Abstract
The emergence of strains of Plasmodium falciparum resistant to the commonly used antimalarials warrants the development of new antimalarial agents. The discovery of type II fatty acid synthase (FAS) in Plasmodium distinct from the FAS in its human host (type I FAS) opened up new avenues for the development of novel antimalarials. The process of fatty acid synthesis takes place by iterative elongation of butyryl-acyl carrier protein (butyryl-ACP) by two carbon units, with the successive action of four enzymes constituting the elongation module of FAS until the desired acyl length is obtained. The study of the fatty acid synthesis machinery of the parasite inside the red blood cell culture has always been a challenging task. Here, we report the in vitro reconstitution of the elongation module of the FAS of malaria parasite involving all four enzymes, FabB/F (β-ketoacyl-ACP synthase), FabG (β-ketoacyl-ACP reductase), FabZ (β-ketoacyl-ACP dehydratase), and FabI (enoyl-ACP reductase), and its analysis by matrix-assisted laser desorption-time of flight mass spectrometry (MALDI-TOF MS). That this in vitro systems approach completely mimics the in vivo machinery is confirmed by the distribution of acyl products. Using known inhibitors of the enzymes of the elongation module, cerulenin, triclosan, NAS-21/91, and (−)-catechin gallate, we demonstrate that accumulation of intermediates resulting from the inhibition of any of the enzymes can be unambiguously followed by MALDI-TOF MS. Thus, this work not only offers a powerful tool for easier and faster throughput screening of inhibitors but also allows for the study of the biochemical properties of the FAS pathway of the malaria parasite.
Malaria afflicts around 500 million people every year and kills almost 1 to 3 million, the majority of which are children under 5 years of age (37). The disease is caused by a protozoan, Plasmodium, which belongs to the phylum Apicomplexa. The situation has worsened with the emergence of drug-resistant strains of Plasmodium and warrants the discovery of either new drugs or new analogues of existing drugs. Novel metabolic pathways specific to Plasmodium and distinct from those of its human host can prove to be a good target for the development of novel antimalarials. The discovery of the type II fatty acid synthase (FAS) pathway in the apicoplast of the parasite, which is distinct from the FAS pathway in its human host, has provided an impetus for the discovery of novel antimalarial agents (2, 34).
Synthesis of fatty acids is a central cellular process. Fatty acids are produced by the iterative condensation of two carbon units by multienzymatic systems called FAS, which can be broadly divided into two types: type I, or the associative type, and type II, or the dissociative type. Type I synthase, which occurs in the cytoplasm of animals, fungi, and certain mycobacteria, consists of a single large, multifunctional protein (10, 19). All the catalytic domains required to catalyze the reactions for the synthesis of fatty acids are present on a single molecule. It can be a homodimer, as in the case of animal FAS (19), or a hexamer, as present in fungal FAS (10). In type II FAS, which is present in bacteria, plants, and protozoans, discrete enzymes encoded by distinct genes catalyze the reactions of fatty acid synthesis (36). Acyl carrier protein (ACP) carries the fatty acyl moieties between various domains or enzymes. ACP is an integral component of the large polypeptide in type I FAS, whereas it exists as an independent moiety in type II FAS. Both the synthases iteratively catalyze the same basic biochemical reactions, viz., condensation, reduction, dehydration, and reduction, for the formation of fatty acids (36).
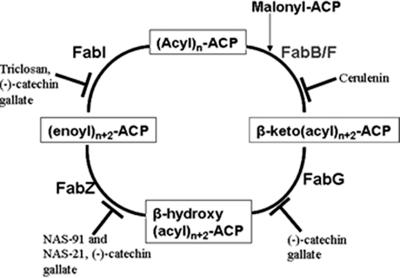
The overall process of fatty acid synthesis can be divided into two parts, the initiation phase and the elongation cycle (36). During the initiation phase, acetyl-coenzyme A (CoA) carboxylase (ACC) catalyzes the conversion of acetyl-CoA to malonyl-CoA. Malonyl-CoA thus formed is converted to malonyl-ACP by malonyl-CoA:ACP transacylase (FabD or MCAT). β-Ketoacyl-ACP synthase III (FabH) then initiates the cycle by condensing acetyl-CoA with malonyl-ACP to give rise to β-ketobutyryl-ACP. This product is then transferred to the elongation module of FAS, which consists of FabG, FabZ/A, FabI, and FabB/F (Fig. 1). FabG (β-ketoacyl-ACP reductase) is a NADPH-dependent reductase which reduces β-ketobutyryl-ACP to β-hydroxybutyryl-ACP, which is then dehydrated to trans-2-butenoyl-ACP by β-hydroxyacyl-ACP dehydratase (FabZ). This product is in turn taken over by an NADH-dependent reductase, FabI (enoyl-ACP reductase), which reduces the double bond, forming butyryl-ACP. This butyryl-ACP is then condensed with malonyl-ACP by the FabB/F class of condensing enzymes, leading to the formation of β-ketohexanoyl-ACP, and the cycle continues until the desired chain length of fatty acid is achieved (36). Thioesterase cleaves the thioester bond with which the acyl chain is bound to ACP and releases the fatty acids. In the case of type II FAS, thioesterases are present as a discrete entity, while in the case of type I FAS, the thioesterase domain is covalently linked to the multifunctional polypeptide (1).
FIG. 1.
Schematic representation of the elongation module of FAS.
