Abstract
We report the capacity of copper oxide-containing filters to reduce infectious titers of a panel of viruses spiked into culture media. Enveloped, nonenveloped, RNA, and DNA viruses were affected, suggesting the possibility of using copper oxide-containing devices to deactivate a wide spectrum of infectious viruses found in filterable suspensions.
Viral transmission through contaminated liquids, such as blood and breast milk, represents an enormous threat to populations worldwide. The problem of the inactivation of viruses in contaminated liquids lacks a straightforward solution, and the development of novel means to inactivate viruses would be highly desirable.
Copper has potent virucidal properties, and copper's neutralization of infectious bronchitis virus, poliovirus, human immunodeficiency virus type 1 (HIV-1), and other enveloped or nonenveloped single- or double-stranded DNA or RNA viruses has been reported (3).
Recently, a durable platform technology was developed that introduces copper oxide into cotton fibers, latex, and other polymeric materials (2), endowing them with potent broad-spectrum antibacterial and antifungal properties (4). Based on the wide-spectrum antiviral potency of copper (3), we hypothesized that passing suspensions spiked with viruses through filters containing copper oxide-impregnated fibers would result in the neutralization of these viruses.
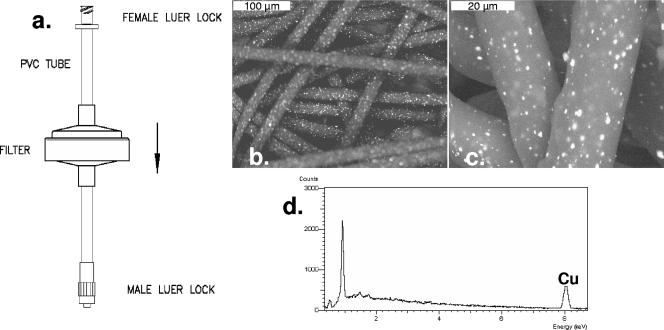
We designed filters with a radius of 2.5 cm containing a 2-cm-thick upper layer of 500 mg of nonwoven polypropylene fibers impregnated with 5% (wt/wt) copper oxide particles (Fig. 1) and a 0.2-cm bottom layer of 100 mg of nonwoven activated carbon fibers. The bottom layer was used to entrap any possible copper particulates or copper ions that might have been released from the first layer during filtration. Filters with polypropylene fibers that were not impregnated with copper oxide, served as negative controls. Clarified cell supernatant fluids containing 105 to 108 50% cell culture infectious doses (CCID50)/ml served as viral inocula. The capacity of the filters to deactivate viruses in physiologically relevant filterable solutions was determined with HIV-1 by using human plasma and human breast milk. All other viruses were diluted 1:10 in appropriate culture medium (minimal essential medium, Dulbecco's modified Eagle's medium, or RPMI 1640) without serum. Ten milliliters of each viral inoculum was put in a syringe attached to a filter and passed through the dry filter by manually applying moderate pressure to the syringe plunger. The retention time of the media within the filter was approximately 2 min. With experiments involving HIV-1, the flow rate was adjusted to 0.25 ml/cm2/min by using a peristaltic pump. The filtrate was collected in sterile tubes and subjected to multiple 4- or 10-fold dilutions in appropriate media containing serum. Each dilution was then added to appropriate target cells (Table 1) in 96-well microplates using three replicate wells per dilution. After 3 to 4 days of culture, the infectious viral titers for vaccinia virus were determined by using a plaque assay and, for all other viruses, by using a cytopathic-effect assay. The titer was calculated by using the Reed-Muench end point dilution method (7). An unfiltered sample from each original clarified viral stock was also titered in parallel. Ten milliliters of culture medium, without virus, passed through the filter was used as a negative control for antiviral activity. Overall, six filters were used for each virus, three containing copper and three not containing copper.
FIG. 1.
(a) Sketch of copper oxide-containing filter. (b and c) Scanning electronic microscope pictures of the copper oxide-impregnated polypropylene fibers. (d) X-ray analysis of the copper oxide-impregnated polypropylene fibers, showing the copper content.
TABLE 1.
