Abstract
An analog of pyrazinamide (PZA), 5-chloropyrazinamide (5-Cl-PZA), has previously been shown to inhibit mycobacterial fatty acid synthase I (FASI). FASI has been purified from a recombinant strain of M. smegmatis (M. smegmatis Δfas1 attB::M. tuberculosis fas1). Following purification, FASI activity and inhibition were assessed spectrophotometrically by monitoring NADPH oxidation. The observed inhibition was both concentration and structure dependent, being affected by both substitution at the 5 position of the pyrazine nucleus and the nature of the ester or N-alkyl group. Under the conditions studied, both 5-Cl-PZA and PZA exhibited concentration and substrate dependence consistent with competitive inhibition of FASI with Kis of 55 to 59 μM and 2,567 to 2,627 μM, respectively. The results were validated utilizing a radiolabeled fatty acid synthesis assay. This assay showed that FASI was inhibited by PZA and pyrazinoic acid as well as by a series of PZA analogs.
Currently, one-third of the world's population is infected with the tuberculosis bacillus, with an ongoing rate of one new infection per second (27). The development of multidrug-resistant strains of Mycobacterium tuberculosis and the prospect of nosocomial transmission make the development of new, better-tolerated therapies ever more important. The inclusion of pyrazinamide (PZA), an agent with a unique sterilizing activity, in the current treatment regimen consisting of isoniazid, rifampin, and ethambutol constitutes the basis for a 6-month short-course therapy for M. tuberculosis (1, 19).
The mechanism of action of PZA is still not clearly understood but is under intense investigation (5, 21, 22, 28-31). PZA is a prodrug of the pharmacologically active agent pyrazinoic acid (POA), which is formed upon hydrolysis of the amide by a mycobacterial pyrazinamidase encoded by the pncA gene (21). Reports on the susceptibility of Mycobacterium bovis, Mycobacterium kansasii, M. tuberculosis (9), and Mycobacterium avium (8) to pyrazinoate esters (POE) suggest that POE may undergo ready hydrolysis to POA, accounting for their activity against pncA-deficient tuberculous bacilli. It is intriguing that M. tuberculosis is the only mycobacterial species that is susceptible to PZA and POA. However, in culture, the antimycobacterial activity of both these compounds is observed only in an acidic medium. It has been shown that acidic pH allows for the intracellular accumulation of POA (30), yet the reason for acidic-pH-dependent POA accumulation remains elusive. M. tuberculosis, unlike M. smegmatis (and probably other mycobacterial species), lacks an efficient POA efflux mechanism, which allows POA accumulation. This deficient POA efflux mechanism has been suggested as the origin of the unique susceptibility of M. tuberculosis to PZA among mycobacteria (30). Nonetheless, since hydrolytically stable POE such as sec-butyl pyrazinoate are more active than POA, under some circumstances hydrolysis is apparently not required for antimycobacterial activity.
5-Chloropyrazinamide (5-Cl-PZA) is an analog of PZA with broad antimycobacterial activity (10, 32). In a study of 5-Cl-PZA resistance in Mycobacterium smegmatis, it was found that, when present on multicopy vectors in M. smegmatis, the fas1 genes from M. avium, M. bovis BCG, and M. tuberculosis confer resistance to 5-Cl-PZA. The observations that 5-Cl-PZA inhibits fatty acid synthase I (FASI) in M. smegmatis and that both 5-Cl PZA and PZA inhibit M. tuberculosis FASI (albeit with the proviso that PZA requires acidic conditions) (32) suggest that FASI is a potential antituberculosis drug target. The inhibition of FASI by 5-Cl-PZA has been verified independently, but the inhibition of FASI by PZA was not confirmed (4).
FAS is essential for cell survival. FAS (E.C. 2.3.1.85) catalyzes the sequential condensation of acetyl-coenzyme A (CoA) and malonyl-CoA to form long chain fatty acids (6, 17). The structure of this enzyme complex differs dramatically in prokaryotes and eukaryotes (14). In most prokaryotes, the synthases are typically composed of at least seven peptides that represent the individual enzyme components and are generally classified as type II synthases (18, 23). However in mammals and mycobacteria the synthase activity is carried out by single high-molecular-weight, multifunctional peptide chains or type I synthases (6, 20, 26). Since fatty acid synthesis in bacteria is essential for cell survival, the enzymes involved in this pathway have emerged as promising targets for antimicrobial agents (11, 12). As mentioned earlier, FASI was inhibited by 5-Cl-PZA (4, 32). Since eukaryotic microbial cells dependent upon endogenously synthesized fatty acids will express type I FAS, inhibitors of type I FAS could reasonably be anticipated to be inhibitors of microbial cell growth (12).
