Abstract
The efficacy of telavancin, a novel lipoglycopeptide, was evaluated in experimental endocarditis in rabbits using two clinical isolates of glycopeptide-intermediate Staphylococcus aureus: ATCC 700788 and HIP 5836. Infected rabbits were treated for 2 days with telavancin (10 mg/kg of body weight once daily intravenously) or vancomycin (1 g twice daily intravenously), administered with a computer-controlled infusion pump system simulating human serum kinetics. Vegetations were harvested at 16 h postinoculation in the control group and at the end of treatment in the drug-treated group. For ATCC 700788, MICs and minimal bactericidal concentrations (MBCs), respectively, were 1 mg/liter and 4 mg/liter for telavancin and 8 mg/liter and 128 mg/liter for vancomycin. For HIP 5836, MICs and MBCs, respectively, were 4 mg/liter and 8 mg/liter for telavancin and 8 mg/liter and 128 mg/liter for vancomycin. Peak and trough levels were 90 μg/ml and 6 μg/ml, respectively, for telavancin and 46 μg/ml and 6 μg/ml, respectively, for vancomycin. In glycopeptide-intermediate S. aureus ATCC 700788, telavancin sterilized 6 of 16 vegetations (37%), whereas vancomycin sterilized 4 of 20 (20%) (P = 0.29) compared with 0 of 17 in the control group. In HIP 5836 experiments, telavancin and vancomycin sterilized 5 of 16 (31%) and 1 of 15 (7%) vegetations (P = 0.17), respectively, compared with none in the control group. Telavancin reduced vegetation titers by 2.0 and 2.3 logs greater than vancomycin for the ATCC 700788 (4.6 [2.0 to 5.8] versus 6.6 [2.0 to 6.9] log CFU/g vegetation; P = 0.05) and HIP 5836 (4.4 [2.0 to 7.4] versus 6.7 [4.5 to 8.7] log CFU/g vegetation; P = 0.09) strains, respectively; these differences did not reach statistical significance. All isolates from vegetations remained susceptible to telavancin after therapy. The results suggest that telavancin may be an effective treatment for endocarditis caused by glycopeptide-intermediate S. aureus.
Vancomycin is currently the American Heart Association's recommended therapy for endocarditis caused by methicillin-resistant Staphylococcus aureus (MRSA) (2). However, a decade has passed since the first documented case of S. aureus with reduced susceptibility to vancomycin surfaced in Japan (18), and strains of S. aureus with intermediate- and high-level resistance to vancomycin and other glycopeptide antibiotics have since appeared in several parts of the world, including Europe and the United States (6, 28, 31, 35, 37, 42). At the same time, MRSA infections have continued to increase dramatically in both health care and community settings worldwide, causing endocarditis and other severe illnesses (1, 22, 24).
In recent years, S. aureus has emerged as the leading cause of infective endocarditis internationally (10, 23), with almost 40% of cases of S. aureus infective endocarditis resulting specifically from MRSA (10), having a high morbidity and mortality (5, 10, 23). Strains of MRSA are often resistant to a wide range of antibiotics, and therapeutic alternatives to vancomycin are limited (19, 33). Additionally, studies have shown that vancomycin is less bactericidal in S. aureus infective endocarditis than penicillinase-resistant β-lactam antibiotics (36) and that glycopeptides have poor diffusion into valve vegetations (7). Therefore, new antibiotics are needed.
Telavancin, a semisynthetic derivative of vancomycin, is a novel lipoglycopeptide with rapid bactericidal activity and multiple mechanisms of action against gram-positive bacteria, including methicillin-resistant, glycopeptide-intermediate, and vancomycin-resistant strains of S. aureus (12, 15-17, 20, 26, 27, 34). Telavancin displays potent antibacterial activity against MRSA in animal models of endocarditis (21), bacteremia (29), soft tissue infections (16), meningitis (40), and pneumonia (30). Telavancin penetrates into skin blister fluid (41), and phase 2 trials have shown that telavancin (7.5 and 10 mg/kg of body weight once daily intravenously [i.v.]) is similar in efficacy to standard therapy for the treatment of complicated skin and skin structure infections (3, 38, 39). Telavancin is being evaluated at a 10-mg/kg i.v. dose in phase 3 complicated skin and skin structure infections and hospital-acquired pneumonia trials and in a phase 2 uncomplicated S. aureus bacteremia trial.
