Abstract
AM3 (Inmunoferon) is an orally effective immunomodulator that influences the regulatory and effector functions of the immune system whose molecular mechanisms of action are mostly unknown. We hypothesized that the polysaccharide moiety of AM3 (IF-S) might affect immune responses by modulating the lectin-dependent pathogen recognition abilities of human dendritic cells. IF-S inhibited binding of viral, fungal, and parasite pathogens by human monocyte-derived dendritic cells in a dose-dependent manner. IF-S specifically impaired the pathogen recognition capabilities of DC-SIGN, as it reduced the attachment of Candida, Aspergillus, and Leishmania to DC-SIGN transfectants. IF-S also inhibited the interaction of DC-SIGN with both its cellular counterreceptor (intercellular adhesion molecule 3) and the human immunodeficiency virus (HIV) type 1 gp120 protein and blocked the DC-SIGN-dependent capture of HIV virions and the HIV trans-infection capability of DC-SIGN transfectants. IF-S promoted DC-SIGN internalization in DCs without affecting mannose receptor expression, and 1D saturation transfer difference nuclear magnetic resonance demonstrated that IF-S directly interacts with DC-SIGN on the cell surface. Therefore, the polysaccharide moiety of AM3 directly influences pathogen recognition by dendritic cells by interacting with DC-SIGN. Our results indicate that DC-SIGN is the target for an immunomodulator and imply that the adjuvant and immunomodulatory actions of AM3 are mediated, at least in part, by alteration of the DC-SIGN functional activities.
Inmunoferon is an immunomodulatory drug whose active principle (AM3) is a glycoconjugate of natural origin composed of a glucomannan polysaccharide from Candida utilis and a storage protein from nongerminated seeds of Ricinus communis (49). In vivo, AM3 enhances lymphocyte proliferation, interleukin-2 (IL-2) production, and NK activity (37); functions as an adjuvant to hepatitis B revaccination in nonresponder healthy persons (32, 38); and partially rescues the defective natural killer and phagocytic activities seen in chronic obstructive pulmonary disease patients (34). Besides, oral administration of AM3 increases IL-10 and reduces lipopolysaccharide (LPS)-induced tumor necrosis factor alpha (TNF-α), IL-1β, and inducible nitric oxide synthase; and thus, AM3 acts as a modulator of the innate immune system by acting on peripheral blood mononuclear cells (5, 25).
Dendritic cells (DCs) are professional antigen-presenting cells which link the innate and adaptive branches of the immune response by virtue of their capacity to recognize pathogen-associated structures and promote the initiation of T-cell-dependent immunity (3). In the steady state, immature myeloid DCs display a potent antigen uptake ability and contribute to the establishment of peripheral tolerance (41), whereas mature DCs display a strong capacity for T-cell stimulation and polarization of the immune response. Pathogen recognition by immature DCs is carried out by a number of cell surface molecules named pathogen-associated molecular pattern receptors, which include the Toll-like receptor (TLR) family (44) and a large number of lectins and lectin-like molecules (48), including the DC-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN; CD209) lectin. DC-SIGN is a type II membrane C-type lectin (13, 16, 21) which recognizes a large array of viral, bacterial, fungal, and parasite pathogens (1, 7, 9, 12, 22-24, 33, 40, 43, 45) in a mannan- and a Lewis oligosaccharide-dependent manner (17, 18) and which mediates DC interactions with naïve T lymphocytes, endothelial cells, and neutrophils by recognition of ICAM-3 (21), ICAM-2 (19), and Mac-1 (46), respectively.
Given its adjuvant and immunomodulatory effects, we have hypothesized that AM3 might have a direct effect on DCs. In fact, AM3 triggers DC maturation and promotes the preferential release of IL-10 from mature human monocyte-derived DCs (P. Majano et al., submitted for publication). Since fungus-derived mannans are capable of inhibiting the lymphoproliferative responses of human mononuclear leukocytes (4, 40) and modulate pathogen recognition by human DCs (40), the effects of the polysaccharide moiety of AM3 (IF-S) on the effector functions of immature human monocyte-derived DCs were analyzed. In the present report we present evidence that the polysaccharide moiety of AM3 directly influences pathogen recognition by human DCs by interacting with DC-SIGN on the cell surface.
MATERIALS AND METHODS
Glucomannan polysaccharide preparation.
The phosphorylated glucomannan polysaccharide from the cell wall of Candida utilis (hereafter termed IF-S) was obtained according to the methods described in patents P9900408 (Spain) and PCT/ES99/00338. Endotoxin contamination of the IF-S preparation was assayed with a Test Pyrogent plus kit (BioWhittaker, Rockland, ME), which has a detection threshold of 0.0625 IU/ml. Endotoxin was not detected even at concentrations of IF-S 1,000 times higher than those used in functional experiments.
Generation of MDDCs and cell culture.
Human peripheral blood mononuclear cells were isolated from buffy coats from healthy donors over a Lymphoprep (Nycomed Pharma, Oslo, Norway) gradient according to standard procedures. Monocytes were purified from peripheral blood mononuclear cells by magnetic cell sorting with CD14 microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). To generate monocyte-derived DCs (MDDCs), CD14+ cells (>95% monocytes) were cultured at 0.5 × 106 to 1 × 106 cells/ml in RPMI 1640 medium with 10% fetal calf serum, 25 mM HEPES, and 2 mM glutamine (complete medium) at 37°C in a humidified atmosphere with 5% CO2. Differentiation into immature MDDCs was accomplished by the addition of granulocyte-macrophage colony-stimulating factor (Immunotools, Friesoythe, Germany) and IL-4 (Immunotools), both at 1,000 U/ml. The medium was replaced, and new cytokines were added every 2 days. After 5 to 7 days, the cells were in suspension and exhibited the phenotypic and functional characteristics of immature DCs.
K562 cells stably transfected with DC-SIGN (K562-CD209) have been described previously (8, 40) and were cultured in complete medium containing 300 μg/ml of G418. Mock-transfected K562 cells (stably transfected with plasmid empty pCDNA3.1−) were used as controls. The Raji DC-SIGN B-cell line (kindly provided by Y. van Kooyk) was grown in RPMI 1640 medium with 10% fetal bovine serum plus 1 mg/ml of G418. The human T-cell line Hut CCR5 (kindly provided by V. N. KewalRamani) was grown in RPMI 1640 medium with 10% fetal bovine serum (FBS) plus G418 (300 μg/ml) and puromycin (1 μg/ml). The Hut CXCR4 and Raji cell lines were grown in RPMI 1640 medium with 10% FBS. Human embryonic kidney (HEK-293T) cells were maintained in Dulbecco modified Eagle medium supplemented with 10% FBS.
Flow cytometry and immunofluorescence.