Since fatty acids are essential primary metabolites, FAS protein components are being intensively scrutinized for development of antibacterial, antitubercular, and in recent studies antimalarial agents (8, 32, 33). Reconstitution experiments have given a great deal of biochemical insight into the in vivo modus operandi of the metabolite synthesis system, be it polyketide synthase (20) or bacterial FAS (7). Though Plasmodium falciparum also has a type II FAS akin to the extensively studied Escherichia coli type II FAS, significant differences occur in overall functionality. Here, for the first time, we report cell-free reconstitution of the elongation module of P. falciparum FAS (PfFAS), consisting of four different proteins, viz., PfFabB/F (MAL6P1.165), PfFabG (PFI_1125c), PfFabZ (PF13_0128), and PfFabI (PFF0730c), for producing C14:0 fatty acids, and the direct determination of the covalently acylated biosynthetic intermediates as well as the final product by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). MALDI-TOF MS can easily detect molecules with molecular masses greater than 1,000 Da. It can differentiate molecules which differ by less than 2 Da in molecular mass, and hundreds of samples can be analyzed in one MALDI plate, which can then be reused. By using type II FAS inhibitors such as cerulenin, triclosan, NAS-21/91, and (−)-catechin gallate, we show that the evolution and accumulation of acyl-ACP intermediates can be followed by using MALDI-TOF MS. Unambiguous characterization of intermediates provides a systems approach for screening of inhibitors for the elongation module of P. falciparum FAS.
MATERIALS AND METHODS
Strains, plasmids, and reagents.
E. coli strain DH5-α was used for all cloning purposes, and BL21(DE3) (Novagen) cells were used for expression of all the recombinant proteins. pET-28a(+), pET-22b, and pET-43.1a(+) vectors and Ni-nitrilotriacetic acid His bind resin were obtained from Novagen. Malonyl-CoA, all acyl-CoAs, NADH, NADPH, dithiothreitol (DTT), and Triton X-100 were purchased from Sigma Chemicals (St. Louis, MO). Hi-Trap desalting columns were from GE Healthcare. All other reagents were of the highest grade available.
Expression and purification of PfFAS enzymes.
In order to function, the elongation module of FAS requires a condensing enzyme, PfFabB/F; a reductase, PfFabG; a dehydratase, PfFabZ; and another reductase, PfFabI. To avoid any time-based activity losses, all four enzymes were purified simultaneously and assayed according to references 12, 14, 16, and 29.
Acyl-ACP synthesis using AcpS.
For the synthesis of various acyl-PfACPs using E. coli holo-ACP synthase and apo-PfACP, the modified protocol of Lambalot and Walsh (17) was followed. AcpS was cloned in pET22b and purified according to reference 17. Pfacp gene was cloned in pET28a(+) (31), and the apo form was purified according to reference 27. Fifty micromolars apo-PfACP, 200 μM acyl-CoA, 20 mM MgCl2, 3 to 5 μg E. coli AcpS in 25 mM Tris-Cl, pH 7.5, and 0.5% Triton X-100 were used for synthesis reactions involving C12-C16Δ1-CoA. All the acyl-ACP synthesis reactions were carried out for 30 min except those for C14, C16, and C16Δ1, which were carried out for 60 min.
In vitro reconstitution of the elongation module of fatty acid synthesis of Plasmodium falciparum.
For reconstitution experiments, malonyl-PfACP, acyl-PfACPs, all FAS enzymes required to complete the cycle, and their cofactors (NADH, NADPH, DTT, and phenylmethylsulfonyl fluoride) were added. The reaction mix included 25 mM Tris-Cl, pH 7.5, 200 μM malonyl-PfACP, 40 μM acyl-PfACP, 5 μg PfFabB/F, 2 μg PfFabG, 3 μg PfFabZ, 2 μg PfFabI, 100 μM NADH, 100 μM NADPH, 1 mM DTT, and 1 mM phenylmethylsulfonyl fluoride in a final volume of 75 μl. The reaction was started by the addition of PfFabB/F. The reaction mixture was incubated at 37°C; aliquots were taken out from the reaction mixture and were snap frozen.
Analysis of reconstituted samples by MALDI-TOF MS.
The reconstituted samples were analyzed by MALDI-TOF MS. Samples were mixed with equal volumes of the matrix, which consisted of a saturated solution of sinnapinic acid in 50% acetonitrile and water containing 0.1% trifluoroacetic acid. The mixture was spotted and allowed to dry before analysis by MALDI-TOF MS (Ultraflex TOF-TOF; Bruker Daltronics).
Inhibition of PfFAS by cerulenin, (−)-catechin gallate, NAS-21/91, and triclosan.
The reconstitution system was individually blocked by inhibitors against each of the participating enzymes. To the reconstitution mixture described in paragraph 4 of Materials and Methods, 50 μM cerulenin, 5 μM (−)-catechin gallate, or 25 μM NAS-21/91 was added. The reaction mixture was incubated at 37°C, and aliquots were taken out at different time points, snap frozen, and analyzed by MALDI-TOF MS. To check for triclosan inhibition, 5 μg PfFabB/F, 2 μg PfFabG, and 3 μg PfFabZ were added one by one, and aliquots were taken out 10 min after each enzyme addition. Finally, 2 μg PfFabI, with or without 5 μM triclosan, was added. Aliquots were removed at each step, snap frozen, and analyzed by MALDI-TOF MS. To validate the utility of the assay for detecting drug-resistant forms of any of the components of the FAS assembly system, the addition of the A217V PfFabI mutant (13) in place of the wild-type (wt) PfFabI was examined. For this purpose, instead of wt PfFabI, 2 μg of A217V was added in the presence of triclosan.