To pursue the study of FASI as a drug target, a cell-free NADPH oxidation assay of M. tuberculosis FASI activity was employed, where FASI was isolated from a recombinant strain of M. smegmatis (M smegmatis Δfas1 attB::M. tuberculosis fas1) (33). The influence of PZA substituents on FASI inhibition and specifically the requirement for 5-chlorine substitution were investigated.
MATERIALS AND METHODS
Media and strains.
A recombinant M. smegmatis fas1 strain, M. smegmatis Δfas1 (attB::M. tuberculosis fas1), previously designated mc22700 (33), which contains the M. tuberculosis fas1 gene, was cultivated in 7H9 medium supplemented with NaCl (0.85 g/liter), glucose (0.2%), glycerol (0.2%), and Tween 80 (0.05%).
Isolation and purification of M. tuberculosis FASI.
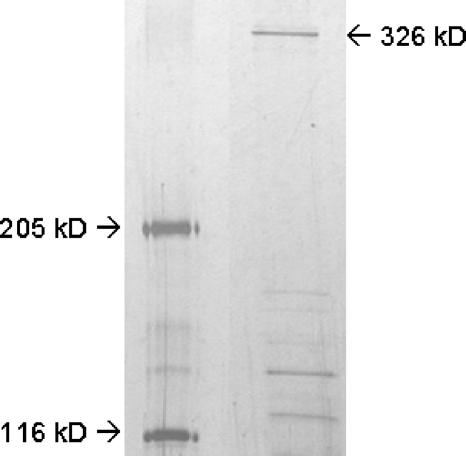
Purification of FASI from M. smegmatis mc22700 was effected using a slight modification of the procedures reported by Wood et al. (25) and Boshoff et al. (4). M. smegmatis mc22700 was grown to an optical density at 600 nm of 1 to 1.3 at 37°C. Cells were pelleted, washed with phosphate-buffered saline, and frozen at −78°C until use. All subsequent isolation and purification steps were done at 4°C. Cells (11 g) were lysed using a bead beater in 0.1 M potassium phosphate buffer (3-ml/g pellet, pH 7.2; 11 mM dithiothreitol [DTT], 1 mM EDTA, 2 mM phenylmethylsulfonyl fluoride). The lysed cells were centrifuged at 6,000 × g for 30 min, and the supernatant was treated with streptomycin sulfate (0.3 mg/ml) and recentrifuged at 18,000 × g for 45 min. The supernatant was then further ultracentrifuged at 40,000 rpm for 90 min. Solid ammonium sulfate was then added slowly to the supernatant to 1.4 M. The mixture was stirred for 30 min and then centrifuged (18,000 × g for 30 min). To induce the precipitation of FASI, the resulting supernatant was then brought to 2.0 M in ammonium sulfate, stirred for 30 min, and centrifuged. The pellet from the 2.0 M ammonium sulfate precipitation was suspended in 0.15 M potassium phosphate buffer (10 ml, pH 7.2; 1 mM DTT, 1 mM EDTA) and dialyzed for 4 h at 1 liter/h against the same buffer. The dialyzed product was centrifuged at 15,000 × g for 30 min, applied to an anion-exchange column (Whatman; DE 52), and eluted with a step gradient of 0.15 to 0.7 M potassium phosphate buffer (pH 7.2; 1 mM DTT, 1 mM EDTA). The fractions showing the expected band (326 kDa) in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 4.2% resolving, 3% stacking) were combined, concentrated (Amicon; Ultra-15; molecular weight cutoff = 100,000), and then applied to an Ultrogel AcA-34 column and eluted with 0.25 M potassium phosphate buffer (pH 7.2; 1 mM DTT, 1 mM EDTA). Fractions showing the desired band in SDS-PAGE were combined and concentrated.
Spectrophotometric assay.
FASI activity was measured using a spectrometric assay following the methods of Lynen (15) and Kawaguchi and Okuda (13), where the consumption of NADPH is monitored. The oxidation of NADPH to NADP+ was determined spectrophotometrically by measuring, over 10 min at 37°C, the linear decrease in the 340-nm absorbance attributed to NADPH. Assay solution contents were as follows: 1 to 3 μg enzyme, 50 μM acetyl-CoA (Km = 12.3 μM), 40 μM malonyl-CoA (Km = 0.28 μM), 100 μM NADPH, 5 mM DTT, and 400 mM potassium phosphate buffer, pH 7.4, in a total volume of 1 ml. The concentration of NADPH employed was that used by Kawaguchi and Okuda (13). One unit of enzyme activity is defined as the amount of material that catalyzes the oxidation of 1 μmol of NADPH per minute. FASI inhibition was defined as the percent reduction in enzymatic activity that results from the addition of inhibitor. Inhibition studies were conducted under the same conditions with the addition of dimethyl sulfoxide (DMSO) solutions of the pyrazinamide analogs up to a concentration of 0.050 ml DMSO/ml assay mixture.