Reduction of infectious viral titers by copper oxide-containing filters
| Virus | Viral strain | Viral family | Genome | E/NEa | Cells used in assay | Virus titer controlb | Virus titer Cu filterb | Log10 reduction (mean ± SD)c | P valued |
|---|---|---|---|---|---|---|---|---|---|
| Rhinovirus 2 | HGP | Picornaviridae | RNA | NE | HeLa Ohio-1 | 4.3/7/7 | 3.3/3/6 | 2 ± 1.7 | 0.2 |
| Yellow fever virus | 17D | Flaviviridae | RNA | E | Vero-76 | 6.3/6.3/5.9 | 5.7/4.7/4.8 | 1.1 ± 0.5 | <0.05 |
| Influenza A virus | Panama/2007/99 H3N2 | Orthomyxoviridae | RNA | E | MDCK | 7.5/7.5/6.8 | 6.7/5/4.8 | 1.77 ± 0.87 | <0.001 |
| Measles virus | Chicago | Paramyxoviridae | RNA | E | CV-1 | 3.67/3.67/3.67 | 0/0/0 | ≥3.67 | <0.001 |
| Respiratory syncytial virus | A2 | Paramyxoviridae | RNA | E | MA-104 | 4/4/4 | 3/2/2.5 | 1.5 ± 0.5 | <0.01 |
| Parainfluenza virus 3 | 14702 | Paramyxoviridae | RNA | E | MA-104 | 8/8/8 | 7.33/6.33/7 | 1.11 ± 0.5 | <0.05 |
| Punta Toro virus | Adames | Bunyaviridae | RNA | E | LLC-MK2 | 7/6.6/6.6 | 3.5/6/5.5 | 1.73 ± 1.55 | 0.09 |
| Pichinde virus | AN 4763 | Arenaviridae | RNA | E | BSC-1 | 7.5/7.6/7 | 4.5/5.6/6.9 | 1.7 ± 1.47 | 0.08 |
| HIV-1 | IIIB | Retroviridae | RNA | E | MT2 | 6/6.5/6 | 0.8/2.5/1.5 | 4.6 ± 0.6 | 0.001 |
| Adenovirus type 1 | Ad-HIVluc | Adenoviridae | DNA | NE | cMAGI | 5/5/5.2 | 2.5/3.2/2.9 | 2.2 ± 0.36 | 0.001 |
| Cytomegalovirus | AD169 | Herpesviridae | DNA | E | Fibroblasts | 6/6/6 | 2/1.5/1.6 | 4.3 ± 0.26 | <0.001 |
| Vaccinia virus | WR | Poxviridae | DNA | E | Vero-76 | 7.4/7.6/7.6 | 7.4/6.7/7.1 | 0.47 ± 0.45 | 0.095 |
E, enveloped viruses; NE, nonenveloped viruses.
Log10 PFU/ml for vaccinia virus; log10 CCID50/ml for the other viruses.
The log reduction was calculated as log10 CCID50/ml of the titer obtained from the control filter minus log10 CCID50/ml of the filter containing copper oxide. The values are the mean and standard deviation obtained in three separate experiments.
t test between viral titers obtained after filtration in control filters not containing copper versus copper-containing filters.
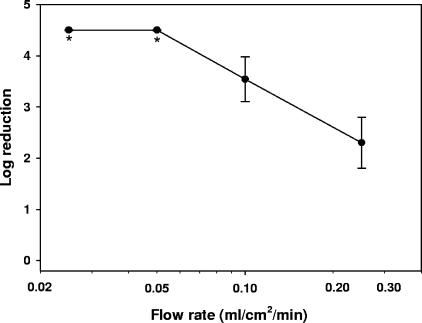
As summarized in Table 1, a passage of the viruses through the filters containing copper oxide resulted in a significant reduction of the infectious viral titers, ranging from 0.47 log10 to 4.6 log10 depending on the virus tested. A further decrease in infectious viral titers could be achieved by reduction of the flow rate. As shown in Fig. 2, an approximately 1.7-fold increase in HIV-1 neutralization was achieved by decreasing the flow rate by 2.5-fold. Residual virus was undetectable by further reducing the flow rate by twofold (Fig. 2).
FIG. 2.
