Abstract
Mice treated with antibiotics early or late after active infection had resolved were examined for chlamydial DNA in endocervical swabs. The early eradication of infection limited oviduct pathology, despite the continued detection of chlamydial DNA by nested PCR. Late antibiotic treatment had no effect on the ability to detect DNA or oviduct pathology.
The host immune response contributes to the pathology of Chlamydia trachomatis infection of the genital tract. Low-grade, chronic infection may elicit an ongoing inflammatory response that could accelerate pathology. The natural course of C. trachomatis infection was recently described in a study of Colombian women followed for a 5-year period. Molano et al. (4) demonstrated that, for 1,995 women who received no antibiotic treatment, the same serovar infection was detected in 46% of them at 1 year, in 18% of them at 2 years, and in 6% of them at 4 years of follow-up as determined by PCR of cervical scrape samples, indicating that a high rate of chronic infection occurs in the absence of antimicrobial therapy. In the Molano study, the detection of chlamydial DNA by PCR in cervical scrape samples was taken to indicate that actively replicating chlamydial organisms were present in the cervical tissue. Evidence against persistent infection after antimicrobial treatment comes from a study of 20 women who were followed for up to 5 months after completion of doxycycline therapy with 384 cervical, rectal, and urethral specimens examined by culture and PCR for chlamydial DNA (9). All specimens were negative except those for one woman with apparent reinfection. Thus, this study found no evidence of persistent chlamydial infection after therapy. However, the majority of the patients had serologic evidence of acute infection, and it is possible that chronic infection, in which organisms may be less metabolically active, would be more difficult to eradicate.
In a study conducted with mice by Ramsey et al. (7), chlamydial DNA was detected by PCR and Southern blot hybridization 120 days subsequent to primary genital tract infection or up to 90 days postresolution of infection as documented by culture of endocervical swabs. Immunosuppressive treatment of immunologically healthy mice at 120 days postinfection did not result in the detection of viable chlamydiae from the lower genital tract. These data suggest that although chlamydial DNA may persist in the genital tracts of mice well past culture-negative states, the detection of DNA by sensitive nucleic acid amplification methods may not indicate the presence of persistent infection as defined by the persistent ability to recover infectious bacteria from a normally sterile site.
The purposes of this study were threefold. First, we sought to determine whether the continued detection of chlamydial DNA by sensitive nucleic acid amplification techniques reflects the detection of persistent infection. Second, if DNA persistence reflects persistent infection, we sought to determine whether it can be prevented by antimicrobial treatment early in the course of an active infection or terminated by the delivery of antimicrobial treatment during a period when infectious organisms can no longer be recovered by culture. Third, we sought to determine, if antimicrobial treatment is able to prevent persistent infection, what effect this might have on the development of disease.
Eight-week-old progesterone-treated female BALB/c mice (Jackson Lab, Bar Harbor, ME) were infected intravaginally with 1 × 107 inclusion forming units (IFU) (1,700 doses that were 50% infective) of C. muridarum (Nigg strain) suspended in 30 μl of 250 mM sucrose-10 mM sodium phosphate-5 mM l-glutamic acid (SPG) buffer (1). Mice were divided into three groups: one received no antibiotic treatment, a second received daily 0.1-ml intraperitoneal injections of doxycycline (3 mg/ml) on days 10 to 24 postinfection, and a third received injections on days 42 to 56 postinfection. Uninfected mice treated with progesterone were used as negative controls. Endocervical swabs were taken on interval days through day 88, and the level of infection was determined by enumeration of IFU in cell culture (2). The presence of chlamydial DNA in endocervical swab eluates was determined by PCR, nested PCR, and Southern blot hybridization. At the time of sacrifice, genital tract tissues were examined grossly for oviduct hydrosalpinx. The tissues were processed and examined for histopathology as previously described (1).
DNA from swab eluates was extracted and amplified using a REDExtract-N-Amp blood PCR kit, following the manufacturer's instructions (Sigma-Aldrich). Nested PCR was used to amplify the target sequence. The initial forward primer sequence used was 5′-AATTCC CTG AGT CAT TCT GTT TAA-3′. This was paired with the reverse primer sequence of 5′-AAAAGA TTC CAT CAT CAA AAG C-3′. The nested forward and reverse primer sequences were 5′-GTCATT CTG TTT AAA AAT CTA GTC AAA-3′ and 5′-TTCATG GAC AGA AGG CAC C-3′, respectively. These primers amplify a portion of the cryptic plasmid of C. muridarum, of which there are multiple copies per genome (6), thereby enhancing the sensitivity of the PCR assay. PCR products were screened using 3% agarose gel electrophoresis for appropriately sized bands. Southern blot hybridization was performed using a 21-bp, 32P end-labeled probe (5′ GAT ATA ACT AGC TGC ACG AAC 3′) complementary to plasmid DNA internal to the forward and reverse primers used for the nested PCR.