RESULTS AND DISCUSSION
Contrary to the earlier widely held view that the parasite imports fatty acids from its human host and is unable to synthesize fatty acids de novo, the presence of type II FAS in Plasmodium has been demonstrated (34). Subsequently, its existence has been confirmed in a number of studies (25, 33). By analyzing the incorporation of [1,214C]malonyl-CoA, Surolia and Surolia (34) concluded that the C10:0, C12:0, and C14:0 fatty acids are the major fatty acids synthesized by the parasite. The reconstitution of the initiation phase of fatty acid synthesis of P. falciparum was reported recently (25). It was shown that PfFabH does not accept branched-chain CoAs as substrates, confirming that Plasmodium FAS can synthesize only straight-chain fatty acids and does not form branched-chain fatty acids. Unlike the initiation phase of FAS, the elongation cycle of fatty acid synthesis in P. falciparum has not been attempted. We have reconstituted the elongation module and showed that it can serve as an important tool for biochemical as well as high-throughput inhibition studies.
Pfacyl-ACP preparation using AcpS.
E. coli AcpS and apo-PfACP were used for the synthesis of acyl-PfACPs (17). AcpS converts inactive apo-ACP to its active holo form by charging it with the phosphopantetheine arm of CoA. When acyl-CoAs are given, apo-ACP is converted to acyl-ACP by AcpS. The enzyme was able to efficiently charge apo-ACP with the whole range of saturated acyl substrates, ranging from C4 to C16 as well as C16Δ1 in length. The reaction was almost completed, as is evident from the absence of a substrate band (apo-PfACP) (Fig. 2a). When various enzymatically synthesized acyl-PfACPs were run on conformation-sensitive polyacrylamide gel electrophoresis (CS-PAGE) gels containing 1 to 5 M urea, unlike acyl-ACPs from other sources, these did not show any significant length-based mobility difference (Fig. 2a). Since the reconstitution of the elongation module of PfFAS involves the formation of acyl-PfACPs of increasing chain lengths with time, conventional CS-PAGE cannot be employed for the study of products.
FIG. 2.
(a) CS-PAGE gel showing the migration of various acyl-PfACPs synthesized using apo-PfACP, respective acyl-CoAs, and the AcpS enzyme. A 13%, 2.5 M urea sample containing native PAGE gel was run at 20 mA at 4°C. Lane 1, malonyl-PfACP, which runs as a doublet; lane 2, C4-PfACP; lane 3, C6-PfACP; lane 4, C8-PfACP; lane 5, C10-PfACP; lane 6, apo-PfACP; lane 7, C12-PfACP; lane 8, C14-PfACP; lane 9, C16-PfACP; lane 10, C16:1-PfACP. As can be seen from the figure, acyl-PfACPs of various chain lengths do not show significant mobility differences, unlike E. coli acyl-ACPs. (b) A 12% sodium dodecyl sulfate-PAGE gel showing the purities of all the enzymes used in the reconstitution experiment along with the E. coli AcpS used for the synthesis of P. falciparum acyl-ACPs. Lane 1, 108-kDa PfFabB/F; lane 2, 36-kDa PfFabI; lane 3, molecular mass marker from MBI Fermentas; lane 4, 29-kDa PfFabG; lane 5, 17-kDa PfFabZ; and lane 6, 14-kDa AcpS.
Purification of FAS enzymes of the elongation module.
Fatty acid synthesis takes place inside a relict plastid, called apicoplast, present in the parasite. The genes coding for the enzymes of the fatty acid synthesis pathway are present on the nuclear genome. All the FAS enzymes are encoded with an N-terminal leader sequence required for targeting to the apicoplast, and thus, they resemble plant proteins more than their bacterial counterparts. Once inside the organelle, this leader peptide is cleaved off and the mature protein is released into the lumen of the apicoplast. Reconstitution of the elongation module requires the activities of PfFabB/F, PfFabG, PfFabZ, and PfFabI.
Mature PfFabB/F, without the leader peptide, was cloned with an N-terminal NusA fusion and purified using metal affinity chromatography. The 108-kDa fusion protein was used for all the experiments. The mature protein sequences, without the leader peptide, of PfFabG, PfFabZ, and PfFabI were cloned with an N-terminal hexa-histidine tag and purified by Ni-nitrilotriacetic acid metal affinity chromatography. All enzymes were purified to homogeneity (Fig. 2b), and their activities were checked by a spectrophotometric assay to confirm their active states prior to their use for the reconstitution experiment. The fact that all FAS enzymes have an N-terminal leader peptide required for apicoplast targeting clearly indicates their functional presence inside the apicoplast.
Reconstitution of the elongation phase and analyses of samples by MALDI-TOF MS.