Determination of Ki and IC50 via Dixon plot.
Inhibition constants were determined under conditions of saturating substrates. For 5-Cl-PZA, the reaction velocity was measured at three NADPH concentrations (75, 100, and 150 μM), each using several 5-Cl-PZA concentrations (0, 10, 25, 40, and 60 μM). For pyrazinamide, the reaction velocity was measured at three NADPH concentrations (50, 100, and 125 μM), where PZA concentrations of 1,000, 2,000, 3,000, and 4,000 μM were employed. The Ki value was determined by the x value at the intersection of the three plots. The values for the concentration of inhibitor that can effect 50% inhibition of enzyme activity (IC50) were obtained from the x intercept of the Dixon plot at 100 μM NADPH (7).
Radiometric assay.
Enzyme activity was monitored by measuring [2-14C]malonyl-CoA incorporation into fatty acid products following the methods of Bloch (3) and Wheeler et al. (24). Assay solution contents were as follows: 6 μg enzyme, 300 μM acetyl-CoA, 20 μM malonyl-CoA, 5 mM DTT, 5 mM EDTA, 1 μM flavin mononucleotide, 0.5 μM α-cyclodextrin, 100 μM NADPH, 200,000 cpm [2-14C]malonyl-CoA, and 0.40 M potassium phosphate buffer, pH 7.2, in a total volume of 0.50 ml. Fatty acid synthesis was allowed to proceed for 30 min at 37°C. Fatty acids were separated following saponification (0.19 ml 40% KOH, 30 min, 100°C), acidification (0.32 ml 6 M HCl), extraction (petroleum ether, four times with 0.75 ml each time), drying (Na2SO4), and filtration, followed by evaporation of volatile organics. Radioactivity was measured by scintillation counting. The extracted fatty acids were dissolved in petroleum ether (0.1 ml) in a 7-ml scintillation vial and were then mixed with the scintillation cocktail (5 ml; Ecoscint H; National Diagnostics). Radiometric inhibition studies were effected with DMSO solutions of the inhibitors (3,000 to 9,000 μM) up to a maximum concentration of 0.015 ml of DMSO per 0.50 ml of assay solution. These concentrations were consistent with those of the spectrophotometric assay.
Inhibitors.
PZA, POA, ethambutol, benzamide, and cerulenin (Sigma Aldrich) were used as supplied. Cerulenin, a known inhibitor of FASI through the condensation reaction, was used as a positive control for FASI inhibition (32). All other analogs were prepared as previously described (8, 10).
RESULTS
M. tuberculosis FASI purification from mc22700.
The specific enzymatic activity was determined following each purification step (Table 1). Following gel filtration the FASI enzyme appeared as a band on SDS-PAGE gel in a size corresponding to the expected molecular mass of 326 kDa (Fig. 1). A decrease in activity was observed with the final purification by gel chromatography. Purification even with a higher phosphate buffer concentration (0.50 M) resulted in a similar decrease in activity. The activity was, however, sufficient for the inhibition studies.
TABLE 1.
FASI purification
| Stepa | Total protein (mg) | Activity (mU/ml) | Total activity (mU) | Yield (%) | Sp act (mU/mg) | Purification factor (fold) |
|---|---|---|---|---|---|---|
| Lysate | 0.130 | 9.09 | 545 | 100 | 69.9 | |
| (NH4)2SO4 | 0.1088 | 19.5 | 341 | 63 | 179 | 3 |
| Anion | 0.0424 | 30.8 | 208 | 38 | 726 | 10 |
| Gel | 0.0338 | 8.67 | 43.4 | 8 | 257 | 4 |
Aliquots were taken after ultracentrifugation (lysate), dialysis of the pellet from 2.0 M ammonium sulfate precipitation [(NH4)2SO4], anion exchange (anion), and gel filtration (gel).
FIG. 1.
SDS-PAGE of purified FAS. Lane 1 (left), molecular weight marker; lane 2, purified FASI fraction. The gel (4.2%) was visualized via silver staining.