Vancomycin and other glycopeptides have a singular mechanism of action, namely inhibition of the synthesis of cell walls by targeting peptidoglycan synthesis. Telavancin, besides inhibiting cell wall synthesis, also causes disruption of cell membrane integrity (17, 26, 27). This multifunctional mechanism of action may help to minimize the potential for the selection of resistance (16, 27). In several studies, telavancin has exhibited in vitro activity superior to that of vancomycin (12, 15, 20, 26, 33).
In vivo data about the activity of telavancin in experimental endocarditis are few, and no clinical data about the efficacy of telavancin in infective endocarditis exist. A study comparing the efficacies of telavancin and vancomycin in a rabbit model of aortic valve infective endocarditis found telavancin to be significantly more effective than vancomycin in endocarditis induced by a strain of vancomycin-intermediate S. aureus and at least as effective in endocarditis caused by a strain of MRSA (21). The researchers, however, did not simulate the human pharmacokinetics of telavancin and vancomycin in their experimental model. Because drugs are eliminated more rapidly in animals than in humans, antibiotic pharmacokinetics in animals and humans may vastly differ. Therefore, the present study was conducted to evaluate the efficacy of telavancin administered using a humanized pharmacokinetics model in the treatment of experimental aortic valve endocarditis in rabbits infected with either of two glycopeptide-intermediate S. aureus (GISA) strains.
(This work was presented in part at the 16th European Congress of Clinical Microbiology and Infectious Diseases, Nice, France, 1 to 4 April 2006 [23a].)
MATERIALS AND METHODS
Antimicrobial agents.
Telavancin powder was obtained from Theravance, Inc. Vancomycin hydrochloride powder was obtained from Sigma-Aldrich Corporation (St. Louis, MO). Agents were prepared for the study experiments according to the manufacturers’ recommendations.
Bacterial strains.
Two strains of S. aureus, both having resistance to methicillin and reduced susceptibility to glycopeptides, were used in the study: GISA strain ATCC 700788, a clinical isolate available from the American Type Culture Collection, and HIP 5836, a GISA strain isolated from a patient in New Jersey and supplied by Theravance, Inc. These strains were kept frozen in skim milk at −80°C. Before each experiment, an aliquot was thawed and inoculated onto plates containing Columbia agar with 5% sheep blood (bioMérieux, Marcy-l'Étoile, France).
In vitro susceptibility studies.
The MICs and minimal bactericidal concentrations (MBCs) of telavancin and vancomycin were determined by the microdilution method in liquid medium cation-adjusted Mueller Hinton broth (Oxoid, Hampshire, England) as described by the guidelines of the Clinical and Laboratory Standards Institute (CLSI; formerly National Committee for Clinical Laboratory Standards [NCCLS]) (25). S. aureus ATCC 29213 was used as the test control strain.
In accordance with CLSI criteria, duplicate time-kill curve studies were performed with each isolate, using an inoculum of 105 CFU/ml. Bactericidal activity was defined as at least a 1,000-fold increase (≥3 log10 CFU/ml) in killing at 24 h in comparison with the initial inoculum. Bacterial viability counts were performed at 0, 4, and 24 h.
Simulation of human serum pharmacokinetics.
A computer-controlled infusion pump system was designed to administer telavancin and vancomycin to rabbits at dosing volumes and intervals that produced pharmacokinetic serum profiles in rabbits that were similar to those observed in humans after i.v. infusion of the antimicrobials. Doses of telavancin and vancomycin that were representative of the pharmacokinetic profiles of the drugs in humans (10 mg/kg i.v. telavancin every 24 h and 1 g i.v. vancomycin every 12 h) were selected.