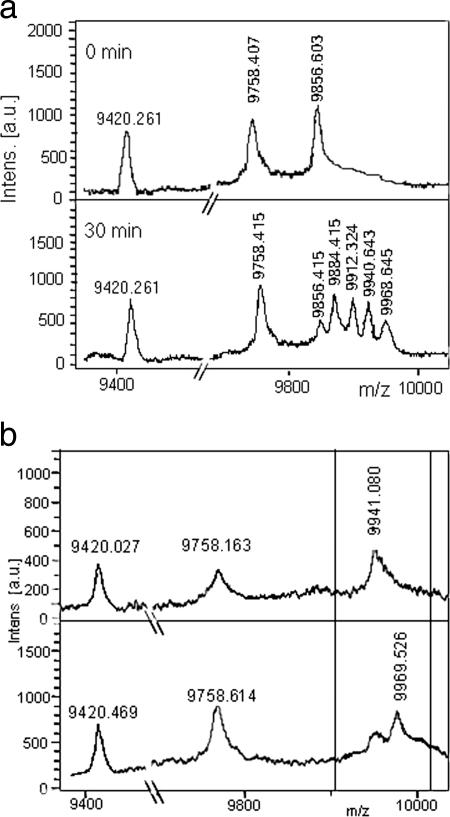
A single round of fatty acid synthesis leads to the addition of an acetyl moiety to the existing acyl chain. Thus, the minimum difference between the successive acyl-PfACPs analyzed will be 28 Da, which can be successfully identified by MALDI-TOF MS. Since ACP exists as an independent identity in type II FAS, all the acyl products linked to it can be analyzed without further processing, unlike for type I FAS, where ACP exists as an integral component and the growing acyl chains are linked to a large polypeptide. As CS-PAGE could not be used for the analysis of the products of the reconstitution experiment, we adopted MS for analysis. The reaction was set up, and aliquots were taken out at different time points, snap frozen, and analyzed by MALDI-TOF MS (Bruker Daltronics). Figure 3a clearly shows the formation of various acyl-PfACPs, viz., the C8, C10, C12, and C14 PfACPs. C14-PfACP was the last and the longest species detected, validating the earlier findings by Surolia and Surolia (34), where it was demonstrated that C10:0, C12:0, and C14:0 are the major fatty acids synthesized by Plasmodium FAS. Additionally, this also authenticates our reconstitution model and the analytical ability of MALDI-TOF MS to detect each intermediate. The profile of fatty acids produced by parasite FAS is in contrast to that for E. coli FAS, in which C16:0, C16Δ9, and C18Δ11 are the major products (18), and mitochondrial FAS, in which short-chain fatty acids (mainly C8) occur as major intermediates, with long chains as the final products (21, 38). Parasite FAS also differs from plant plastid FAS, as the latter synthesizes long-chain fatty acids in abundance (11), which suggests the evolutionary divergence of parasite FAS from that of the primary symbiont from which the apicoplast has evolved.
FIG. 3.
(a) MALDI-TOF spectra showing in vitro reconstitution of the fatty acid synthesis cycle of Plasmodium falciparum. C6-PfACP (9,856 Da) + malonyl-PfACP → C8-PfACP (9,884 Da) + C10-PfACP (9,912 Da) + C12-PfACP (9,940 Da) + C14-PfACP (9,968 Da) + holo PfACP (9,758 Da). The peak at 9,420 Da corresponds to apo-ACP. (b) MALDI-TOF spectra showing in vitro reconstitution of the fatty acid synthesis cycle of Plasmodium falciparum. C12-PfACP (9,941 Da) + malonyl-PfACP → C14-PfACP (9,969 Da) + PfACP (9,758 Da). The peak at 9,420 Da corresponds to apo-ACP.
To rule out the possibility of limiting substrates, we reconstituted the cycle starting from C12-PfACP and malonyl-PfACP, but only the C14-PfACP peak can be seen (Fig. 3b). Thus, our in vitro-reconstituted cycle showed the presence of C14-PfACP as the longest-chain-length fatty acid synthesized by Plasmodium FAS.
Inhibition of reconstituted PfFAS.
The fatty acid pathway has come up as one of the major targets for the development of novel antimicrobial chemotherapeutics (8, 33). Some of the potent inhibitors of the fatty acid synthesis pathway are cerulenin and thiolactomycin, which inhibit condensing enzymes (24); NAS-91 and NAS-21, which inhibit FabZ from Plasmodium falciparum (29); catechins and flavonoids, which inhibit FabG, FabZ, and FabI (30, 35); and triclosan and diazoborines, which inhibit FabI (9, 12). Thus, after establishing the ability of our reconstitution model to mimic the in vivo situation, we decided to check whether this novel approach can be used for the screening of inhibitors. We used cerulenin, triclosan, NAS-21/91, and (−)-catechin gallate to inhibit our reconstituted system and study the distribution of end products accumulated.
Cerulenin is a fungal product synthesized by the fungus Cephalosporium ceruleans. It forms a covalent adduct with the active site cysteine thiol and thus mimics the acyl-enzyme intermediate state (22). Cerulenin, an irreversible inhibitor of synthases, has been shown to inhibit the growth of P. falciparum in vitro with a 50% inhibitory concentration (IC50) of 20 μM (34). Upon addition of cerulenin was added to the reconstitution mixture, only the substrate peak (for C6-PfACP) could be detected, which suggested a complete inhibition of fatty acid synthesis by cerulenin (Fig. 4). This is quite obvious, as PfFabB/F, being the condensing enzyme, catalyzes the first step and inhibition of its activity by cerulenin is expected to bring the entire cycle to a halt. Thus, the assay readily validates the site of inhibition.
FIG. 4.