Inhibition of FASI activity as determined by NADPH oxidation.
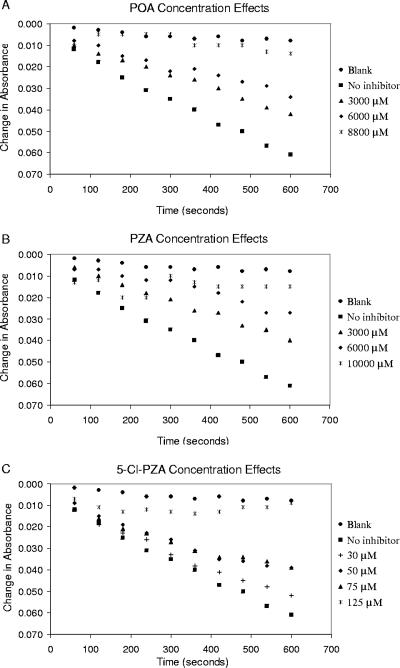
The NADPH oxidation assay is widely used in the study of fatty acid synthesis (13, 15). In a typical assay there is a spontaneous oxidation of NADPH at a rate of 1.29 × 10−4 μmol/min, while the enzyme causes a further decrease of about 9.81 × 10−4 μmol/min (Fig. 2). The decrease in the 340-nm absorbance of NADPH on oxidation to NADP+ is an indicator of the progress of fatty acid homologation. These studies showed that the FASI inhibition assay was not influenced by the presence or absence of NADH.
FIG. 2.
Effect of increasing inhibitor concentration on NADPH oxidation. Blank runs with no enzyme are included for reference. Results were obtained from spectrometric assays using purified FASI (3 μg/ml).
5-Cl-PZA, POA, and PZA show the expected increases in inhibition (Fig. 2) with increasing inhibitor concentration. Benzamide was a significantly less potent inhibitor in the spectrophotometric assay, with only 15% inhibition even at concentrations up to 100 mM (for comparison, see reference 5). POA and PZA show increased inhibition with increased concentration (Fig. 2A and B). The higher potency of 5-Cl PZA relative to POA and PZA in inhibition of FASI is clearly illustrated in Fig. 2C.
Inhibition of M. tuberculosis FASI by different classes of PZA analogs and cerulenin.
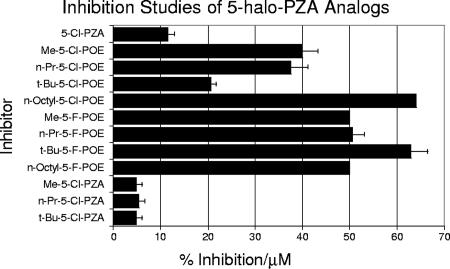
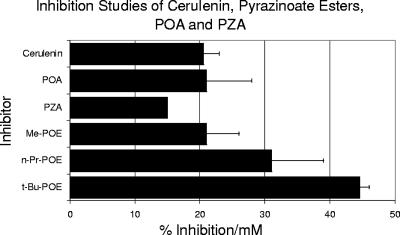
Four general classes of compounds were tested for inhibitory effects on purified FASI: nonhalogenated pyrazinoates (POE), 5-chloropyrazinoates (5-Cl-POE), 5-fluoropyrazinoates (5-F-POE), and 5-Cl-PZA analogs. Results of inhibition studies (Fig. 3 and 4) showed 5-Cl-PZA (Fig. 3) to be about 500 times more potent than either cerulenin or POA (Fig. 4) at inhibiting purified FASI. Of the esters examined, the pyrazine nuclear halogenated esters were considerably more potent inhibitors of FASI than the nonhalogenated compounds. Furthermore, the former were found to be more potent inhibitors than 5-Cl-PZA itself (ca. four times more active by mole) with purified FASI (Fig. 3). The chlorinated and fluorinated esters exhibited dissimilar trends. For the chloroesters, decreasing inhibition correlated with increasing bulk of the alkyl substituents (methyl, n-propyl [n-Pr], and tert-butyl [t-Bu]). However the, n-octyl derivative was a very effective inhibitor. The activity of the fluorinated esters was relatively insensitive to the ester functional group.
FIG. 3.
Activity of 5-substituted pyrazinamide analogs on inhibition of FASI as assessed by NADPH oxidation. Results were obtained from spectrometric assays using purified FASI (1 μg/ml) (13, 15).
FIG. 4.
Activity of cerulenin, POE, and PZA or POA on inhibition of FASI as assessed by NADPH oxidation. Results were obtained from spectrometric assays using purified FASI (1 μg/ml) (13, 15).