Cells were collected, washed in ice-cold phosphate-buffered saline (PBS), resuspended in 100 μl of complete medium containing 50 μg/ml of human immunoglobulin G (IgG), and incubated for 15 min at 4°C to prevent binding through the Fc portion of the antibodies. Then, 100 μl of a solution containing 10 μg/ml of monoclonal antibody was added and the mixture was incubated for 30 min on ice. After three washing steps in PBS, the cells were resuspended in 100 μl of complete medium containing fluorescein isothiocyanate (FITC)-labeled F(ab′)2 rabbit anti-mouse IgG, kept on ice for 30 min, washed, and resuspended in 200 μl of PBS for flow cytometry. Monoclonal antibodies included anti-CD209 (DC-SIGN, MR1) and anti-mannose receptor 2.1D10 (anti-CD206 mannose receptor; generously provided by S. J. Sung, Department of Internal Medicine, University of Virginia Health Sciences Center, Charlottesville). The cells were also incubated with isotype-matched control antibodies and the supernatant of the nonproducing myeloma P3X63 (X63) to determine the basal level of fluorescence. Flow cytometry analysis was performed with an EPICS-CS flow cytometer (Coulter Científica, Madrid, Spain) by using log amplifiers.
For immunofluorescence, the cells were resuspended in PBS and allowed to adhere to poly-l-lysine-coated coverslips (50 × 103 cells/coverslip) for 60 min at 37°C. After a brief washing with PBS, the cells were fixed with 2% paraformaldehyde for 10 min at room temperature. The coverslips were mounted in fluorescent mounting medium (DakoCytomation, Carpinteria, CA), and representative fields were photographed through an oil immersion lens on a Nikon Eclipse E800 microscope equipped for epifluorescence or by confocal microscopy.
Viral stocks and plasmids.
Single-round infectious pseudotyped human immunodeficiency virus (HIV) type 1 (HIV-1) stocks (HIVJRFL/NL43-Luc) were generated by cotransfecting the envelope-deficient proviral vector pNL4-3.Luc.R−E− (obtained from N. Landau through the NIH AIDS Research and Reference Reagent Program) containing the firefly luciferase reporter gene with plasmid pJRFL, which expresses the envelope glycoprotein of the CCR5-tropic strain HIV-1JRFL (kindly provided by V. N. KewalRamani). Replication-competent full-length HIV-1 stocks (HIVNFN-SX) were generated by transfecting the proviral construct NFN-SX, a HIV-1NL43 provirus with the HIV-1JRFL envelope glycoprotein (kindly provided by W. O'Brien). Briefly, 2.5 × 106 HEK 293T cells were transfected with calcium phosphate in 10-cm2 well plates by adding up to 20 μg of plasmid DNA. The cells were washed at 4 h posttransfection; and the supernatants, which contained HIVJRFL/NL43-Luc or HIVNFN-SX, were collected 2 days later and frozen at −80°C until use. The titers of all viruses were determined on a Ghost CCR5 indicator cell line, which contains an HIV-2 long terminal repeat linked to a green fluorescent protein gene, and the p24gag antigen was measured by enzyme-linked immunosorbent assay (ELISA; Perkin-Elmer).
DC-SIGN internalization assays.
MDDCs were washed, resuspended in complete medium (2.5 × 105 cells per time point), and incubated with IF-S at distinct concentrations for 1 h at 4°C to prevent internalization. After the cells were extensively washed, they were placed at 37°C to allow internalization to occur. At the indicated time points, internalization was stopped by adding 4 volumes (200 μl) of cold PBS, and the cells were immediately placed at 4°C. Then, the cells were subjected to DC-SIGN and mannose receptor cell surface detection by flow cytometry with the MR1 and 2.1D10 antibodies and a 1:100 dilution of an FITC-labeled goat anti-mouse antibody (Serotec). All incubations were done in the presence of 50 μg/ml of human IgG to prevent binding through the Fc portion of the antibodies.
Aspergillus fumigatus- and Candida albicans-binding assays.
Conidia from A. fumigatus or C. albicans were washed twice, resuspended, and incubated in PBS containing 0.1 mg/ml FITC for 1 h at room temperature. The fungi were then extensively washed and either used immediately or stored at −20°C until use. Cells (MDDCs or K562 transfectants) were washed, resuspended in complete medium (3 × 105 cells/well), and pretreated for 20 min at room temperature with anti-DC-SIGN antibody (antibody MR1; 5 μg/ml), IF-S, Saccharomyces cerevisiae mannan, or the control X63 antibody at distinct concentrations. Then, the cells were incubated with FITC-labeled fungi at various ratios for 30 min at room temperature. After fixation with 2% paraformaldehyde for 1 h at 4°C, the cells were washed and analyzed by flow cytometry.
Leishmania amastigote-binding assay.
MDDCs or K562-CD209 transfectants were washed in PBS with 1 mM EDTA, resuspended in complete medium, aliquoted, and placed in 24-well plates (2 × 105 cells/well). 5,6-Carboxyfluorescein succinimidyl ester (CFSE)-labeled Leishmania pifanoi amastigotes were added to the cells at a 5:1 (amastigote-to-cell) ratio, and the mixture was incubated at room temperature for 30 min. Afterwards, the cells were fixed (with 2% paraformaldehyde in PBS) for 1 h at room temperature, and analyzed by flow cytometry with an EPICS-CS flow cytometer (Coulter Científica). For inhibition assays, the cells were washed with PBS with 1 mM EDTA and preincubated for 20 min at room temperature with either the anti-DC-SIGN MR1 antibody (5 μg/ml), distinct concentrations of IF-S, or the control X63 antibody in complete medium before parasite addition.
Gp120-Fc-binding assays.
MDDCs or K562 transfectants were washed in PBS with 1 mM EDTA, resuspended in complete medium, aliquoted, and placed in 96-well plates (2 × 105 cells/well). The plates were incubated with gp120-Fc either alone or in the presence of a monoclonal antibody against DC-SIGN (MR1; 5 μg/ml), supernatant from the mouse myeloma P3X63Ag8 (negative control), S. cerevisiae mannan, IF-S, or laminarin at the indicated concentrations for 20 min at room temperature. The cells were then washed, incubated with FITC-labeled polyclonal antisera against human IgG Fc (Beckman Coulter), washed again, and analyzed by flow cytometry.
Virus capture and transmission assays.
After a 2 h-incubation of 3 × 105 Raji DC-SIGN (or control Raji) cells with 50 ng of HIVNFN-SX p24gag antigen at 37°C, the cells were washed three times with PBS and lysed with 0.5% Triton X-100. The lysates were cleared of cell debris by centrifugation, and the p24gag antigen content was measured by ELISA. To inhibit virus capture, the cells were preincubated for 30 min at 4°C with mannan (500 μg/ml; Sigma), anti-DC-SIGN monoclonal antibody MR1, or IF-S at different concentrations (5 to 500 μg/ml) and then processed as described above. To characterize viral transmission efficiencies, Raji DC-SIGN cells (or Raji cells as a control) were counted, pulsed with 200 ng of HIVJRFL/NL43-Luc p24gag antigen for 3 h at 37°C, and washed three times with PBS. The pulsed cells were then cocultured in four replicates with the target Hut CCR5 cell line at a 1:1 ratio (1 × 105 cells per well in a 96-well plate) in the presence of 10 μg/ml of Polybrene. The cells were assayed for luciferase activity (BrightGLo luciferase system; Promega) 48 h later in a Fluoroskan Ascent FL luminometer. To detect the possible direct infection of pulsed cells, the CCR5 target cells in the coculture were replaced by CXCR4 target cells as a control. Inhibition of viral transfer was assayed by preincubation with mannan (500 μg/ml), MR1, or IF-S (5 to 500 μg/ml) for 30 min at 4°C before viral addition.