Inhibition of PfFAS by cerulenin. The boxed area shows the presence of only the substrate peak (C6-ACP, 9,856 Da) with time and no sign of the appearance of any of the product peaks (β-keto C8-ACP [9,898 Da], β-hydroxy C8-ACP [9,900 Da], octenoyl-ACP [9,882 Da], C8-ACP [9,884 Da]), indicating inhibition of the first condensation step by cerulenin. The 9,758-Da peak corresponds to holo-ACP, and the 9,420-Da peak corresponds to apo-ACP.
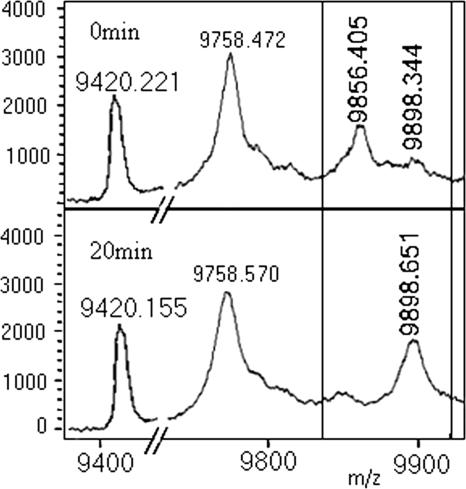
The PfFAS cycle was next inhibited with (−)-catechin gallate, which is known to inhibit PfFabG, PfFabZ, and PfFabI. The catalytic cycle is clearly blocked at the β-ketoacyl-ACP step (9,898 Da, a product of PfFabB/F reaction), resulting from the inhibition of PfFabG (the next enzyme of the module) (Fig. 5). Thus, in the context of the elongation phase of fatty acid synthesis, inhibition of FabG by catechin gallate appears to predominate over the inhibitory effect on PfFabZ and PfFabI, highlighting the unique ability of the present approach to pinpoint the precise site of action of an inhibitor with multiple targets in the reconstituted FAS system.
FIG. 5.
Inhibition of PfFAS by (−)-catechin gallate. The boxed area shows the conversion of the substrate peak, C6-PfACP (9,856 Da) to β-keto C8-PfACP (9,898 Da) (a product of PfFabB/F) but no sign of β-hydroxy C8-PfACP (9,900 Da) (a product of PfFabG), octenoyl-ACP (9,882 Da), or C8-ACP (9,884 Da), indicating inhibition of the PfFabG step. The 9,758-Da peak corresponds to holo-ACP, and the 9,420-Da peak corresponds to apo-ACP.
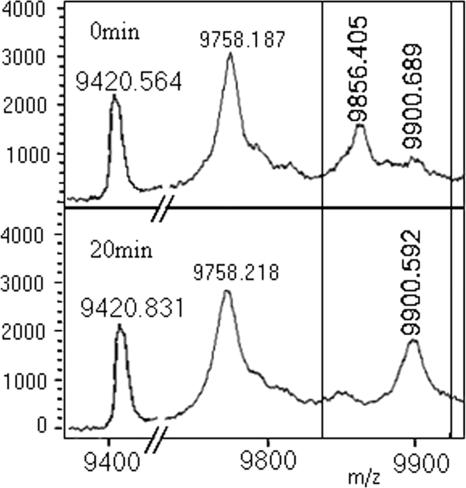
The reconstituted PfFAS was also inhibited with NAS-21 and NAS-91, inhibitors of PfFabZ. As can be seen from Fig. 6, the substrate peak is converted from C6-PfACP (9,856 Da) to β-hydroxy C8-ACP (9,900 Da) (a product of FabG reaction). This clearly indicates that the substrate C6-ACP is condensed by PfFabB/F and reduced by FabG to β-hydroxy C8-ACP but is not further dehydrated to trans-2-octenoyl-ACP, indicating inhibition of the PfFabZ step.
FIG. 6.
Inhibition of PfFAS by NAS-21 and NAS-91. The boxed area shows the conversion of the substrate peak C6-PfACP (9,856 Da) to β-hydroxy C8-PfACP (9,900 Da) (a product of PfFabG) but no sign of octenoyl-ACP (9,882 Da) or C8-ACP (9,884 Da), indicating inhibition of the PfFabZ step. The 9,758-Da peak corresponds to holo-ACP, and the 9,420-Da peak corresponds to apo-ACP.
2,4,4′-Trichloro-2′-hydroxy-diphenyl ether, commonly known as ergasan or triclosan, is a potent inhibitor of type II fatty acid synthesis. It inhibits recombinant PfFabI with an IC50 of 70 nM and arrests the growth of P. falciparum in red blood cell cultures with an IC50 of 0.7 μM (12, 34). Its potency as a systemic antibacterial agent has also been explored (28). For studying the inhibition by triclosan, all of the FAS enzymes were added sequentially, in contrast to previous reaction setups in which all of the FAS enzymes were added together in the reaction mixture. As can be seen from Fig. 7A, at 0 min, the substrate peak (C10-ACP, 9,913 Da) can be seen. After addition of PfFabB/F, the peak of β-ketoacyl-ACP (9,955 Da) can be seen (Fig. 7B), followed by the β-hydroxyacyl-ACP peak (9,957 Da) after addition of PfFabG (Fig. 7C). However, a peak for enoyl-ACP after the addition of PfFabZ could not be detected (Fig. 7D). It has been shown previously in the cases of E. coli FabZ and PfFabZ that the reverse reactions catalyzed by FabZ enzymes are nine and seven times faster than the forward reactions, respectively, and the presence of FabI is essential to pull the reaction in the forward direction, which ultimately leads to the formation of a saturated acyl-ACP product (7, 29). This readily explains the lack of the appearance of an enoyl-ACP product in the absence of FabI. In the last step, when PfFabI was preincubated with 5 μM triclosan, there was no formation of a saturated acyl-ACP product, which is required to begin the next round of the synthesis cycle (Fig. 7E). The last panel of Fig. 7 shows the control reaction (PfFabI without triclosan), and thus, the formation of higher acyl-ACPs (C12-ACP, 9,941 Da; C14-ACP, 9,969 Da) can be seen (Fig. 7F). This clearly indicates that triclosan blocked the activity of PfFabI and stopped the fatty acid synthesis cycle. The A217V mutant of PfFabI has a Ki of 232 nM for triclosan, about 7,000 times higher than that of the wt enzyme (13). When the A217V FabI mutant was added to the reconstitution mixture in the presence of triclosan, the spectra obtained were similar to those in Fig. 7F. Increasing the concentration of triclosan to even 100-fold did not change the product pattern, indicating the sensitivity of the reconstitution assay for identifying the resistant enzyme.