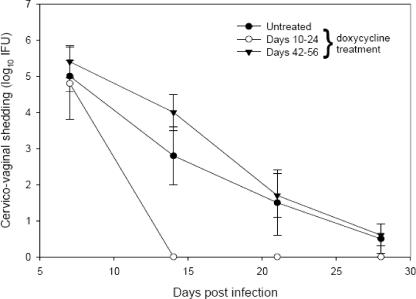
Mice that received no or late antibiotic treatment resolved active infection (as determined by the recovery of infectious organisms from cervicovaginal swabs) by day 28 (Fig. 1). The early antibiotic treatment group resolved infection by day 14. Chlamydial plasmid DNA was detected by first-round PCR in the majority of mice from each group up to 2 weeks postresolution of active infection (data not shown).
FIG. 1.
Intensity and duration of in vivo genital tract infection in BALB/c mice that received no doxycycline treatment, early (days 10 to 24) doxycycline treatment, or late (days 42 to 56) doxycycline treatment following vaginal inoculation of C. muridarum. Data points represent the means ± the standard errors of the means of the results from quantitative isolations of duplicate determinations from lower-genital-tract swabs taken from five animals examined on each day.
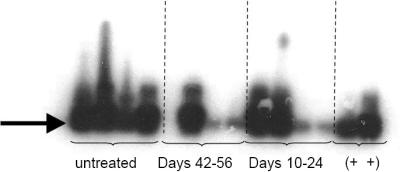
The use of nested PCR and Southern blot hybridization permitted the detection of chlamydial plasmid DNA in samples taken up to 88 days postinfection regardless of antibiotic treatment (Fig. 2). The specificity of the PCR was confirmed by Southern blot hybridization (Fig. 2). Although a single, day 88 sample from the group of mice treated from days 42 to 56 was negative and DNA in samples 3 and 4 from the antibiotic-treated groups was not as readily visualized in the Southern blot, chlamydial DNA was detected in four of five mice in the late treatment group and in five of five mice in the early treatment group on day 88 postinfection. Similar results were seen for eluates from swabs taken on multiple days prior to day 88, whereas swabs from uninfected controls were always negative by PCR. The amplification of DNA by first-round PCR, followed by second-round nested PCR, with confirmation of specificity by Southern blot analysis greatly amplifies minute quantities of chlamydial DNA and gives no indication of the quantity of DNA present in the original swab eluate. Chlamydial DNA was detected when actively replicating organisms were readily detected from swabs as well as during a period when infectious organisms were not recoverable by cell culture.
FIG. 2.
Detection of chlamydial plasmid DNA in eluates from cervicovaginal swabs taken on day 88 postinfection. Nested PCR followed by Southern blot hybridization of amplicands detected the presence of chlamydial plasmid DNA in five of five samples in the untreated group, four of five samples in the late treatment group, and five of five samples in the early treatment group. Four randomly selected samples from each group and day 4 culture-positive swabs used as positive controls (+ +) were loaded in the gel apparatus, transferred, and subsequently hybridized to the chlamydial plasmid DNA probe. Similar results were seen for eluates from swabs taken on multiple days prior to day 88, whereas swabs from uninfected controls were negative.
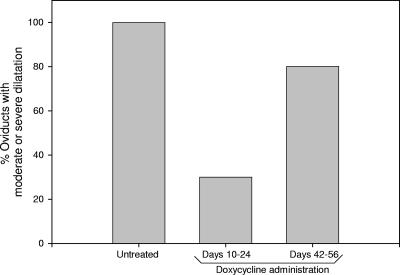
Mice were sacrificed 89 days postinfection, and tissues examined histologically revealed moderate or severe oviduct dilatation in 100% of the oviducts from the untreated group, in 80% of the oviducts from the late treatment group, and in 30% of the oviducts from the early treatment group (Fig. 3). Thus, early eradication of active infection helped to prevent pathology, as observed in a prior murine study (8), but late treatment had no effect, as seen in prior monkey studies (5).