DC-SIGN-dependent adhesion assays: adhesion to ICAM-3- or polysaccharide-coated plates.
DC-SIGN-dependent adhesion was evaluated by using ICAM-3/Fc (kindly provided by Donald Staunton, ICOS Corporation, Bothwell, WA), S. cerevisiae mannan, or IF-S as ligands. Ninety-six-well microtiter EIA II-Linbro plates were coated overnight at 4°C with ICAM-3/Fc (3 μg/ml in 100 mM NaHCO3, pH 8.8), mannan, or IF-S at distinct concentrations (0.05 to 50 μg/ml) in PBS; and the remaining sites were blocked with 0.4% bovine serum albumin (BSA) for 2 h at 37°C. The cells were labeled in complete medium with the fluorescent dye 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (Molecular Probes) and then preincubated for 20 min at 37°C in RPMI 1640 medium containing 0.4% BSA and containing or not containing the function-blocking antibody MR1 against DC-SIGN (MR1), mannan, or IF-S at distinct concentrations. The cells were allowed to adhere to each well for 15 min at 37°C. Unbound cells were removed by three washes with 0.5% BSA in RPMI 1640 medium, and adherent cells were quantified by using a fluorescence analyzer.
For the aggregation experiments, K562-CD209 cells were washed, maintained in PBS with 1 mM EDTA for 5 min, and resuspended in complete medium at 2 × 105 cells/ml. Five hundred microliters of this cell suspension was then seeded onto tissue culture plates containing either 500 μl of complete medium or 500 μl of complete medium containing A. fumigatus galactomannan (100 μg/ml), a blocking antibody against DC-SIGN (MR1 at 10 μg/ml), or IF-S at distinct concentrations. Homotypic aggregation was allowed to proceed for 20 min, and the plates were photographed.
Determination of cytokine levels.
The level of TNF-α was determined by using a TNF-α ELISA set (Immunotools), according to the manufacturer's recommendations. Based on the results of preliminary experiments, supernatants from MDDCs were assayed either undiluted or diluted 1/25 in complete medium.
NMR experiments.
The results of all the nuclear magnetic resonance (NMR) imaging experiments were recorded on a Bruker 500-MHz apparatus at 298 K. A basic saturation transfer difference (STD) sequence was used, with the on-resonance frequency varied between 6.8 ppm and 1.3 ppm (27). The success of the STD experiments depends on the kinetics of the dissociation process and the molar ratio of the ligand versus that of the receptor (10, 28). The off-resonance frequency was maintained fixed at 100 ppm. A train of 40 gaussian-shaped pulses of 50 ms each was employed, with a total saturation time of the protein envelope of 2 s. On- and off-resonance scans were alternated and recorded separately. In order to perform the STD experiments, 3.6 × 106 cells (K562-CD209 or mock-transfected K562cells) were washed, dissolved in 250 μl of deuterated PBS at pH 7.3 (containing 1 mM CaCl2 previously exchanged with D2O), and mixed with 2.5 mg of the IF-S preparation dissolved in 250 μl of the same deuterated PBS (26). A typical NMR tube volume was 450 μl. The estimated concentration of DC-SIGN in the NMR tube was approximately 0.1 μM. Robust data were obtained with 2 × 32 scans, corresponding to a total experimental time of 5 min. In order to determine the biological stability of the cells that were employed, these were checked by optical microscopy before and after the NMR experiments, and cell viability was evaluated by trypan blue exclusion.
RESULTS
IF-S inhibits C. albicans and A. fumigatus binding to human DCs.
Given the origin of the polysaccharide moiety of AM3 (IF-S), we initially set up an experiment to analyze whether IF-S could affect the capture of pathogenic strains of Candida by DCs. IF-S inhibited the binding of C. albicans to human MDDCs in a dose-dependent manner and to a similar extent as a function-blocking anti-DC-SIGN monoclonal antibody (Fig. 1A and C). Furthermore, IF-S also diminished the number of fungi bound per cell, as evidenced by the reduction of the mean fluorescence intensity of the cell population (Fig. 1B). Besides, IF-S reduced the binding of A. fumigatus conidia to human MDDCs in a dose-dependent manner, and the degree of inhibition was comparable to that obtained with equivalent concentrations of S. cerevisiae mannan or with a blocking antibody against DC-SIGN antibody (Fig. 1D). Therefore, the polysaccharide component of AM3 inhibits the DC-SIGN-dependent binding of C. albicans and A. fumigatus to MDDCs (7).
FIG. 1.
IF-S inhibits the binding of C. albicans and A. fumigatus to MDDCs. (A) Flow cytometry analysis of the binding of FITC-labeled C. albicans to MDDCs in the presence of IF-S or the indicated antibodies. The percentage of cells with bound C. albicans is indicated. MOI, multiplicity of infection. (B) Mean fluorescence intensities (MFI) of MDDCs in the binding assay whose results are shown in panel A. (C) Phase-contrast images (left panels) and epifluorescence visualization (right panels) of the experiment whose results are shown in panel A. (D) Flow cytometry analysis of the binding of FITC-labeled A. fumigatus to MDDCs in the presence of S. cerevisiae mannan, IF-S, or the indicated antibodies. The percentage of cells with bound Aspergillus is indicated in each case.
The polysaccharide component of AM3 inhibits DC-SIGN-dependent pathogen-binding activities of human DCs.
To determine whether IF-S was directly affecting DC-SIGN-dependent fungal recognition (7, 40), binding assays were performed with K562 cells stably transfected with DC-SIGN. IF-S also diminished the binding of C. albicans or A. fumigatus to the DC-SIGN transfectants in a dose-dependent manner, with 50% inhibition at the maximal concentration assayed (Fig. 2A and C), and reduced both the number of cells with bound fungi and the number of fungi bound per cell (Fig. 2B). In fact, IF-S decreased fungal binding as effectively as mannan, a well-known inhibitor of all DC-SIGN-dependent functions (Fig. 2C). These results demonstrate that IF-S inhibits fungal binding to human DCs by preventing the recognition ability of DC-SIGN, suggesting that DC-SIGN directly binds to IF-S.
FIG. 2.
IF-S inhibits the binding of C. albicans and A. fumigatus to K562-CD209 cells. (A) Flow cytometry analysis of the binding of FITC-labeled C. albicans to K562 and K562-CD209 cells in the presence of IF-S or the indicated antibodies. The percentage of cells with bound C. albicans is indicated in each case. MOI, multiplicity of infection. (B) Mean fluorescence intensity (MFI) of K562-CD209 cells after the binding assay whose results are shown in panel A. (C) Flow cytometry analysis of the binding of FITC-labeled A. fumigatus conidia to K562-CD209 cells in the presence of S. cerevisiae mannan, IF-S, or the indicated antibodies. The percentage of cells with bound A. fumigatus is indicated in each case.