FIG. 7.
Inhibition of PfFAS reaction with triclosan, using C10-ACP as the primer. The reaction was initiated by the addition of 5 μg PfFabB/F, and the first aliquot was taken out at 0 min (A). After 10 min of incubation, another aliquot was taken out (B) and 2 μg PfFabG was added to the same reaction mixture. After incubation for 10 min, another aliquot was taken out (C) and 3 μg PfFabZ was added to the reaction mixture. The mixture was incubated for another 10 min before an aliquot was taken out (D). Finally 2 μg PfFabI plus triclosan was added to the mixture and was incubated for 10 min before termination of the reaction (E). (F) The reaction in which PfFabI was added without triclosan. All the aliquots were snap frozen and analyzed by MALDI-TOF MS. Results are shown for C10-PfACP (9,913 Da), β-keto C12-PfACP (9,955 Da), β-hydroxy C12-ACP (9,957 Da), C12-PfACP (9,941 Da), and C14-PfACP (9,969 Da). The molecular weights of acylated-ACPs were calculated keeping holo ACP (9,758 Da) as the standard.
Taken together, these inhibition studies underscore the versatility and power of MALDI-TOF MS as a tool for the study of FAS and its potential for screening of FAS inhibitors. Hundreds of reaction mixtures can be analyzed simultaneously, which validates its ability for high-throughput analysis. Though MALDI-TOF MS is not a quantitative technique, because of differential ionization of molecules, the addition of an internal standard, like apo-ACP, allowed us to test the consistency of the results (<7% standard deviation). Since a single reaction mixture contains all the enzymes, and inhibition of any will stop the cycle, molecules can be studied for their inhibitory activities against all four enzymes together and their generic inhibitory activities against PfFAS can be determined rapidly. As can be seen in the case of the A217V FabI mutant, the reconstitution approach can readily identify the drug-resistant enzyme of the cycle.
It will be interesting to find out why PfFAS is biased toward the synthesis of shorter-chain fatty acids. Like Toxoplasma gondii FAS, P. falciparum FAS could be speculated as the supplier of octanoyl PfACP for the synthesis of lipoic acid, an essential cofactor of alpha-ketoacid dehydrogenase complexes (4). However, lipoic acid (R/S) and its derivatives are unable to rescue the parasite from death caused by some antimalarial agents by inhibiting its FAS (26). Therefore, this pathway is perhaps required for meeting an additional metabolic necessity of the organism and one such requirement could be the synthesis of sphingolipids (6, 23). Using tritiated serine and glucosamine in metabolic labeling studies, Gerold and Schwarz demonstrated that P. falciparum is capable of synthesizing glycosphingolipids de novo (5). A key step in the synthesis of glycosphingolipids involves the transfer of glucosyl moiety from UDP-glucose to ceramide by the enzyme glucosylceramide transferase. Glucosyl-ceramide then acts as a precursor for the synthesis of higher sphingolipids (15). Couto and coworkers showed the existence of functional glucosylceramide transferase in P. falciparum (3). Upon analysis of ceramide and various glucosyl-ceramides isolated from the parasite by UV-MALDI-TOF MS, the authors concluded that these ceramides mainly contain long-chain base d18:0 or d20:0 and fatty acids with lengths of C10:0, C12:0, and C14:0, with or without one-three hydroxyl residues. Since host ceramides contain mostly long-chain fatty acids, it seems plausible that the type II FAS of Plasmodium is the major supplier of these fatty acids.
Conclusion.
We have in vitro reconstituted the elongation module of the fatty acid synthesis cycle of Plasmodium falciparum. Incidentally, this report involves a comprehensive study of a type II FAS other than the E. coli type II FAS, which has been extensively studied (18, 36). This study demonstrates that the Plasmodium FAS machinery can elongate fatty acids with lengths of up to 14 carbon atoms, thus proving that in vitro assembly of the active FAS enzymes from Plasmodium falciparum can successfully mimic the in vivo situation. Cerulenin, catechin gallate, NAS-21/91, and triclosan inhibited PfFabB/F, PfFabG, PfFabZ, and PfFabI, respectively, and blocked the operation of the reconstituted fatty acid synthesis cycle, and the pattern of accumulated acyl products can pinpoint the identity of the enzyme inhibited. This in vitro reconstitution system can thus serve as a fast and efficient tool for the simultaneous screening of hundreds of molecules active against any of the enzymes of the elongation module. Such a screen can easily be extended for testing of inhibitors for FAS type II systems in bacteria, including those for Mycobacterium tuberculosis.