Telavancin concentrations in plasma were assayed by Theravance, Inc., using a validated liquid chromatography-tandem mass spectrometry method with a lower limit of quantitation of 0.25 μg/ml (16). Vancomycin concentrations were assayed at the Centre de Diagnòstic Biomèdic, Hospital Clínic, Barcelona, Spain. Vancomycin concentrations were measured by an immunoturbidimetric method with boosting by latex (ADVIA Chemistry, Bayer Health Care LLC, Germany). The limit of detection of the procedure was 0.8 μg/ml (range from 0.8 to 72.9 μg/ml), and the precision coefficients represented by the inter- and intra-assay coefficients of variation ranged from 2.2% to 2.7%, evaluated for three levels of concentrations: 10.9, 39.3, and 73.1 μg/ml.
In vivo experimental pharmacokinetic studies were performed in five healthy rabbits to simulate the pharmacokinetic profiles of vancomycin and telavancin in humans at doses of 1 g i.v. twice daily and 7.5 mg/kg i.v. once daily, respectively. Two polyethylene catheters (inner diameter, 0.81 mm; outer diameter, 1.27 mm [Portex SA, Hythe, England]) were inserted: one through the carotid artery for sampling and the other into the cava vein through the jugular vein for infusion. Both lines were tunneled subcutaneously and brought to the interscapular region. The external portion of the jugular catheter was connected to a flowthrough swivel, and the other portion was connected to a computer-controlled infusion pump system, in accordance with in vivo experimental pharmacokinetic studies previously described by Gavaldà and colleagues (14). The pump system was programmed to deliver an i.v. infusion at previously calculated flow rates. To determine the antibiotic concentrations, 1 ml of blood was sampled at 0, 0.25, 0.5, 1, 2, 3, 4, 5, and 6 h after the start of the infusion. Because the telavancin pharmacokinetic disposition in rabbits exhibits dose linearity, infusion parameters for the 10-mg/kg i.v. human dose were projected from the pharmacokinetic data obtained from the simulated 7.5-mg/kg i.v. human dose.
Experimental endocarditis model.
This research project fulfills the requirements stipulated in Spanish Royal Decree 223/1988 on the protection of animals used in experiments. The Ethical Committee on Animal Research of the University of Barcelona approved the animal studies. New Zealand White rabbits, 2 kg each, were obtained from San Bernardo Farm, Pamplona, Spain. The animals were housed in the animal facilities of the Faculty of Medicine, University of Barcelona, and nourished ad libitum.
Experimental aortic valve infective endocarditis was induced in the rabbits according to the technique described by Garrison and Freedman (13). Briefly, a polyethylene catheter was inserted through the right carotid artery into the left ventricle and was kept in place during the experiment. One or two catheters (inner diameter, 0.81 mm; outer diameter, 1.27 mm; Portex SA) were inserted into the inferior cava vein through the jugular vein, in accordance with the method of Garrison and Freedman, to administer the test antimicrobials.
The infusion pump delivered 2 ml/h of 0.9% saline solution to keep the catheter accessible until the initiation of dosing; 24 h after the placement of the intracardiac catheter, the animals were infected via the marginal ear vein with 1 ml of saline solution containing 7 × 105 CFU/ml of ATCC 700788 (n = 40) or strain HIP 5836 (n = 32).
A 1-ml sample of blood was obtained 18 h after infection and just before the initiation of antimicrobial therapy to confirm the presence of endocarditis. The bacteremia was interpreted as indicative of infective endocarditis. Infected rabbits were randomly assigned to one of three groups: control without treatment, treatment with telavancin simulating 10 mg/kg i.v. every 24 h, and treatment with vancomycin simulating 1 g i.v. every 12 h. Antimicrobial therapy administered using the computer-controlled infusion pump system was initiated 18 h after inoculation and maintained for 48 h. Following 48 h of treatment and 6 half-lives of the antibiotic after ending antimicrobial therapy, the rabbits in the treatment groups were killed with a lethal i.v. injection of sodium pentobarbital. The animals in the control group were killed 16 h after infection.