To further study the ability of IF-S to impair DC-SIGN-dependent functions, we determined its effect on the DC-SIGN-dependent Leishmania binding (11, 12) by DCs and DC-SIGN transfectants. At concentrations as low as 10 μg/ml, IF-S partly blocked the binding of Leishmania amastigotes to DCs from two distinct donors (Fig. 3A and B) and to DC-SIGN transfectants, with levels of inhibition of at least 50% at the highest concentrations assayed (Fig. 3E). As expected, an anti-DC-SIGN antibody completely blocked Leishmania attachment to both cell types (Fig. 3A, B, and E). The inhibitory effect of IF-S correlated with the level of expression of DC-SIGN (compare the inhibition in Fig. 3A and B with the DC-SIGN expression data in Fig. 3C and D), again supporting the involvement of DC-SIGN in the inhibitory action of IF-S. Fluorescence microscopy experiments also revealed the effects of IF-S: binding of Leishmania to K562-CD209 cells results in the formation of cellular aggregates caused by the simultaneous binding of several cells to a single Leishmania amastigote, whereas the presence of IF-S prevented the formation of such aggregates (Fig. 3F). Altogether, this set of results further supports the possibility that IF-S impairs pathogen recognition by DCs by virtue of its ability to inhibit DC-SIGN-dependent functions.
FIG. 3.
IF-S inhibits the binding of Leishmania pifanoi amastigotes to MDDCs and K562-CD209 cells. (A and B) Flow cytometry analysis of the binding of CFSE-labeled L. pifanoi amastigotes to MDDCs in the presence of IF-S or the indicated antibodies. The percentage of cells with bound amastigotes is indicated. MOI, multiplicity of infection. (C and D) DC-SIGN cell surface expression (percentage of marker-positive cells and mean fluorescence intensity) on the MDDCs analyzed and for which the results are shown in panels A and B, as determined by flow cytometry. (E) Flow cytometry analysis of the binding of CFSE-labeled L. pifanoi amastigotes to K562-CD209 cells (multiplicity of infection [MOI], 5:1) in the presence of IF-S or an anti-DC-SIGN antibody. The percentage of cells with bound amastigotes is indicated. (F) Phase-contrast images (left panels) and epifluorescence visualization (right panels) of the experiment whose results are shown in panel E.
IF-S inhibits recognition of HIV-1 by DC-SIGN.
The ability of IF-S to block the pathogen recognition ability of DC-SIGN prompted us to determine whether IF-S could also inhibit the binding of HIV to DC-SIGN (20). IF-S reduced the binding of HIV gp120 to K562-CD209 cells in a dose-dependent manner, with the degree of inhibition similar to that caused by S. cerevisiae mannan (Fig. 4A). More importantly, binding of gp120 to monocyte-derived DCs was greatly inhibited by the anti-DC-SIGN MR1 antibody, and a similar inhibitory effect was observed in the presence of IF-S (Fig. 4B). As a control, the β-glucan-containing dectin-1 polysaccharide ligand laminarin (50) had no effect on gp120 binding to DC-SIGN (Fig. 4A). Next, we asked if IF-S was able to inhibit the binding of replication-competent HIV virions to DC-SIGN. Like mannan or anti-DC-SIGN antibodies, IF-S abrogated the binding of HIVNFN-SX virions to Raji DC-SIGN cells at all concentrations assayed (5 to 500 μg/ml) (Fig. 4C). Finally, and to better reflect the physiological role of DC-SIGN in HIV pathobiology, we evaluated the ability of IF-S to block the HIV trans-infection ability of Raji DC-SIGN cells. IF-S fully abrogated the transmission of HIVJRFL/NL43-Luc from Raji DC-SIGN cells to Hut CCR5 target cells at concentrations as low as 5 μg/ml (Fig. 4D). Therefore, IF-S inhibits the binding of HIV-1 gp120 to DC-SIGN on either transfectants or human MDDCs and blocks the capture and the transmission of HIV virions from DC-SIGN-expressing cells to target cells expressing HIV correceptors.
FIG. 4.
IF-S inhibits DC-SIGN-dependent recognition of HIV-1. (A and B) Binding of gp120-Fc to K562 and K562-CD209 cells (A) or MDDCs (B) in the presence of S. cerevisiae mannan (Man), laminarin (Lam), IF-S, anti-DC-SIGN, or an irrelevant antibody, as determined by flow cytometry. The percentage of marker-positive cells (B) and the mean fluorescence intensity (MFI) (A and B) are indicated. (C) Binding of HIVNFN-SX to Raji and Raji DC-SIGN cells in the presence of DC-SIGN blocking agents, as determined by a p24gag ELISA. (D) HIVJRFL/NL43-Luc transmission from Raji DC-SIGN or Raji cells to Hut CCR5 target cells in the presence of DC-SIGN blocking agents. As a control of direct infection, pulsed Raji DC-SIGN cells were also cultured with Hut CXCR4 target cells. Dotted line, background levels of luciferase (RLU, relative light units) observed with nonpulsed Raji DC-SIGN cells.
IF-S diminishes the ICAM-3-binding ability of DC-SIGN.
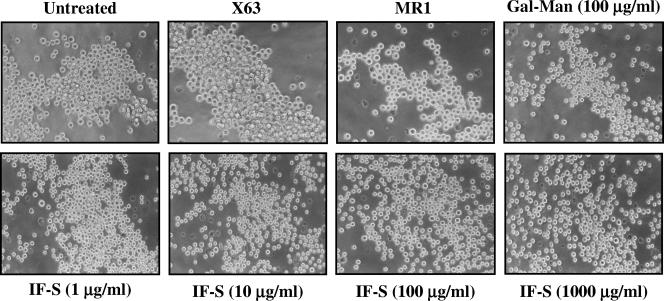
DC-SIGN also functions as a cell adhesion molecule, mediating DC adhesion to lymphocytes, endothelial cells, and neutrophils by interactions with ICAM-3, ICAM-2, and Mac-1, respectively (19, 21, 46). In an effort to evaluate whether IF-S influenced DC-SIGN-dependent adhesive functions, MDDC, K562-CD209, and Jurkat-CD209 cells were allowed to bind to ICAM-3 in adhesion assays in the presence of IF-S. Although the distinct cell types exhibited different levels of cell surface DC-SIGN (Fig. 5A and C), their binding to ICAM-3 was consistently reduced by at least 50% in the presence of IF-S (Fig. 5B and D), with inhibitory effects that were in some cases higher than those exhibited by mannan (Fig. 5D). In addition, IF-S at 10 μg/ml prevented the DC-SIGN-dependent homotypic aggregation of K562-CD209 cells (14), whereas IF-S at 1 μg/ml inhibited aggregation to a similar extent as the MR1 blocking antibody (Fig. 6). Therefore, IF-S prevents the DC-SIGN-dependent cell adhesion to ICAM-3 and the DC-SIGN-mediated homotypic aggregation.