Acknowledgments
This work is supported by grants from the Department of Biotechnology (DBT), Government of India (GOI), to A.S. and N.S. and a grant from the Centre of Excellence to A.S. S.S. and S.K.S. are senior research fellows of the Council of Scientific and Industrial Research (CSIR), India. A.S. is a J. C. Bose fellow of Department of Science and Technology, Government of India.
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Asturias, F. J., J. Z. Chadick, I. K. Cheung, H. Stark, A. Witkowski, A. K. Joshi, and S. Smith. 2005. Structure and molecular organization of mammalian fatty acid synthase. Nat. Struct. Mol. Biol. 12225-232. [DOI] [PubMed] [Google Scholar]
- 2.Chhibber, M., G. Kumar, P. Parasuraman, T. N. Ramya, N. Surolia, and A. Surolia. 2006. Novel diphenyl ethers: Design, docking studies, synthesis and inhibition of enoyl ACP reductase of Plasmodium falciparum and Escherichia coli. Bioorg. Med. Chem. 148086-8098. [DOI] [PubMed] [Google Scholar]
- 3.Couto, A. S., C. Caffaro, M. L. Uhrig, E. Kimura, V. J. Peres, E. F. Merino, A. M. Katzin, M. Nishioka, H. Nonami, and R. Erra-Balsells. 2004. Glycosphingolipids in Plasmodium falciparum. Presence of an active glucosylceramide synthase. Eur. J. Biochem. 2712204-2214. [DOI] [PubMed] [Google Scholar]
- 4.Crawford, M. J., N. Thomsen-Zieger, M. Ray, J. Schachtner, D. S. Roos, and F. Seeber. 2006. Toxoplasma gondii scavenges host-derived lipoic acid despite its de novo synthesis in the apicoplast. EMBO J. 253214-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerold, P., and R. T. Schwarz. 2001. Biosynthesis of glycosphingolipids de-novo by the human malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 11229-37. [DOI] [PubMed] [Google Scholar]
- 6.Hakomori, S. 2003. Structure, organization and function of glycosphingolipids in membrane. Curr. Opin. Hematol. 1016-24. [DOI] [PubMed] [Google Scholar]
- 7.Heath, R. J., and C. O. Rock. 1995. Enoyl-acyl carrier protein reducatse (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J. Biol. Chem. 27026538-26542. [DOI] [PubMed] [Google Scholar]
- 8.Heath, R. J., S. W. White, and C. O. Rock. 2002. Inhibitors of fatty acid synthesis as antimicrobial chemotherapeutics. Appl. Microbiol. Biotechnol. 58695-703. [DOI] [PubMed] [Google Scholar]
- 9.Heath, R. J., J. R. Rubin, D. R. Holland, E. Zhang, M. E. Snow, and C. O. Rock. 1999. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 27411110-11114. [DOI] [PubMed] [Google Scholar]
- 10.Jenni, S., M. Leibundgut, T. Maier, and N. Ban. 2006. Architecture of a fungal fatty acid synthase at 5 A resolution. Science 3111263-1267. [DOI] [PubMed] [Google Scholar]
- 11.Kannangara, C. G., and P. K. Stumpf. 1971. The formation of fatty acyl thioesters during 14 C-1-acetate incorporation into long chain fatty acids by isolated spinach chloroplasts. Biochem. Biophys. Res. Commun. 441544. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor, M., M. J. Dar, A. Surolia, and N. Surolia. 2001. Kinetic determinants of the interaction of enoyl-ACP reductase from Plasmodium falciparum with its substrates and inhibitors. Biochem. Biophys. Res. Commun. 289832-837. [DOI] [PubMed] [Google Scholar]
- 13.Kapoor, M., J. Gopalakrishnapai, N. Surolia, and A. Surolia. 2004. Mutational analysis of the triclosan-binding region of enoyl-ACP (acyl-carrier protein) reductase from Plasmodium falciparum. Biochem. J. 381735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karmodiya, K., and N. Surolia. 2006. Analyses of co-operative transitions in Plasmodium falciparum beta-ketoacyl acyl carrier protein reductase upon co-factor and acyl carrier protein binding. FEBS J. 2734093-4103. [DOI] [PubMed] [Google Scholar]
- 15.Labaied, M., A. Dagan, M. Dellinger, M. Geze, S. Egee, S. L. Thomas, C. Wang, S. Gatt, and P. Grellier. 2004. Anti-Plasmodium activity of ceramide analogs. Malar. J. 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lack, G., E. Homberger-Zizzari, G. Folkers, L. Scapozza, and R. Perozzo. 2006. Recombinant expression and biochemical characterization of the unique elongating beta-ketoacyl-acyl carrier protein synthase involved in fatty acid biosynthesis of Plasmodium falciparum using natural and artificial substrates. J. Biol. Chem. 2819538-9546. [DOI] [PubMed] [Google Scholar]
- 17.Lambalot, R. H., and C. T. Walsh. 1995. Cloning, overproduction, and characterization of the Escherichia coli holo-acyl carrier protein synthase. J. Biol. Chem. 27024658-24661. [DOI] [PubMed] [Google Scholar]