Each animal having proper placement of the catheter, macroscopic evidence of vegetations at the time of death, and S. aureus in cultures of blood obtained before the start of antimicrobial therapy was studied. The chest cavity was immediately opened, the heart was excised and opened, and the aortic valves were removed aseptically. Aortic valve vegetations were weighed and homogenized with 2 ml of tryptic soy broth (Difco Laboratories Incorporated, Detroit, MI) in a tissue homogenizer (Stomacher 80; Seward Limited, London, England). Homogenates were quantitatively cultured onto plates containing Columbia agar with 5% sheep blood (bioMérieux). The plates were incubated over 48 h at 37°C in room air. Two additional plates were cultured with 0.1 ml of the homogenate. The remaining homogenate was qualitatively cultured in tryptic soy broth for a week. The bacteria recovered were retested to confirm their telavancin and vancomycin MICs.
Data analysis.
The results were expressed as log10 CFU of ATCC 700788 or HIP 5836 per gram of vegetation. Vegetations were assigned a value of 2 log10 CFU/g when growth was detected in the culture of the remaining homogenate in tryptic soy broth but not detected in the quantitative cultures on plates containing Columbia agar with 5% sheep blood. Vegetations in which no growth was detected in any of the cultures were assigned a value of 0 log10 CFU/g and considered sterile.
Results were expressed as the median (interquartile range) of the number of log10 CFU/g tissue of ATCC 700788 or HIP 5836. The Fisher exact test was used to compare the rate of sterile vegetations and assess whether there were differences between treatment groups. The Mann-Whitney rank sum test was used to compare the log10-CFU/g tissue values between the different treatment groups.
RESULTS
In vitro susceptibility studies.
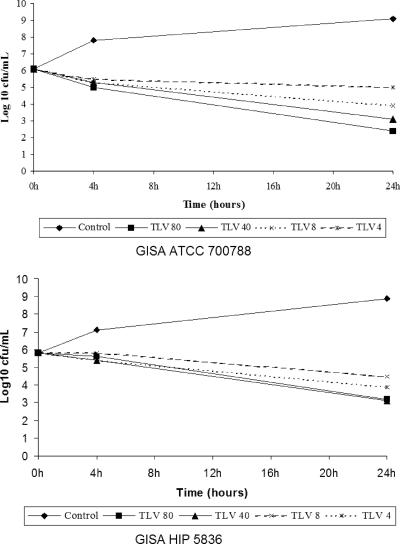
The respective MICs/MBCs of telavancin and vancomycin were 1/4 and 8/128 for ATCC 700788 and 4/8 and 8/128 for HIP 5836. The in vitro activity of telavancin for ATCC 700788 is presented in Fig. 1A, and that for HIP 5836 is presented in Fig. 1B. At the concentrations tested, telavancin demonstrated activity to some extent and caused a reduction in bacterial counts of approximately 2 to 2.5 log10 CFU/ml at 24 h (Fig. 1). Vancomycin was bacteriostatic.
FIG. 1.
Time-kill curves with telavancin.
Simulation of human serum pharmacokinetics.
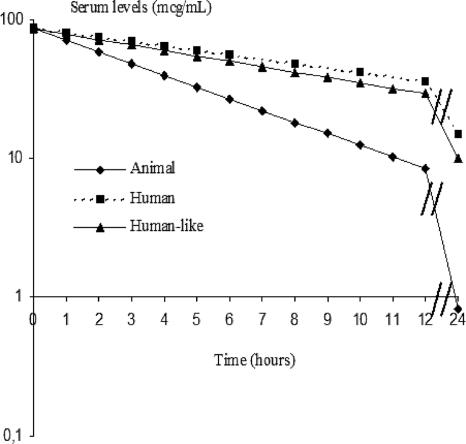
The rabbit pharmacokinetic data used in the mathematical model are shown in Table 1. The computer-controlled infusion pump system produced serum kinetics in rabbits similar to those found in humans for telavancin (Fig. 2). The pharmacokinetic parameters obtained from the humanized model in rabbits were similar to those in humans (Table 1).