While the N-substituted 5-Cl-PZAs were less potent than the esters, they were still 2 orders of magnitude (ca. 200 times) more effective than cerulenin, POA, and PZA. The nonhalogenated pyrazinoates were the least potent inhibitors among the groups tested but nonetheless were about 1.5 to 2 times more effective than cerulenin, POA, and PZA (Fig. 4).
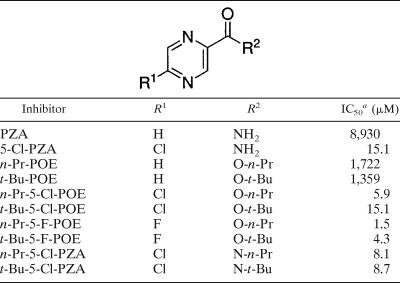
The IC50 values for n-Pr and t-Bu derivatives from each group of pyrazinamide analogs (Table 2) serve as a better gauge of the inhibitory strength. As expected, the IC50 values were found to be lowest for 5-F-POE, followed by 5-Cl-POE and then the 5-chloropyrazinamides, with the n-Pr-substituted derivatives being slightly more active for each case.
TABLE 2.
Comparison of IC50 values for PZA analogs
a IC50 values obtained at 100 μM NADPH. Results were obtained from spectrometric assays using purified FASI (3 μg/ml).
Determination of Kis (inhibition constants) of 5-Cl-PZA and PZA activity against FASI in the spectrophotometric assay.
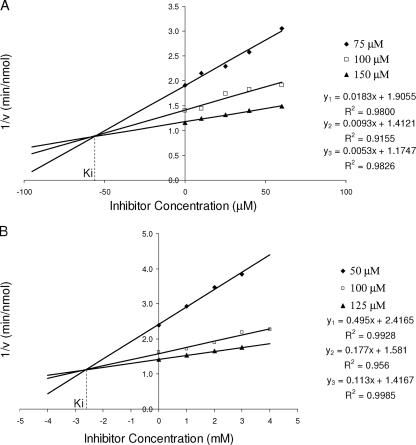
Application of the method of Dixon (7) as shown in Fig. 5 allowed estimation of Kis for both 5-Cl-PZA and PZA. For each concentration of NADPH, a variety of inhibitor concentrations were used. A plot of the reciprocal velocity (1/v) against inhibitor concentration made possible the estimation of Kis, 55 to 59 μM for 5-Cl-PZA and 2,567 to 2,627 μM for PZA. From the Dixon plots, it is also possible to determine IC50 values (7). The IC50 values for 5-Cl-PZA and PZA were 151 μM and 8,930 μM, respectively, at an NADPH concentration of 100 μM.
FIG. 5.
Dixon plots for 5-Cl-PZA (A) and pyrazinamide (B) inhibition of FASI. The results are indicative of competitive inhibition of NADPH oxidation, with Kis of 55 to 59 μM and 2,567 to 2,627 μM, respectively. Results were obtained from spectrometric assays using cell extracts of FASI (3 μg/ml).
Radiometric assays.
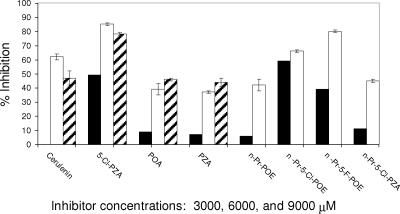
The incorporation of [2-14C]malonyl-CoA into fatty acids confirms that the NADPH oxidation activity is due to FASI activity. The published radiometric assay conditions (3, 24) were employed, even though those conditions differed modestly from those used in the spectrophotometric assay, in order to maintain the validity of the radiometric assay as a check on enzyme function. The inhibition studies using [2-14C]malonyl-CoA incorporation into fatty acids show the same general trend for the effectiveness for the different inhibitors (Fig. 6). There was an observed depression of the inhibitory effects at very high inhibitor concentrations (9,000 μM). While cerulenin, a known FASI inhibitor, exhibited no inhibitory effect at 3,000 μM, it did give a 62% inhibition at 6,000 μM. For POA, PZA, n-Pr-POE, and n-Pr-5Cl-PZA, which had only modest effects on the synthesis of fatty acids at 3,000 μM (6 to 11%), at 6,000 μM inhibition increased to 37 to 42%. 5-Cl-PZA and the halogenated esters n-Pr-5-Cl-POE and n-Pr-5-F-POE exhibited inhibition in the range of 39 to 59% at 3,000 μM and 66 to 80% at 6,000 μM. Thus, the synthesis of organic soluble fatty acids is inhibited in a concentration-dependent manner by 5-Cl-PZA, as well as PZA and POA, albeit at lower concentrations. Ethambutol, an antituberculosis agent known to act by another mechanism (2), was used as a negative control. Ethambutol had no inhibitory effect on FASI activity at concentrations up to 6,000 μM.
