the drug are well known to physicians all over the world. If increasing the dose of rifampin proves worthwhile, this intervention could be implemented broadly and quickly to the benefit of many patients.
Only a few clinical studies have addressed the concept of high-dose rifampin in TB treatment so far (14). In one study, a short regimen that incorporated a high dose of rifampin (1,200 mg daily or every other day) yielded very high sputum culture negativity by 2 months (5). On the other hand, another study demonstrated no difference in effectiveness between patients who used 600 (10 mg/kg) or 750 mg rifampin daily. In the latter study, 750 mg of rifampin was well tolerated (6). Past attempts to use large intermittent doses of rifampin met with a high incidence of a “flu-like syndrome,” but this was ascribed to the intermittency of dosing rather than the size of the dose (1, 14).
In the current study, a moderate (one-third) increase in the dose of rifampin was evaluated. We chose to be cautious, considering the paucity of clinical data regarding high-dose rifampin in general and taking into account that there are only scarce pharmacokinetic and tolerability data for even a standard dose of rifampin in Asian populations. A high dose of rifampin (20 mg/kg) is used in the treatment of brucellosis (14), but it should be considered that tolerability data for high-dose rifampin in brucellosis cannot be directly extrapolated to TB. In the treatment of brucellosis, high-dose rifampin is combined with just one other drug (doxycycline) instead of several toxic TB drugs, and treatment of the infection takes only 45 days.
The moderate increase in the rifampin dose, as applied in this study, resulted in a relatively strong, more than dose-proportional increase in plasma rifampin concentrations, which is consistent with the nonlinear pharmacokinetics of rifampin (1, 9, 11). Increasing the dose of rifampin also caused a more than proportional (around twofold) increase in the AUC0-24 of the active metabolite desacetylrifampin and an increase in the desacetylrifampin/rifampin ratio, which is in agreement with previous data (9). Considering the relatively small contribution of the active metabolite to the exposure and effectivness of rifampin, these findings do not seem to be clinically relevant. Importantly, the increase in the rifampin dose did not affect the mean AUC0-24 and Cmax of pyrazinamide and ethambutol, despite the observed correlations between the pharmacokinetics of rifampin on the one hand and those of pyrazinamide and ethambutol on the other.
The toxicity of rifampin is known to be related to the dose and administration interval (1). In this study, the incidence of adverse events was relatively high in both the higher- and standard-dose groups. This high incidence may be attributable in part to the active and frequent questioning of patients that we applied to guarantee their safety. The large majority of adverse events were mild in severity. The incidence of grade 1 or 2 hepatotoxicity was higher in the higher-dose group, but this was transient and did not cause treatment interruption or alteration. More importantly, this study did not show evidence for an increase in the incidence of serious hepatotoxicity related to a higher dose of rifampin, which is in agreement with previous data (6).
Nausea and vomiting occurred some 10% more often in the higher-dose arm, which reflects a (nonsignificant) difference of only two or three patients in this small phase II study. These adverse events should be monitored carefully in follow-up studies, as they may possibly affect adherence and the absorption of rifampin, which may offset the advantages of high-dose rifampin. In the current study, nausea or vomiting was not associated with exposure to the TB drugs. This may not be surprising, considering that no vomiting occurred during the pharmacokinetic assessments.
A limitation of this study, inherent in phase II studies in general, is the relatively small number of participants. This means that only large differences in the incidence of adverse events became statistically significant. Furthermore, the number of participants, in combination with a relatively short (6-month) follow-up, did not allow a valid comparison of bacteriological responses between the two treatment arms. As another limitation, it should be considered that all participants were Indonesian. Although rifampin doses were based on body weight, the possibility that the pharmacokinetics and/or tolerability of high-dose rifampin may be different in people of another race or genetic background cannot be excluded. As a third limitation, the effect of high-dose rifampin on the pharmacokinetics of isoniazid was not assessed in this study.
In conclusion, this study showed that an increase in the rifampin dose from 10 to 13 mg/kg daily causes a more than dose-proportional increase in the mean AUC0-24 and Cmax of rifampin without causing an increase in severe adverse events of the TB treatment. Therefore, increasing the dose of rifampin is feasible and effective from a pharmacokinetic point of view. Follow-up phase II studies should be performed to evaluate an even higher dose of rifampin. These studies should be carefully monitored, considering the nonlinear pharmacokinetics of rifampin. Eventually, high-dose rifampin is to be tested in larger phase III trials. For certain risk groups, e.g., patients with drug resistance or patients with HIV infection (17) or diabetes mellitus (10), a higher dose of rifampin may be useful as part of the current 6-month TB treatment regimen. In the absence of such risk factors, a higher dose of rifampin (possibly in conjunction with new TB drugs) may allow shortening of TB treatment.
Acknowledgments
We thank the patients for their participation in this study. The staff at the outpatient clinic Balai Pengobatan Penyakit Paru-paru (BP4) Bandung is warmly thanked for their cooperation. Marga de Graaff-Teulen and Robert Bijlsma are acknowledged for the analysis of plasma samples.
This study was financially supported by a grant from PRIOR, a fellowship for Rovina Ruslami from the Netherlands Foundation for Tropical Research (NWO-WOTRO) and from Radboud University Nijmegen, and a clinical fellowship for Reinout van Crevel from the Netherlands Organization for Health Research and Development (ZonMW).