Effect of flow rate on HIV-1 deactivation. Ten milliliters of human plasma containing ∼2 × 105 CCID50 of HIV-1 was added at room temperature to copper oxide-containing filters or to control filters containing no copper oxide. The plasma was eluted from the filters via a peristaltic pump at flow rates of 0.25 ml/cm2/min to 0.025 ml/cm2/min. The eluates were then subjected to sequential fourfold dilutions and added to MT2 cells. The CCID50 was determined after 4 days of culture as previously described (2). The y axis shows the difference between the log10 CCID50s obtained by the eluates of the control filters minus the log10 CCID50s obtained by the eluates of the copper oxide-containing filters. The average and standard deviation of triplicate experiments for each flow rate are shown. *, undetectable residual virus.
The results of this study demonstrate the potential of copper oxide-based filters to reduce infectious titers of viruses found in filterable suspensions. No correlation was observed between susceptibility to copper oxide and the virus family, whether enveloped or nonenveloped, RNA or DNA virus. Interestingly, some viruses were significantly more susceptible than others to passage through the filters, indicating that a key viral component may be readily damaged during filtration. This is in accordance, for example, with the finding that stoichiometric concentrations of copper ions inactivate HIV-1 protease (5, 6), which is an essential protein for viral replication.
Excess copper in humans at the level that is expected to be leached out into the milk, blood, or plasma as it passes through the copper filters (1 to 20 ppm) (data not shown) is not toxic. This represents only a minor perturbation to the normal serum copper levels in blood, which range from approximately 80 to 160 μg/dl (1, 8). Obviously, the effect of the copper filters on the important components found in breast milk, such as the protecting immunoglobulins, or those components found in blood, such as coagulation factors, should be thoroughly studied before implementing this technology. Preliminary data indicate that the copper oxide filters do not cause damage to red blood cells or coagulation factors found in plasma.
For most applications, if this technology is to be useful, complete neutralization of viruses in a given solution should be achieved. Depending on the solution composition and volume and the viruses that may contaminate these solutions, appropriate filters will have to be developed. Clearly, larger volumes of the active copper oxide-impregnated fibers are needed. The retention time of the solution in the filter should be increased by decreasing the flow rate and/or increasing the filter volume. This copper-based technology may, therefore, represent a novel and inexpensive means to quickly deactivate viruses in contaminated liquids.
Acknowledgments
This work was supported in part by Cupron, Inc., and contract NO1-AI-30048 from the Virology Branch, NIAID, NIH. G.B. is the Chief Scientist of Cupron, Inc., and J.G. is the CEO of Cupron, Inc., the company developing the copper oxide-based filter technology.
Footnotes
Published ahead of print on 30 April 2007.
REFERENCES
- 1.Ahmed, M. J., I. Jahan, and S. Banoo. 2002. A simple spectrophotometric method for the determination of copper in industrial, environmental, biological and soil samples using 2,5-dimercapto-1,3,4-thiadiazole. Anal. Sci. 18805-810. [DOI] [PubMed] [Google Scholar]
- 2.Borkow, G., and J. Gabbay. 2004. Putting copper into action: copper-impregnated products with potent biocidal activities. FASEB J. 181728-1730. [DOI] [PubMed] [Google Scholar]
- 3.Borkow, G., and J. Gabbay. 2005. Copper as a biocidal tool. Curr. Med. Chem. 122163-2175. [DOI] [PubMed] [Google Scholar]
- 4.Gabbay, J., J. Mishal, E. Magen, R. C. Zatcoff, Y. Shemer-Avni, and G. Borkow. 2006. Copper oxide impregnated textiles with potent biocidal activities. J. Ind. Textiles 35323-335. [Google Scholar]
- 5.Karlstrom, A. R., and R. L. Levine. 1991. Copper inhibits the protease from human immunodeficiency virus 1 by both cysteine-dependent and cysteine-independent mechanisms. Proc. Natl. Acad. Sci. USA 885552-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlstrom, A. R., B. D. Shames, and R. L. Levine. 1993. Reactivity of cysteine residues in the protease from human immunodeficiency virus: identification of a surface-exposed region which affects enzyme function. Arch. Biochem. Biophys. 304163-169. [DOI] [PubMed] [Google Scholar]
- 7.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 8.Yilmaz, M. E., M. Kiraz, and I. H. Kara. 2000. The evaluation of serum zinc and copper levels in hemodialysis patients in Southeast Turkey. Dialysis Transplant. 29718-721. [Google Scholar]