FIG. 5.
IF-S inhibits DC-SIGN-dependent adhesive functions. (A) Flow cytometry determination of the DC-SIGN cell surface expression on immature MDDCs, K562 cells, and K562-CD209 cells. The percentage of marker-positive cells and the mean fluorescence intensity are indicated in each case. (B) Adhesion of MDDCs, K562 cells, and K562-CD209 cells to recombinant ICAM-3-Fc in the presence of mannan, IF-S, an anti-DC-SIGN antibody (MR1), or an irrelevant antibody. Each condition was assayed in triplicate, and the data shown are the means ± standard deviations. (C) Flow cytometry determination of the DC-SIGN cell surface expression on Jurkat and Jurkat-CD209 cells. The percentage of marker-positive cells and the mean fluorescence intensity are indicated in each case. (D) Adhesion of Jurkat-CD209 cells to recombinant ICAM-3-Fc performed as described in the legend to panel B.
FIG. 6.
IF-S inhibits the DC-SIGN-dependent homotypic aggregation of K562-CD209 cells. K562-CD209 cells were resuspended and allowed to aggregate for 20 min in the presence of A. fumigatus galactomannan (Gal-Man), IF-S, or the indicated antibodies.
The presence of IF-S promotes DC-SIGN internalization and triggers TNF-α production by human DCs.
All the previous results indicated that IF-S inhibits DC-SIGN functional activities on either DCs or transfected cells. Given the ability of DC-SIGN to internalize its cognate ligands (15, 42), DCs were incubated with IF-S at 37°C and the cell surface expression level of DC-SIGN was determined by flow cytometry. In the presence of IF-S, DC-SIGN expression was dramatically reduced, with 50% of the molecules being internalized after only 5 min and with further reductions at later time points (Fig. 7A and B). By contrast, no significant change in the expression of either CD29 (Fig. 7B) or, more importantly, the mannose receptor was detected at any time point (Fig. 7B and C). Therefore, although DC-SIGN and the mannose receptor display similar adhesive and internalization capabilities, the presence of the polysaccharide moiety of AM3 promotes only the internalization of DC-SIGN in DCs, suggesting the specificity of the interaction.
FIG. 7.
IF-S promotes DC-SIGN internalization in MDDCs. (A to C) MDDCs (2.5 × 105 cells per time point) were incubated with IF-S at 4°C for 1 h, washed, and placed at 37°C for the indicated time points to allow internalization. The cell surface expression of DC-SIGN (A and B), CD29 (B), or mannose receptor (C) was then determined by flow cytometry. Each experiment was performed twice, with similar results each time, and the results of representative experiments are shown. (D) Production of TNF-α by MDDCs exposed for 18 h to LPS, IF-S, anti-DC-SIGN antibody (MR1), or both MR1 and IF-S. Each treatment was assayed in triplicate, and the data shown are the means ± standard deviations.
The influence of IF-S on DCs was evaluated with human MDDCs, where IF-S (at 100 μg/ml) promoted the release of TNF-α to a higher extent than LPS (at 100 ng/ml) did (Fig. 7D). This effect did not appear to be a direct consequence of the interaction of IF-S with DC-SIGN, as the blocking anti-DC-SIGN antibody MR1, which by itself did not induce TNF-α, was not able to prevent the IF-S-induced release (Fig. 7D). Therefore, IF-S promotes DC activation independently of DC-SIGN, probably because it interacts with other pathogen-associated molecular pattern receptors (P. Majano et al., submitted for publication).
IF-S binds to and directly contacts DC-SIGN on the cell surface.
To directly test the direct interaction between IF-S and DC-SIGN, adhesion assays were performed with DC-SIGN transfectants on IF-S, using ICAM-3 and mannan as positive controls. As shown in Fig. 8A, K562-CD209 cells bound to IF-S in a dose-dependent manner, reaching maximal adhesion at 5 μg/ml. As expected from a direct interaction, cell binding to IF-S was prevented in the presence of either mannan or the MR1 blocking monoclonal antibody against DC-SIGN (Fig. 8B). Therefore, DC-SIGN mediates cell binding to IF-S. To more definitively demonstrate the DC-SIGN-IF-S interaction, we used an alternative strategy. It has recently been demonstrated that STD NMR experiments might be used, in favorable cases, to detected ligand binding to receptors expressed at the surface of living cells (10), allowing the study of cell surface DC-SIGN-mannan interactions by following the magnetization transfer between protons of mannan and cell surface DC-SIGN (26). Therefore, one-dimensional STD NMR experiments were performed with IF-S and either K562-CD209 or mock-transfected K562 cells. The regular one-dimensional 1H NMR spectrum of IF-S alone or in the presence of cells (K562 or K562-CD209) is shown in Fig. 8C (upper left panel). The STD control spectrum of the IF-S confirmed that the on-resonance irradiation (7 ppm, aromatic region) did not affect the polysaccharide signals, and identical results were obtained by using saturation at −0.3 ppm (aliphatic side chain region) (data not shown). When control data for mock-transfected K562 cells were taken in the presence of IF-S, no polysaccharide signal was evident in the difference spectrum (Fig. 8C, lower left panel), indicating that IF-S does not interact with mock-transfected K562 cells. In contrast, the STD spectrum of the mixture of IF-S with K562-CD209 cells unambiguously revealed the presence of polysaccharide signals upon irradiation at either −0.3 ppm (Fig. 8C, upper right panel) or 7 ppm (Fig. 8C, lower right panel). Therefore, irradiation at the aromatic or aliphatic region protons of the DC-SIGN receptor protein expressed on living cells produces the transfer of magnetization to the polysaccharide protons of IF-S, thus demonstrating the interaction of the IF-S polysaccharide with cell surface DC-SIGN. Since DC-SIGN-negative K562 cells do not produce any NMR signal, it can be concluded that the STD signals observed are due to the interaction of IF-S with the DC-SIGN receptor on the cell surface.
FIG. 8.
IF-S binds and directly contacts DC-SIGN on the cell surface. (A) Adhesion of K562 K562-CD209 cells to IF-S-, mannan-, and ICAM-3-coated wells. (B) Adhesion of K562 and K562-CD209 cells to IF-S-coated wells in the presence of mannan or the indicated antibodies. Each adhesion condition was assayed in triplicate, and the data shown are the means ± standard deviations. (C) Binding of IF-S to cell surface DC-SIGN as determined by one-dimensional STD NMR. (Upper left panel) 1H NMR spectrum of IF-S with K562-CD209 cells in PBS at 298 K; (lower left panel) STD reference spectrum of IF-S with K562 cells (signal enhancement = 32×, NS [number of scans] = 128, on-resonance frequency = −0.3 ppm, off-resonance frequency = 100 ppm, total saturation time = 2 s); (upper right panel) STD spectrum of IF-S with K562-CD209 cells (signal enhancement = 16×, NS = 64, on-resonance frequency = −0.3 ppm, off-resonance frequency = 100 ppm); (lower right panel) STD spectrum of IF-S with K562-CD209 cells (signal enhancement = 32×, NS = 64, on-resonance frequency = 6.8 ppm, off-resonance frequency = 100 ppm).