FIG. 6.
Results of radiometric studies via inhibition of [2-14C]malonyl-CoA incorporation in fatty acids. Assay solution contents were as follows: crude lysate (12 μg/ml) and 3,000 (▪), 6,000 (□), and 9,000 (▒) μM of inhibitor. Radioactivity was measured via scintillation counting.
DISCUSSION
All the PZA or POA analogs studied here were found to inhibit FASI in the cell-free assay, albeit modestly for the nonsubstituted lead compounds PZA and POA. Moreover, the inhibition was concentration and structure dependent, being affected by either substitution at the 5 position or ester or N-alkyl group. 5-Cl-POE and 5-F-POE, as well as the 5-Cl-PZA analogs, were found to be potent inhibitors of purified FASI. The potency of FASI inhibition, however, was strongly dependent upon the structure of the derivative, with substitution at the 5 position of the POA nucleus having the most profound effect on activity. These observations correlate with tests of the susceptibility of mycobacteria to these compounds, i.e., increased inhibition of FASI in our in vitro studies was consistent with lower observed MICs (9). This enhancement could be further modulated by structural variation of the ester or N-alkyl groups in a manner consistent with our previously published results on the inhibition of growth of mycobacteria (8-10). Furthermore, the greater FASI inhibition by unsubstituted POA esters relative to POA suggests that hydrolysis of the POE to POA is not required for FASI inhibition in the cell-free system.
POA was also found to be an effective inhibitor of FASI activity at 0.37 mg/ml (or 3 mM), which is about 6 to 23 times the MIC (10), in both the spectrophotometric and radiometric assays. Even though these assays are conducted with substrate or FASI cofactor concentrations likely manyfold higher than physiologic intracellular concentrations, POA and PZA have a consistent dose-dependent inhibitory activity. The relatively low potency of FASI inhibition by POA or PZA is not necessarily evidence against the role of FASI inhibition in intact cells. A relative lack of potency in cell-free assays in comparison with whole-cell assays has been shown with isoniazid. Despite a very low MIC for M. tuberculosis (<0.1 μg/ml), isoniazid required concentrations as high as 100 μM (13.7 μg/ml) for inhibition of the dissociated fatty acid elongation (FASII) system containing inhA (16). POA accumulation in M. tuberculosis was found to reach 350 μM (31), which probably far exceeds the intracellular physiologic concentrations of any FASI substrate.
The studies of [2-14C]malonyl incorporation into fatty acids verify the fatty acid synthesis activity of the purified enzyme and measure the inhibitory efficacy of the analogs investigated. In both experiments, PZA and POA were only modest inhibitors of FASI activity while 5-substituted analogs were much superior. Importantly, both assays, the NADPH oxidation assay and the radiolabeled-fatty acid synthesis assay, demonstrated a consistent structure-activity relation. The observed higher dose requirements in the radiometric assays suggest an interruption in the chain elongation process. Since the radiometric assay depends upon the extraction of more hydrophobic fatty acids, such as those formed when at least four successive full cycles of FASI activity are completed, if the proportion of intermediate-length fatty acids increases as a result of tendency of the inhibitors to interrupt elongation, the isolation of these still easily extracted acids would cause the radiometric assay to underreport FASI inhibition. NADPH oxidation takes place in each cycle of FASI activity, and thus an assay based on NADPH consumption (the spectrophotometric assay) is not biased toward fatty acid molecular weight.
The Dixon plots for the inhibition of NADPH oxidation by 5-Cl-PZA and PZA indicate a competitive process. This is in contrast to a previous report that 5-Cl-PZA was a noncompetitive inhibitor based upon a series of preincubation experiments where FAS activity, as determined radiometrically, was not recovered even after dialysis of the preincubated enzyme (4). It should be noted that the earlier-reported observations of Boshoff et al. (4), where the irreversible binding of 5-Cl-PZA was described, required incubation times (30 min) with 5-Cl-PZA longer than the total period of the inhibition experiments of the Dixon plot. In this work, the inhibition was nearly linear with time over the much shorter experimental duration (10 min). While it is certainly possible, given the complexity of FASI activity, that 5-Cl-PZA may act in more than one manner, the results with 5-Cl-PZA are internally consistent with inhibition of FASI by PZA, POA, and unsubstituted POA esters.