TABLE 1.
Pharmacokinetic parameters
| Parametera | Result for:
|
||
|---|---|---|---|
| Vancomycin | Telavancin
|
||
| Dose 1 | Dose 2 | ||
| Humansb | |||
| Dose | 1 g i.v. | 7.5 mg/kg i.v. | 10 mg/kg i.v. |
| Cmax/Cmin (μg/ml) | 52/10 | 89/6 | |
| k (h−1) | 0.15 | 0.110 | 0.095 |
| t1/2β (h) | 4.6 | 6.18 | 7.41 |
| AUC (μg·h/ml) | 358.6 | 604 | 785 |
| Protein binding (%) | 50 | 90 | 90 |
| Animals (n = 5) | |||
| Dose | 25 mg/kg i.v. | 15 mg/kg i.v. | |
| k (h−1) | 0.53 ± 0.14 | 0.57 ± 0.06 | |
| t1/2β (h) | 1.3 ± 0.4 | 1.2 ± 0.11 | |
| AUC (μg·h/ml) | 101 ± 16 | 269.8 ± 52.2 | |
| Protein binding (%) | 65 | 90 | 90 |
| Humanlike (n = 3 or 5) | |||
| Dose | 1 g i.v. | 7.5 mg/kg i.v. | 10 mg/kg i.v. |
| k (h−1) | 0.18 ± 0.03 | 0.19 ± 0.09 | NDc |
| t1/2β (h) | 3.8 ± 1.2 | 4.6 ± 2.8 | ND |
| AUC (μg·h/ml) | 298.8 ± 18 | 638 ± 38 | ND |
| Cmax/Cmin (μg/ml) | 46/6 | 90/6 | 114/6 |
| AUC/MIC | |||
| HIP 5836 | 37.3 | 159.5 | |
| ATCC700788 | 37.3 | 638 | |
k, first-order elimination rate constant; t1/2β, half-life at β phase; AUC, area under the concentration-time curve; Cmax, maximum concentration of drug; Cmin, minimum concentration of drug.
Vancomycin values were obtained from Blouin and colleagues (4); telavancin values were obtained from data on file at Theravance, Inc.
ND, not determined. Infusion parameters for the 10-mg/kg i.v. human dose were projected from the pharmacokinetic data obtained from the simulated 7.5-mg/kg i.v. human dose.
FIG. 2.
Serum pharmacokinetics of telavancin.
Experimental endocarditis model.
The results of the therapeutic regimens tested in the experimental model of GISA endocarditis are shown in Table 2. All control rabbits had infected vegetations with high mean bacterial counts (>9 log CFU) per gram of vegetation. For both vancomycin and telavancin, the bacterial counts after 2 days of treatment were reduced in the vegetations of treated animals, compared with those of the control group (P < 0.001). Telavancin also reduced vegetation titers by 2.0 and 2.3 log CFU greater than vancomycin for the ATCC and HIP strains, respectively, but the difference did not reach statistical significance (P = 0.09 and P = 0.05, respectively). All isolates from vegetations remained susceptible to telavancin.
TABLE 2.