DISCUSSION
The immunomodulatory action of polysaccharides has been known for decades, but the precise molecular mechanism has not been fully determined. In the present study we have addressed the biological activities of the phosphorylated glucomannan polysaccharide from the cell wall of Candida utilis (IF-S), a constituent of AM3 which acts as an orally effective immunomodulator. IF-S reduces the binding and capture of fungal (Candida, Aspergillus) and parasite (Leishmania) pathogens by human MDDCs in a dose-dependent manner, an effect that is mediated through its interaction with the DC-SIGN pathogen attachment factor. Besides, IF-S prevents the activity of DC-SIGN as a mediator of cell adhesion by weakening its interaction with ICAM-3, impairs the binding of HIV gp120 to DC-SIGN on DCs, and completely blocks the ability of DC-SIGN-expressing cells to capture and transmit replication-competent HIV virions. Therefore, our results demonstrate that the polysaccharide moiety of AM3 directly influences pathogen recognition by DCs by interacting with DC-SIGN on the cell surface and suggest that the adjuvant and immunomodulatory actions of AM3 are mediated, at least in part, by altering the functional capabilities of DC-SIGN.
Structurally, IF-S is a mannan from the cell wall of Candida utilis, and mannan-type polysaccharides from plant, bacterial, and fungal sources have been described to have immunomodulatory effects, although their macrophage-activating potential appears to be weaker than that of β-glucans. As an example, acemannan, a polydispersed β-(1,4)-linked mannan used for the treatment of fibrosarcoma, wounds, and burns, is an immunostimulant which causes macrophage activation (35). In the case of mycobacteria, lipoarabinomannans (LAMs) affect a wide array of biological functions (31). However, subtle differences in LAM structure result in opposite functional properties. Whereas mannosyl cap-containing LAMs (ManLAMs) are anti-inflammatory molecules and inhibit TNF-α and IL-12 production by mononuclear phagocytes, phosphoinositol-capped LAMs (PILAMs), which lack mannooligosaccharide caps, are proinflammatory molecules capable of stimulating the production of TNF-α and IL-12 (31). These differential effects of ManLAMs and PILAMs underline the correlation between the presence of mannan and their immunomodulatory effects (31). Importantly, only mannosylated LAMs have been shown to be recognized by DC-SIGN on the surface of human DCs (22). Regarding Inmunoferon, IF-S has previously been shown to reduce LPS-induced TNF-α, IL-1β, and inducible nitric oxide synthase production by human mononuclear cells (5, 25); but the present results indicate that it is also capable of promoting TNF-α production by MDDCs in a DC-SIGN-independent manner. Our results therefore confirm the immunomodulatory action of IF-S and suggest the existence of additional recognition receptors for IF-S on the surface of human DCs.
The ability of IF-S to block all the pathogen recognition and adhesive capabilities of DC-SIGN assayed is in line with the recently described ability of A. fumigatus cell wall galactomannan to inhibit not only the capture of fungal conidia by DC-SIGN but also the DC-SIGN-ICAM-3 interaction (40). Interestingly, the structures of IF-S and the A. fumigatus galactomannan differ from those of the LewisX [Galβ1-4(Fucα1-3)GlcNAc] and pseudo-LewisY [Fucα1-3Galβ1-4(Fucα1-3)GlcNAc] determinants, which are the DC-SIGN glycolipid ligands in Schistosoma mansoni cercariae (29). The flexibility in the sugar recognition activity of DC-SIGN is thought to be the basis for its ability to recognize a large array of pathogens, all of which target DC-SIGN probably as a means of evading the immune response (47). From this point of view, the ability of IF-S to block pathogen binding to DC-SIGN indicates that it constitutes a useful tool for prevention of the access of clinically relevant pathogens to DCs, whose regulated migratory behavior contributes to pathogen dissemination. Given the therapeutic benefits of AM3 (2, 6, 32, 38), which acts as an adjuvant after oral ingestion and which causes no side effects in clinical studies, it might be worth determining whether it exhibits additional applications as inhibitor of the initial stages of infection and dissemination of pathogens which enter DCs via DC-SIGN.
The previously reported adjuvant activity of AM3 can be directly linked to two other relevant aspects of DC-SIGN, namely, its function as an endocytic receptor and its signaling capability in DCs. Upon ligation by pathogenic or endogenous ligands, DC-SIGN is rapidly internalized from the cell surface and is found in intracellular vesicles, where DC-SIGN mediates antigen delivery into endocytic and lysosomal compartments for subsequent loading of major histocompatibility complex molecules and effective antigen presentation (15, 42). For this reason, DC-SIGN has been proposed to be an efficient target for the antibody-mediated delivery of T-cell epitopes in vaccine development (39). In fact, monoclonal antibodies against DC-SIGN are extremely potent at inducing antigen-specific CD4+ T-cell proliferation (39), and humanized anti-DC-SIGN antibodies have been shown to be effective inducers of naïve and recall T-cell responses (42). Based on these findings, it can be anticipated that IF-S binding to DC-SIGN might contribute to an enhanced rate of antigen capture and internalization by DCs, thus explaining its previously described adjuvant activity. The identification of DC-SIGN as a specific receptor for IF-S will certainly help provide an understanding of the molecular mechanisms for this adjuvant activity and might allow the generation of IF-S-derived molecules with improved adjuvant efficacy.
On the other hand, the IF-S-binding ability of DC-SIGN and its intracellular signaling capability can also explain some of the previously described effects of AM3. Lectin receptors on DCs trigger intracellular signals which modulate those arising from TLR molecules (30). As a representative example, ligation of the β-glucan receptor dectin-1 acts in synergy with TLR2 to induce TNF-α and IL-12 and promotes IL-10 synthesis through recruitment of the Syk kinase (36). In the case of DC-SIGN, recognition of mycobacterial LAM leads to the production of IL-10 and the suppression of DC activity (22), an effect that might be explained by the ERK activation that takes place upon DC-SIGN cross-linking on the DC membrane (8). Moreover, the simultaneous presence of LPS and anti-DC-SIGN cross-linking antibodies results in enhanced production of IL-10 by human MDDCs, without significantly affecting the release of IL-12 p70 (8). As a consequence, it can be hypothesized that the immunomodulatory activities of IF-S could be also explained by its ability to ligate DC-SIGN on the surfaces of DCs and macrophages. In this manner, IF-S could promote intracellular signals favoring the production of IL-10 and modulate the signals arising from other pathogen recognition receptors. This hypothesis is supported by the induction of maturation that takes place upon addition of IF-S onto MDDCs, which results in IL-10-producing mature MDDCs with an enhanced ability to stimulate T-cell proliferation (Majano et al., unpublished). The determination of the gene expression profile induced by AM3 in human DCs will be extremely helpful for the determination of its precise mechanism of action and evaluation of its potential application as a general immunomodulator and in preventing the entry of pathogens in DCs and DC-SIGN-positive macrophages.