- 18.Magnuson, K., S. Jackowski, C. O. Rock, and J. E. Cronan, Jr. 1983. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol. Rev. 57522-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier, T., S. Jenni, and N. Ban. 2006. Architecture of mammalian fatty acid synthase at 4.5 A resolution. Science 3111258-1262. [DOI] [PubMed] [Google Scholar]
- 20.Meadows, E. S., and C. Khosla. 2001. In vitro reconstitution and analysis of the chain initiating enzymes of the R1128 polyketide synthase. Biochemistry 4014855-14861. [DOI] [PubMed] [Google Scholar]
- 21.Mikolajczyk, S., and S. Brody. 1990. De novo fatty acid synthesis mediated by acyl-carrier protein in Neurospora crassa mitochondria. Eur. J. Biochem. 187431-437. [DOI] [PubMed] [Google Scholar]
- 22.Moche, M., G. Schneider, P. Edwards, K. Dehesh, and Y. Lindqvist. 1999. Structure of the complex between the antibiotic cerulenin and its target, β-ketoacyl-acyl carrier protein synthase. J. Biol. Chem. 2746031-6034. [DOI] [PubMed] [Google Scholar]
- 23.Pessi, G., G. Kociubinski, and C. B. Mamoun. 2004. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc. Natl. Acad. Sci. USA 1016206-6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price, A. C., K. Choi, R. J. Heath, Z. Li, S. W. White, and C. O. Rock. 2001. Inhibition of β-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin. J. Biol. Chem. 2766551-6559. [DOI] [PubMed] [Google Scholar]
- 25.Prigge, S. T., X. He, L. Gerena, N. C. Waters, and K. A. Reynolds. 2003. The initiating steps of a type II fatty acid synthase in Plasmodium falciparum are catalyzed by pfACP, pfMCAT, and pfKASIII. Biochemistry 421160-1169. [DOI] [PubMed] [Google Scholar]
- 26.Ramya, T. N., S. Mishra, K. Karmodiya, N. Surolia, and A. Surolia. 2006. Inhibitors of nonhousekeeping functions of the apicoplast defy delayed death in Plasmodium falciparum. Antimicrob. Agents Chemother. 51307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revill, W. P., and P. F. Leadley. 1991. Cloning, characterization, and high-Level expression in Escherichia coli of the Saccharopolyspora erythraea gene encoding an acyl carrier protein potentially involved in fatty acid biosynthesis. J. Bacteriol. 1734379-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma, S., T. N. C. Ramya, N. Surolia, and A. Surolia. 2003. Triclosan as a systemic antibacterial agent in a mouse model of acute bacterial challenge. Antimicrob. Agents Chemother. 473859-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma, S. K., M. Kapoor, T. N. C. Ramya, S. Kumar, G. Kumar, R. Modak, S. Sharma, N. Surolia, and A. Surolia. 2003. Identification, characterization and inhibition of Plasmodium falciparum β-hydroxyacyl-acyl carrier protein dehydratase (FabZ). J. Biol. Chem. 27845661-45671. [DOI] [PubMed] [Google Scholar]
- 30.Sharma, S. K., P. Parasuraman, G. Kumar, N. Surolia, and A. Surolia. 2007. Green tea catechins potentiate triclosan binding to enoyl-ACP reductase from Plasmodium falciparum (PfENR). J. Med. Chem. 50765-775. [DOI] [PubMed] [Google Scholar]
- 31.Sharma, S. K., R. Modak, S. Sharma, A. K. Sharma, S. P. Sarma, A. Surolia, and N. Surolia. 2005. A novel approach for over expression, characterization and isotopic enrichment of a homogeneous species of acyl carrier protein from Plasmodium falciparum. Biochem. Biophys. Res. Commun. 3301019-1026. [DOI] [PubMed] [Google Scholar]
- 32.Slayden, R. A., R. E. Lee, and C. E. Barry. 2000. Isoniazid affects multiple components of the type II fatty acid synthase system of Mycobacterium tuberculosis. Mol. Microbiol. 38514-525. [DOI] [PubMed] [Google Scholar]
- 33.Surolia, A., T. N. C. Ramya, V. Ramya, and N. Surolia. 2004. ′FAS't inhibition of malaria. Biochem. J. 383401-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surolia, N., and A. Surolia. 2001. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 7167-173. [DOI] [PubMed] [Google Scholar]
- 35.Tasdemir, D., G. Lack, R. Brun, P. Reudi, L. Scapozza, and R. Perozzo. 2006. Inhibition of Plasmodium falciparum fatty acid biosynthesis: evaluation of FabG, FabZ, and FabI as drug targets for flavonoids. J. Med. Chem. 493345-3353. [DOI] [PubMed] [Google Scholar]
- 36.White, S. W., J. Zheng, Y.-M. Zhang, and C. O. Rock. 2005. The structural biology of type II fatty acid biosynthesis. Annu. Rev. Biochem. 74791-831. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. 2002. The World Health Report 2002: reducing risks, promoting healthy life. World Health Organization, Geneva, Switzerland.
- 38.Yasuno, R., P. von-Wettstein Knowles, and H. Wada. 2004. Identification and molecular characterization of the β-ketoacyl-[acyl carrier protein] synthase component of the Arabidopsis mitochondrial fatty acid synthase. J. Biol. Chem. 2798242-8251. [DOI] [PubMed] [Google Scholar]