Treatment of experimental endocarditis caused by ATCC 700788 and HIP 5836 strainsa
| Treatment groupb | No. surviving/total (%) | No. with sterile vegetation/total (%) | Median log CFU/g of vegetation (interquartile range) |
|---|---|---|---|
| GISA ATCC 700788 | |||
| Control | 0/17 (0) | 9.5 (8.3-9.8) | |
| Telavancin | 16/17 (94) | 6/16 (37)* | 4.6 (2.0-5.8)† |
| Vancomycin | 20/23 (87) | 4/20 (20)* | 6.6 (2.0-6.9)† |
| HIP 5836 | |||
| Control | 0/20 (0) | 9.1 (9.1-9.4) | |
| Telavancin | 16/17 (94) | 5/16 (31)* | 4.4 (2.0-7.4)‡ |
| Vancomycin | 15/15 (100) | 1/15 (7)* | 6.7 (4.5-8.7)‡ |
*, not significant; †, P = 0.05; ‡, P = 0.09.
Results for telavancin represent simulation of a dose of 10 mg/kg every 24 h i.v. Results for vancomycin represent simulation of a dose of 1 g every 12 h i.v.
DISCUSSION
Telavancin exhibited rapid action against the two GISA strains both in vivo and in vitro. The MICs/MBCs of telavancin for HIP 5836 were 4 mg/liter and 8 mg/liter, respectively, and 1 mg/liter and 4 mg/liter for ATCC 700788, respectively, versus vancomycin MICs/MBCs of 8 mg/liter and 128 mg/liter for both strains. In vivo results show that after 2 days of therapy, telavancin sterilized more vegetations and reduced more vegetation titers to a greater degree than vancomycin, although the difference was not statistically significant.
Madrigal and colleagues conducted a previous study of telavancin in a rabbit model of aortic valve endocarditis caused by COL, an MRSA strain, or by HIP 5836, and found that after 4 days of therapy a twice-daily regimen of 30 mg/kg telavancin reduced mean aortic valve vegetation titers and sterilized vegetations of COL at least as effectively as vancomycin at 30 mg/kg twice daily. However, the observed difference was not statistically significant (21). The researchers also found that telavancin was significantly more effective than vancomycin in endocarditis due to HIP 5836, resulting in a 5.5-log10 CFU/g reduction in vegetation titers, versus no reduction in CFU with vancomycin. The investigators concluded that telavancin may be an effective treatment for endocarditis and other serious staphylococcal infections. However, the results of the present study support the assessment of the efficacy of telavancin for staphylococcal endocarditis because it is the first study to use a humanized pharmacokinetics model to compare the activity of telavancin with that of vancomycin in GISA endocarditis. The present study also tested the activity of telavancin in vegetations of a greater density in control rabbits than those tested in the previous rabbit S. aureus endocarditis study (∼9.5 log CFU/g ATCC 700788 and ∼9.1 log CFU/g HIP 5836, versus ∼7.4 log CFU/g COL and ∼6.7 log CFU/g HIP 5836). Furthermore, the present study was of a shorter duration than the earlier study, showing a clear trend of more potent activity by telavancin compared with vancomycin in only 2 days of antimicrobial therapy, versus 4 days of therapy in the prior study. Additionally, resistance against telavancin was not selected in vivo after 2 days of therapy. The results of the present study of GISA-induced endocarditis can also be extrapolated to MRSA infections, as both GISA strains used in the study were resistant to methicillin.
The bactericidal activity of telavancin may be further enhanced by the addition of gentamicin or rifampin, as suggested by recommendations for prosthetic valve endocarditis and in vitro synergy data (8, 9). In addition, controversial clinical outcomes associated with other drugs for gram-positive infections, including linezolid and daptomycin, have heightened the need for an alternative to vancomycin for the treatment of S. aureus endocarditis and other serious staphylococcal infections. Linezolid has been associated with adverse events and reports of clinical failure that have rendered it inappropriate for MRSA endocarditis (9, 19). Daptomycin has showed a good in vivo activity against MRSA experimental endocarditis (32), and in a recent published trial (11), the efficacy of daptomycin in patients with S. aureus bacteremia or right-sided or left-sided endocarditis was similar to that of comparator-based therapy (nafcillin or vancomycin). However, for methicillin-susceptible S. aureus or MRSA left-sided endocarditis, its efficacy was poor and similar to those of the comparators (nafcillin or vancomycin). Furthermore, the daptomycin MIC increases in some patients with microbiological failure were a cause of concern (11).