Footnotes
Published ahead of print on 23 April 2007.
REFERENCES
- 1.Burman, W. J., K. Gallicano, and C. A. Peloquin. 2001. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 40327-341. [DOI] [PubMed] [Google Scholar]
- 2.Burman, W. J., S. Goldberg, J. L. Johnson, G. Muzanye, M. Engle, A. W. Mosher, S. Choudhri, C. L. Daley, S. S. Munsiff, Z. Zhao, A. Vernon, and R. E. Chaisson. 2006. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 174331-338. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health, Republic of Indonesia. 2000. National guidelines for tuberculosis, 5th ed. Department of Health, Jakarta, Republic of Indonesia.
- 4.Jayaram, R., S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, A. Bharat, R. K. Shandil, E. Kantharaj, and V. Balasubramanian. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 472118-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreis, B., S. Pretet, J. Birenbaum, P. Guibout, J. J. Hazeman, E. Orin, S. Perdrizet, and J. Weil. 1976. Two three-month treatment regimens for pulmonary tuberculosis. Bull. Int. Union Tuberc. 5171-75. [PubMed] [Google Scholar]
- 6.Long, M. W., D. E. Snider, and L. S. Farer. 1979. US Public Health Service Cooperative trial of three rifampin-isoniazid regimens in treatment of pulmonary tuberculosis. Am. Rev. Respir. Dis. 119879-894. [DOI] [PubMed] [Google Scholar]
- 7.McIlleron, H., P. Wash, A. Burger, J. Norman, P. I. Folb, and P. Smith. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob. Agents Chemother. 501170-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchison, D. A. 2000. Role of individual drugs in the chemotherapy of tuberculosis. Int. J. Tuberc. Lung Dis. 4796-806. [PubMed] [Google Scholar]
- 9.Mouton, R. P., H. Mattie, K. Swart, J. Kreukniet, and J. de Wael. 1979. Blood levels of rifampicin, desacetylrifampicin and isoniazid during combined therapy. J. Antimicrob Chemother. 5447-454. [DOI] [PubMed] [Google Scholar]
- 10.Nijland, H. M. J., R. Ruslami, J. E. Stalenhoef, J. N. Nelwan, B. Alisjahbana, R. H. H. Nelwan, A. J. A. M. van der Ven, H. Danusantoso, R. E. Aarnoutse, and R. van Crevel. 2006. Exposure to rifampicin is strongly reduced in tuberculosis patients with type 2 diabetes. Clin. Infect. Dis. 43848-854. [DOI] [PubMed] [Google Scholar]
- 11.Pargal, A., and S. Rani. 2001. Non-linear pharmacokinetics of rifampicin in healthy Asian Indian volunteers. Int. J. Tuberc. Lung Dis. 570-79. [PubMed] [Google Scholar]
- 12.Peloquin, C. A. 2001. Pharmacological issues in the treatment of tuberculosis. Ann. N. Y. Acad. Sci. 953156-164. [DOI] [PubMed] [Google Scholar]
- 13.Peloquin, C. A. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 622169-2183. [DOI] [PubMed] [Google Scholar]
- 14.Peloquin, C. A. 2003. What is the right dose of rifampicin? Int. J. Tuberc. Lung Dis. 73-5. [PubMed] [Google Scholar]
- 15.Rosenthal, I. M., K. Williams, S. Tyagi, C. A. Peloquin, A. A. Vernon, W. R. Bishai, J. H. Grosset, and E. L. Nuermberger. 2006. Potent twice-weekly rifapentine-containing regimens in murine tuberculosis. Am. J. Respir. Crit. Care Med. 17494-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruslami, R., H. Nijland, R. Aarnoutse, and R. van Crevel. 2006. Evaluation of high- versus standard-dose rifampin in Indonesian patients with pulmonary tuberculosis. Antimicrob. Agents Chemother. 50822-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tappero, J. W., W. Z. Bradford, T. B. Agerton, P. Hopewell, A. L. Reingold, S. Lockman, A. Oyewo, E. A. Talbot, T. A. Kenyon, T. L. Moeti, H. J. Moffat, and C. A. Peloquin. 2005. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin. Infect. Dis. 41461-469. [DOI] [PubMed] [Google Scholar]
- 18.van Crevel, R., B. Alisjahbana, W. C. de Lange, F. Borst, H. Danusantoso, J. W. van der Meer, D. Burger, and R. H. Nelwan. 2002. Low plasma concentrations of rifampicin in tuberculosis patients in Indonesia. Int. J. Tuberc. Lung Dis. 6497-502. [DOI] [PubMed] [Google Scholar]
- 19.van Crevel, R., F. Borst, E. Sahiratmadja, J. Cox, W. van der Meij, M. de Graaf, B. Alisjahbana, W. C. de Lange, R. H. Nelwan, and D. Burger. 2004. Bioavailability of rifampicin in Indonesian subjects: a comparison of different local drug manufacturers. Int. J. Tuberc. Lung Dis. 4500-503. [PubMed] [Google Scholar]
- 20.World Health Organization. 2006. Global tuberculosis control: surveillance, planning, financing. WHO report 2006. Report no. WHO/HTM/TB/2006.362. WHO, Geneva, Switzerland.