These results of cell-free assays, as well as the studies by Zimhony et al. (32, 34) with whole-cell assays using [1-14C]acetate incorporation into fatty acids, showed that, although 5 substitution significantly enhances the potency of PZA analogs in FASI inhibition, particularly in the cell-free system, this substituent is not a prerequisite for FASI inhibition by PZA analogs. These studies involving the cell-free and whole-cell assays illustrate clearly that hydrolysis to POA is not required for FASI inhibition.
Taken together, FASI inhibition cannot be discounted in discussion of the mechanism of the antimycobacterial activity of PZA and related analogs. Furthermore, the enhanced FASI inhibition by POE compared to POA does not support the hypothesis that the reactivity of the chlorine atom at the 5 position of the pyrazine ring as in 5-Cl-PZA, which renders the ipso-carbon susceptible to attack by a suitable nucleophile, is uniquely responsible for the activity observed (4).
These studies of M. tuberculosis FASI in a cell-free system suggest that the NADPH oxidation assay could be used to screen compounds as potential FASI inhibitors. Potent inhibitors of FASI could then be tested for antimycobacterial activity in whole cells and subsequently in animal models.
Acknowledgments
We gratefully acknowledge the helpful comments and suggestions of John Blanchard of the Albert Einstein College of Medicine and James K. Coward of the University of Michigan in the preparation of the manuscript.
This research was supported by grant AI052777-02 (J.T.W.) and grant AI43268 (W.R.J.) and the Centers for AIDS Research (W.R.J.).
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Bass, J. B., Jr., L. S. Farer, P. C. Hopewell, R. O'Brien, R. F. Jacobs, F. Ruben, D. E. Snider, Jr., and G. Thornton. 1994. Treatment of tuberculosis and tuberculosis infection in adults and children. Am. J. Respir. Crit. Care Med. 1491359-1374. [DOI] [PubMed] [Google Scholar]
- 2.Belanger, A. E., G. S. Besra, M. E. Ford, K. Mikusova, J. T. Belisle, P. J. Brennan, and J. M. Inamine. 1996. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc. Natl. Acad. Sci. USA 9311919-11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloch, K. 1975. Fatty acid synthases from Mycobacterium phlei. Methods Enzymol. 3584-90. [DOI] [PubMed] [Google Scholar]
- 4.Boshoff, H. I., V. Mizrahi, and C. E. Barry III. 2002. Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase I. J. Bacteriol. 1842167-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boshoff, H. I. M., and V. Mizrahi. 2000. Expression of Mycobacterium smegmatis pyrazinamidase in Mycobacterium tuberculosis confers hypersensitivity to pyrazinamide and related amides. J. Bacteriol. 1825479-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brindley, D. N., S. Matsumura, and K. Bloch. 1969. Mycobacterium phlei fatty acid synthetase; a bacterial multienzyme complex. Nature 224666-669. [Google Scholar]
- 7.Burlingham, B. T., and T. S. Widlanski. 2003. An intuitive look at the relationship of Ki and IC50: a more general use of the Dixon plot. J. Chem. Educ. 80214-218. [Google Scholar]
- 8.Cynamon, M. H., R. Gimi, F. Gyenes, C. A. Sharpe, K. E. Bergmann, H. J. Han, L. B. Gregor, R. Rapolu, G. Luciano, and J. T. Welch. 1995. Pyrazinecarboxylate esters with broad spectrum in vitro antimycobacterial activity. J. Med. Chem. 383902-3907. [DOI] [PubMed] [Google Scholar]
- 9.Cynamon, M. H., S. P. Klemens, T. S. Chou, R. H. Gimi, and J. T. Welch. 1992. Antimycobacterial activity of a series of pyrazinoic acid esters. J. Med. Chem. 351212-1215. [DOI] [PubMed] [Google Scholar]
- 10.Cynamon, M. H., R. J. Speirs, and J. T. Welch. 1998. In vitro antimycobacterial activity of 5-chloropyrazinamide. Antimicrob. Agents Chemother. 42462-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dick, J. D., and F. P. Kuhajda. January 1995. Inhibitors of fatty acid synthesis as antimicrobial agents. U.S. patent 9,519,706.