In summary, telavancin was efficacious in a rabbit model of S. aureus endocarditis simulating antimicrobial pharmacokinetics in humans. Telavancin was bactericidal in vitro and in vivo against two GISA isolates that are resistant to methicillin. Telavancin was as effective as vancomycin in the treatment of experimental endocarditis from GISA. These results suggest that telavancin may be an effective alternative to vancomycin in the treatment of GISA endocarditis and other serious staphylococcal infections, including those with decreased susceptibility to vancomycin.
Acknowledgments
This work was supported in part by a medical school grant from Theravance, Inc.: Red Española de Investigación en Patología Infecciosa (V-2003-REDC14A-O). This work was also supported in part by Fondo de Investigaciones Sanitarias (FIS) grants FIS 00/0475, FIS 02/0322, and FIS 05/0170. J. M. Miró was a recipient of a research grant from the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and the Conselleria de Salut de la Generalitat de Catalunya, Barcelona (Spain).
The authors also thank Fundación Máximo Soriano Jiménez for its technical support.
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Appelbaum, P. C. 2006. MRSA—the tip of the iceberg. Clin. Microbiol. Infect. 12(Suppl. 2)3-10. [DOI] [PubMed] [Google Scholar]
- 2.Baddour, L. M., W. R. Wilson, A. S. Bayer, V. G. Fowler, Jr., A. F. Bolger, M. E. Levison, P. Ferrieri, M. A. Gerber, L. Y. Tani, M. H. Gewitz, D. C. Tong, J. M. Steckelberg, R. S. Baltimore, S. T. Shulman, J. C. Burns, D. A. Falace, J. W. Newburger, T. J. Pallasch, M. Takahashi, and K. A. Taubert. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111e394-e434. [DOI] [PubMed] [Google Scholar]
- 3.Bayés, M., X. Rabasseda, and J. R. Prous. 2005. Gateways to clinical trials: April 2005. Methods Find. Exp. Clin. Pharmacol. 27193-219. [PubMed] [Google Scholar]
- 4.Blouin, R. A., L. A. Bauer, D. D. Miller, K. E. Record, and W. O. Griffin, Jr. 1982. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob. Agents Chemother. 21575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabell, C. H., J. G. Jollis, G. E. Peterson, G. R. Corey, D. J. Anderson, D. J. Sexton, C. W. Woods, L. B. Reller, T. Ryan, and V. G. Fowler, Jr. 2002. Changing patient characteristics and the effect on mortality in endocarditis. Arch. Intern. Med. 16290-94. [DOI] [PubMed] [Google Scholar]
- 6.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin for the Vancomycin-Resistant Staphylococcus aureus Investigative Team. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 3481342-1347. [DOI] [PubMed] [Google Scholar]
- 7.Cremieux, A. C., B. Maziere, J. M. Vallois, M. Ottaviani, A. Azancot, H. Raffoul, A. Bouvet, J. J. Pocidalo, and C. Carbon. 1989. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J. Infect. Dis. 159938-944. [DOI] [PubMed] [Google Scholar]
- 8.Eliopoulos, G., and R. Moellering. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 5th ed. Williams & Wilkins, Baltimore, MD.