FIG. 3.
Incidence of moderate or severe oviduct dilation in female mice after chlamydial intravaginal infection. Mice (five per treatment group) received either no antimicrobial treatment or doxycycline injections early (days 10 to 24) or late (days 42 to 56) postinfection. Results shown are the percentages of oviducts in each group exhibiting moderate (grade 3) or severe (grade 4) dilatation histologically in tissues processed from animals sacrificed 89 days postinfection. P = 0.07 for early versus late treatment; P = 0.005 for early versus no treatment, as performed by z test for the determination of significant differences in sample proportions.
In a paper by H. K. Maxion et al. (3), the kinetics of oviduct infection were revealed in BALB/c mice infected vaginally with 1 × 107 IFU of C. muridarum, parameters mirrored in this study. Marked infection of the oviducts (5 × 103 IFU/mg tissue) was detected by day 7 after vaginal inoculation, and at 21 days postinoculation, organisms were detected at a level of 1 × 103 IFU/mg tissue. These data, considered together with ours, indicate that early treatment with doxycycline does not prevent infection of the oviduct but aids in the prevention of disease likely by inhibition of ongoing active infection at this vulnerable site.
Nested PCR allowed for the detection of chlamydial DNA in the majority of mice in all groups, indicating that persistent low levels of chlamydial DNA may not reflect the presence of persistent infection as defined by recoverable infectious organisms. The continued ability to detect chlamydial plasmid DNA did not affect disease outcome, whereas the inhibition of actively replicating organisms had a protective effect.
Acknowledgments
This work was supported by the Horace C. Cabe Foundation, the Bates-Wheeler Foundation, the Arkansas Children's Hospital Research Institute and the University of Arkansas for Medical Sciences, and an Arkansas Children's Hospital Summer Science Research Award (D.R.).
We acknowledge the superb technical assistance of James D. Sikes.
Footnotes
Published ahead of print on 14 May 2007.
REFERENCES
- 1.Darville, T., C. W. Andrews, Jr., K. K. Laffoon, W. Shymasani, L. R. Kishen, and R. G. Rank. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 653065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly, K. A., E. A. Robinson, and R. G. Rank. 1996. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect. Immun. 644976-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maxion, H. K., W. Liu, M. H. Chang, and K. A. Kelly. 2004. The infecting dose of Chlamydia muridarum modulates the innate immune response and ascending infection. Infect. Immun. 726330-6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molano, M., C. J. Meijer, E. Weiderpass, A. Arslan, H. Posso, S. Franceschi, M. Ronderos, N. Munoz, and A. J. van den Brule. 2005. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J. Infect. Dis. 191907-916. [DOI] [PubMed] [Google Scholar]
- 5.Patton, D. L., Y. C. Sweeney, N. J. Bohannon, A. M. Clark, J. P. Hughes, A. Cappuccio, L. A. Campbell, and W. E. Stamm. 1997. Effects of doxycycline and antiinflammatory agents on experimentally induced chlamydial upper genital tract infection in female macaques. J. Infect. Dis. 175648-654. [DOI] [PubMed] [Google Scholar]
- 6.Pickett, M. A., J. S. Everson, P. J. Pead, and I. N. Clarke. 2005. The plasmids of Chlamydia trachomatis and Chlamydophila pneumoniae (N16): accurate determination of copy number and the paradoxical effect of plasmid-curing agents. Microbiology 151893-903. [DOI] [PubMed] [Google Scholar]
- 7.Ramsey, K. H., G. S. Miranpuri, I. M. Sigar, S. Ouellette, and G. I. Byrne. 2001. Chlamydia trachomatis persistence in the female mouse genital tract: inducible nitric oxide synthase and infection outcome. Infect. Immun. 695131-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su, H., R. Morrison, R. Messer, W. Whitmire, S. Hughes, and H. D. Caldwell. 1999. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. J. Infect. Dis. 1801252-1258. [DOI] [PubMed] [Google Scholar]
- 9.Workowski, K. A., M. F. Lampe, K. G. Wong, M. B. Watts, and W. E. Stamm. 1993. Long-term eradication of Chlamydia trachomatis genital infection after antimicrobial therapy. Evidence against persistent infection. JAMA 2702071-2075. [PubMed] [Google Scholar]