- 12.Heath, R. J., S. W. White, and C. O. Rock. 2001. Lipid biosynthesis as a target for antibacterial agents. Prog. Lipid Res. 40467-497. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi, A., and S. Okuda. 1977. Fatty acid synthetase from Brevibacterium ammoniagenes: formation of monounsaturated fatty acids by a multienzyme complex. Proc. Natl. Acad. Sci. USA 743180-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linn, T. C. 1981. Purification and crystallization of rat liver fatty acid synthetase. Arch. Biochem. Biophys. 209613-619. [DOI] [PubMed] [Google Scholar]
- 15.Lynen, F. 1969. Yeast fatty acid synthase. Methods Enzymol. 1417-33. [Google Scholar]
- 16.Marrakchi, H., G. Laneelle, and A. Quemard. 2000. InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. Microbiology 146289-296. [DOI] [PubMed] [Google Scholar]
- 17.Nelson, D. L., and M. M. Cox. 2000. Lehninger principles of biochemistry. Worth Publishers, New York, NY.
- 18.Prescott, D. J., and P. R. Vagelos. 1972. Acyl carrier protein. Adv. Enzymol. 36269-311. [DOI] [PubMed] [Google Scholar]
- 19.Steele, M. A., and R. M. Des Prez. 1988. The role of pyrazinamide in tuberculosis chemotherapy. Chest 94845-850. [DOI] [PubMed] [Google Scholar]
- 20.Stoops, J. K., M. J. Arslanian, Y. H. Oh, K. C. Aune, T. C. Vanaman, and S. J. Wakil. 1975. Presence of two polypeptide chains comprising fatty acid synthetase. Proc. Natl. Acad. Sci. USA 721940-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun, Z., A. Scorpio, and Y. Zhang. 1997. The pncA gene from naturally pyrazinamide-resistant Mycobacterium avium encodes pyrazinamidase and confers pyrazinamide susceptibility to resistant M. tuberculosis complex organisms. Microbiology 1433367-3373. [DOI] [PubMed] [Google Scholar]
- 22.Sun, Z., and Y. Zhang. 1999. Reduced pyrazinamidase activity and the natural resistance of Mycobacterium kansasii to the antituberculosis drug pyrazinamide. Antimicrob. Agents Chemother. 43537-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpe, J. J., and P. R. Vagelos. 1973. Saturated fatty acid biosynthesis and its regulation. Annu. Rev. Biochem. 4221-60. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler, P. R., K. Bulmer, and C. Ratledge. 1990. Enzymes for biosynthesis de novo and elongation of fatty acids in mycobacteria grown in host cells: is Mycobacterium leprae competent in fatty acid biosynthesis? J. Gen. Microbiol. 136211-217. [DOI] [PubMed] [Google Scholar]
- 25.Wood, W. I., D. O. Peterson, and K. Bloch. 1978. Subunit structure of Mycobacterium smegmatis fatty acid synthetase. Evidence for identical multifunctional polypeptide chains. J. Biol. Chem. 2532650-2656. [PubMed] [Google Scholar]
- 26.Wood, W. I., D. O. Peterson, and K. Bloch. 1977. Mycobacterium smegmatis fatty acid synthetase. A mechanism based on steady state rates and product distributions. J. Biol. Chem. 2525745-5749. [PubMed] [Google Scholar]
- 27.World Health Organization. 2004. Tuberculosis. Fact sheet no. 104. World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs104/en/.
- 28.Zhang, Y., and D. Mitchison. 2003. The curious characteristics of pyrazinamide: a review. Int. J. Tuberc. Lung Dis. 76-21. [PubMed] [Google Scholar]
- 29.Zhang, Y., S. Permar, and Z. Sun. 2002. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J. Med. Microbiol. 5142-49. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Y., A. Scorpio, H. Nikaido, and Z. Sun. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 1812044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, Y., M. M. Wade, A. Scorpio, H. Zhang, and Z. Sun. 2003. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J. Antimicrob. Chemother. 52790-795. [DOI] [PubMed] [Google Scholar]
- 32.Zimhony, O., J. S. Cox, J. T. Welch, C. Vilcheze, and W. R. Jacobs, Jr. 2000. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 61043-1047. [DOI] [PubMed] [Google Scholar]
- 33.Zimhony, O., C. Vilcheze, and W. R. Jacobs, Jr. 2004. Characterization of Mycobacterium smegmatis expressing the Mycobacterium tuberculosis fatty acid synthase I (fas1) gene. J. Bacteriol. 1864051-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimhony, O., C. Vilcheze, M. Arai, J. T. Welch and W. R. Jacobs, Jr. 2007. Pyrazinoic acid and its n-propyl ester inhibit fatty acid synthase type I in replicating tubercle bacilli. Antimicrob. Agents Chemother. 51752-754. [DOI] [PMC free article] [PubMed] [Google Scholar]