Acknowledgments
This work was supported by the Ministerio de Educación y Ciencia (grants SAF2005-0021, AGL2004-02148-ALI, and GEN2003-20649-C06-01/NAC), the Fundación para la Investigación y Prevención del SIDA en España (grant FIPSE 36422/03), the Fundación de Investigación Médica Mutua Madrileña (grant 20060789), and the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (Spanish Network for the Research in Infectious Diseases, grant REIPI RD06/0008), to A.L.C. and grant SAF2004-06991 to J.M.-P. D.S.-G. was supported by a predoctoral grant (grant AP2002-2151) from the Ministerio de Educación y Ciencia (Spain). R.T.M.-N. was funded by Beca de Introducción a la Investigación fellowship from the Consejo Superior de Investigaciones Científicas (CSIC).
Footnotes
Published ahead of print on 23 April 2007.
REFERENCES
- 1.Alvarez, C. P., F. Lasala, J. Carrillo, O. Muniz, A. L. Corbi, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 766841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Mon, M., M. Miravitlles, J. Morera, L. Callol, and J. L. Alvarez-Sala. 2005. Treatment with the immunomodulator AM3 improves the health-related quality of life of patients with COPD. Chest 1271212-1218. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 4.Blake, J. S., M. V. Dahl, M. J. Herron, and R. D. Nelson. 1991. An immunoinhibitory cell wall glycoprotein (mannan) from Trichophyton rubrum. J. Investig. Dermatol. 96657-661. [DOI] [PubMed] [Google Scholar]
- 5.Brieva, A., A. Guerrero, J. L. Alonso-Lebrero, and J. P. Pivel. 2001. Immunoferon, a glycoconjugate of natural origin, inhibits LPS-induced TNF-alpha production and inflammatory responses. Int. Immunopharmacol. 11979-1987. [DOI] [PubMed] [Google Scholar]
- 6.Brieva, A., A. Guerrero, and J. P. Pivel. 2002. Inmunoferon, a glycoconjugate of natural origin, regulates the liver response to inflammation and inhibits TNF-alpha production by an HPA axis-dependent mechanism. Int. Immunopharmacol. 2807-813. [DOI] [PubMed] [Google Scholar]
- 7.Cambi, A., K. Gijzen, J. M. de Vries, R. Torensma, B. Joosten, G. J. Adema, M. G. Netea, B. J. Kullberg, L. Romani, and C. G. Figdor. 2003. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur. J. Immunol. 33532-538. [DOI] [PubMed] [Google Scholar]
- 8.Caparros, E., P. Munoz, E. Sierra-Filardi, D. Serrano-Gomez, A. Puig-Kroger, J. L. Rodriguez-Fernandez, M. Mellado, J. Sancho, M. Zubiaur, and A. L. Corbi. 2006. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood 1073950-3958. [DOI] [PubMed] [Google Scholar]
- 9.Chehimi, J., Q. Luo, L. Azzoni, L. Shawver, N. Ngoubilly, R. June, G. Jerandi, M. Farabaugh, and L. J. Montaner. 2003. HIV-1 transmission and cytokine-induced expression of DC-SIGN in human monocyte-derived macrophages. J. Leukoc. Biol. 74757-763. [DOI] [PubMed] [Google Scholar]
- 10.Claasen, B., M. Axmann, R. Meinecke, and B. Meyer. 2005. Direct observation of ligand binding to membrane proteins in living cells by a saturation transfer double difference (STDD) NMR spectroscopy method shows a significantly higher affinity of integrin alpha(IIb)beta3 in native platelets than in liposomes. J. Am. Chem. Soc. 127916-919. [DOI] [PubMed] [Google Scholar]
- 11.Colmenares, M., A. L. Corbi, S. J. Turco, and L. Rivas. 2004. The dendritic cell receptor DC-SIGN discriminates among species and life cycle forms of Leishmania. J. Immunol. 1721186-1190. [DOI] [PubMed] [Google Scholar]
- 12.Colmenares, M., A. Puig-Kroger, O. M. Pello, A. L. Corbi, and L. Rivas. 2002. Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human DCs, is a receptor for Leishmania amastigotes. J. Biol. Chem. 27736766-36769. [DOI] [PubMed] [Google Scholar]
- 13.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA. 898356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Rosa, G., M. Yanez-Mo, R. Samaneigo, D. Serrano-Gomez, L. Martinez-Munoz, E. Fernandez-Ruiz, N. Longo, F. Sanchez-Madrid, A. L. Corbi, and P. Sanchez-Mateos. 2005. Regulated recruitment of DC-SIGN to cell-cell contact regions during zymosan-induced human dendritic cell aggregation. J. Leukoc. Biol. 77699-709. [DOI] [PubMed] [Google Scholar]
- 15.Engering, A., T. B. Geijtenbeek, S. J. van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C. G. Figdor, V. Piguet, and Y. van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 1682118-2126. [DOI] [PubMed] [Google Scholar]
- 16.Engering, A., S. J. Van Vliet, T. B. Geijtenbeek, and Y. Van Kooyk. 2002. Subset of DC-SIGN(+) dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood 1001780-1786. [DOI] [PubMed] [Google Scholar]
- 17.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 2942163-2166. [DOI] [PubMed] [Google Scholar]
- 18.Frison, N., M. E. Taylor, E. Soilleux, M. T. Bousser, R. Mayer, M. Monsigny, K. Drickamer, and A. C. Roche. 2003. Oligolysine-based oligosaccharide clusters: selective recognition and endocytosis by the mannose receptor and dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin. J. Biol. Chem. 27823922-23929. [DOI] [PubMed] [Google Scholar]
- 19.Geijtenbeek, T. B., D. J. Krooshoop, D. A. Bleijs, S. J. van Vliet, G. C. van Duijnhoven, V. Grabovsky, R. Alon, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 1353-357. [DOI] [PubMed] [Google Scholar]
- 20.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100587-597. [DOI] [PubMed] [Google Scholar]
- 21.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100575-585. [DOI] [PubMed] [Google Scholar]
- 22.Geijtenbeek, T. B., S. J. Van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. Van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 1977-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geijtenbeek, T. B., S. J. van Vliet, G. C. van Duijnhoven, C. G. Figdor, and Y. van Kooyk. 2001. DC-SIGN, a dentritic cell-specific HIV-1 receptor present in placenta that infects T cells in trans—a review. Placenta 22(Suppl. A)S19-S23. [DOI] [PubMed] [Google Scholar]
- 24.Lozach, P. Y., H. Lortat-Jacob, A. de Lacroix de Lavalette, I. Staropoli, S. Foung, A. Amara, C. Houles, F. Fieschi, O. Schwartz, J. L. Virelizier, F. Arenzana-Seisdedos, and R. Altmeyer. 2003. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J. Biol. Chem. 27820358-20366. [DOI] [PubMed] [Google Scholar]
- 25.Majano, P., J. L. Alonso-Lebrero, A. Janczyk, S. Martin-Vichez, F. Molina-Jimenez, A. Brieva, J. P. Pivel, S. Gonzalez, M. Lopez-Cabrera, and R. Moreno-Otero. 2005. AM3 inhibits LPS-induced iNOS expression in mice. Int. Immunopharmacol. 51165-1170. [DOI] [PubMed] [Google Scholar]
- 26.Mari, S., D. Serrano-Gomez, F. J. Canada, A. L. Corbi, and J. Jimenez-Barbero. 2004. 1D saturation transfer difference NMR experiments on living cells: the DC-SIGN/oligomannose interaction. Angew. Chem. Int. Ed. Engl. 44296-298. [DOI] [PubMed] [Google Scholar]
- 27.Mayer, M., and B. Meyer. 2001. Group epitope mapping by saturation transfer difference NMR to identify segments of a ligand in direct contact with a protein receptor. J. Am. Chem. Soc. 1236108-6117. [DOI] [PubMed] [Google Scholar]
- 28.Meyer, B., and T. Peters. 2003. NMR spectroscopy techniques for screening and identifying ligand binding to protein receptors. Angew. Chem. Int. Ed. Engl. 42864-890. [DOI] [PubMed] [Google Scholar]
- 29.Meyer, S., E. van Liempt, A. Imberty, Y. van Kooyk, H. Geyer, R. Geyer, and I. van Die. 2005. DC-SIGN mediates binding of dendritic cells to authentic pseudo-LewisY glycolipids of Schistosoma mansoni cercariae, the first parasite-specific ligand of DC-SIGN. J. Biol. Chem. 28037349-37359. [DOI] [PubMed] [Google Scholar]
- 30.Meyer-Wentrup, F., A. Cambi, G. J. Adema, and C. G. Figdor. 2005. “Sweet talk”: closing in on C type lectin signaling. Immunity 22399-400. [DOI] [PubMed] [Google Scholar]
- 31.Nigou, J., M. Gilleron, M. Rojas, L. F. Garcia, M. Thurnher, and G. Puzo. 2002. Mycobacterial lipoarabinomannans: modulators of dendritic cell function and the apoptotic response. Microbes Infect. 4945-953. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Garcia, R., A. Perez-Garcia, D. Verbeelen, E. D. Bernstein, V. G. Villarrubia, and M. Alvarez-Mon. 2002. AM3 (Inmunoferon) as an adjuvant to hepatitis B vaccination in hemodialysis patients. Kidney Int. 611845-1852. [DOI] [PubMed] [Google Scholar]
- 33.Pohlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 774070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prieto, A., E. Reyes, E. D. Bernstein, B. Martinez, J. Monserrat, J. L. Izquierdo, L. Callol, P. de Lucas, R. Alvarez-Sala, J. L. Alvarez-Sala, V. G. Villarrubia, and M. Alvarez-Mon. 2001. Defective natural killer and phagocytic activities in chronic obstructive pulmonary disease are restored by glycophosphopeptical (Inmunoferon). Am. J. Respir. Crit. Care Med. 1631578-1583. [DOI] [PubMed] [Google Scholar]
- 35.Ramamoorthy, L., M. C. Kemp, and I. R. Tizard. 1996. Acemannan, a beta-(1,4)-acetylated mannan, induces nitric oxide production in macrophage cell line RAW 264.7. Mol. Pharmacol. 50878-884. [PubMed] [Google Scholar]
- 36.Rogers, N. C., E. C. Slack, A. D. Edwards, M. A. Nolte, O. Schulz, E. Schweighoffer, D. L. Williams, S. Gordon, V. L. Tybulewicz, G. D. Brown, and E. S. C. Reis. 2005. Syk-dependent cytokine induction by dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22507-517. [DOI] [PubMed] [Google Scholar]
- 37.Rojo, J. M., M. T. Rejas, G. Ojeda, P. Portoles, and I. Barasoain. 1986. Enhancement of lymphocyte proliferation, interleukin-2 production and NK activity by Inmunoferon (AM-3), a fungal immunomodulator: variations in normal and immunosuppressed mice. Int. J. Immunopharmacol. 8593-597. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez, L., E. Pena, A. Civantos, G. Sada, M. M. Alvarez, M. A. Chirigos, and V. G. Villarrubia. 1995. AM3, an adjuvant to hepatitis B revaccination in non-responder healthy persons. J. Hepatol. 22119-121. [DOI] [PubMed] [Google Scholar]
- 39.Schjetne, K. W., K. M. Thompson, T. Aarvak, B. Fleckenstein, L. M. Sollid, and B. Bogen. 2002. A mouse C kappa-specific T cell clone indicates that DC-SIGN is an efficient target for antibody-mediated delivery of T cell epitopes for MHC class II presentation. Int. Immunol. 141423-1430. [DOI] [PubMed] [Google Scholar]
- 40.Serrano-Gomez, D., A. Dominguez-Soto, J. Ancochea, J. A. Jimenez-Heffernan, J. A. Leal, and A. L. Corbi. 2004. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin mediates binding and internalization of Aspergillus fumigatus conidia by dendritic cells and macrophages. J. Immunol. 1735635-5643. [DOI] [PubMed] [Google Scholar]
- 41.Steinman, R. M., D. Hawiger, and M. C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21685-711. [DOI] [PubMed] [Google Scholar]
- 42.Tacken, P. J., I. J. de Vries, K. Gijzen, B. Joosten, D. Wu, R. P. Rother, S. J. Faas, C. J. Punt, R. Torensma, G. J. Adema, and C. G. Figdor. 2005. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood 1061278-1285. [DOI] [PubMed] [Google Scholar]
- 43.Tailleux, L., O. Schwartz, J. L. Herrmann, E. Pivert, M. Jackson, A. Amara, L. Legres, D. Dreher, L. P. Nicod, J. C. Gluckman, P. H. Lagrange, B. Gicquel, and O. Neyrolles. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21335-376. [DOI] [PubMed] [Google Scholar]
- 45.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Gisbergen, K. P., M. Sanchez-Hernandez, T. B. Geijtenbeek, and Y. van Kooyk. 2005. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2011281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Kooyk, Y., A. Engering, A. N. Lekkerkerker, I. S. Ludwig, and T. B. Geijtenbeek. 2004. Pathogens use carbohydrates to escape immunity induced by dendritic cells. Curr. Opin. Immunol. 16488-493. [DOI] [PubMed] [Google Scholar]
- 48.van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3697-709. [DOI] [PubMed] [Google Scholar]
- 49.Varela, J., M. L. Navarro Pico, A. Guerrero, F. Garcia, G. Gimenez Gallego, and J. P. Pivel. 2002. Identification and characterization of the peptidic component of the immunomodulatory glycoconjugate Immunoferon. Methods Find. Exp. Clin. Pharmacol. 24471-480. [DOI] [PubMed] [Google Scholar]
- 50.Yoshitomi, H., N. Sakaguchi, K. Kobayashi, G. D. Brown, T. Tagami, T. Sakihama, K. Hirota, S. Tanaka, T. Nomura, I. Miki, S. Gordon, S. Akira, T. Nakamura, and S. Sakaguchi. 2005. A role for fungal β-glucans and their receptor dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201949-960. [DOI] [PMC free article] [PubMed] [Google Scholar]