- 9.Ellis, M. W., and J. S. Lewis II. 2005. Treatment approaches for community-acquired methicillin-resistant Staphylococcus aureus infections. Curr. Opin. Infect. Dis. 18496-501. [DOI] [PubMed] [Google Scholar]
- 10.Fowler, V. G., Jr., J. M. Miró, B. Hoen, C. H. Cabell, E. Abrutyn, E. Rubinstein, G. R. Corey, D. Spelman, S. F. Bradley, B. Barsic, P. A. Pappas, K. J. Anstrom, D. Wray, C. Q. Fortes, I. Anguera, E. Athan, P. Jones, J. T. M. van der Meer, T. S. J. Elliott, D. P. Levine, and A. S. Bayer for the ICE Investigators. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2933012-3021. [DOI] [PubMed] [Google Scholar]
- 11.Fowler, V. G., Jr., H. W. Boucher, G. R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Tally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, S. G. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Prince, G. N. Forrest, G. Fätkenheuer, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, and S. E. Cosgrove for the S. aureus Endocarditis and Bacteremia Study Group. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355653-665. [DOI] [PubMed] [Google Scholar]
- 12.Gander, S., A. Kinnaird, and R. Finch. 2005. Telavancin: in vitro activity against staphylococci in a biofilm model. J. Antimicrob. Chemother. 56337-343. [DOI] [PubMed] [Google Scholar]
- 13.Garrison, P. K., and L. R. Freedman. 1970. Experimental endocarditis. I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in right side of the heart. Yale J. Biol. Med. 42394-410. [PMC free article] [PubMed] [Google Scholar]
- 14.Gavaldà, J., P. J. Cardona, B. Almirante, J. A. Capdevila, M. Laguarda, L. Pou, E. Crespo, C. Pigrau, and A. Pahissa. 1996. Treatment of experimental endocarditis due to Enterococcus faecalis using once-daily dosing regimen of gentamicin plus simulated profiles of ampicillin in human serum. Antimicrob. Agents Chemother. 40173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein, E. J. C., D. M. Citron, C. V. Merriam, Y. A. Warren, K. L. Tyrrell, and H. T. Fernandez. 2004. In vitro activities of the new semisynthetic glycopeptide telavancin (TD-6424), vancomycin, daptomycin, linezolid, and four comparator agents against anaerobic gram-positive species and Corynebacterium spp. Antimicrob. Agents Chemother. 482149-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hegde, S. S., N. Reyes, T. Wiens, N. Vanasse, R. Skinner, J. McCullough, K. Kaniga, J. Pace, R. Thomas, J.-P. Shaw, G. Obedencio, and J. K. Judice. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 483043-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 491127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40135-136. [DOI] [PubMed] [Google Scholar]
- 19.Kanafani, Z. A., and V. G. Fowler, Jr. 2006. Staphylococcus aureus infections: new challenges from an old pathogen. Enferm. Infecc. Microbiol. Clin. 24182-193. [DOI] [PubMed] [Google Scholar]
- 20.King, A., I. Phillips, and K. Kaniga. 2004. Comparative in vitro activity of telavancin (TD-6424), a rapidly bactericidal, concentration-dependent anti-infective with multiple mechanisms of action against Gram-positive bacteria. J. Antimicrob. Chemother. 53797-803. [DOI] [PubMed] [Google Scholar]
- 21.Madrigal, A. G., L. Basuino, and H. F. Chambers. 2005. Efficacy of telavancin in a rabbit model of aortic valve endocarditis due to methicillin-resistant Staphylococcus aureus or vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 493163-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maltezou, H. C., and H. Giamarellou. 2006. Community-acquired methicillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2787-96. [DOI] [PubMed] [Google Scholar]
- 23.Miró, J. M., I. Anguera, C. H. Cabell, A. Y. Chen, J. A. Stafford, G. R. Corey, L. Olaison, S. Eykyn, B. Hoen, E. Abrutyn, D. Raoult, A. Bayer, V. G. Fowler, Jr., and the International Collaboration on Endocarditis Merged Database Study Group. 2005. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin. Infect. Dis. 41507-514. [DOI] [PubMed] [Google Scholar]
- 23a.Miró, J. M., F. Marco, C. García de la Mària, Y. Armero, E. Amat, D. Soy, A. Moreno, A. del Rio, M. Almela, C. A. Mestres, J. Gatell, and M. T. Jímenez de Anta. 2006. Efficacy of telavancin in the treatment of experimental endocarditis due to glycopeptide-intermediately susceptible Staphylococcus aureus (GISA). Clin. Microbiol. Infect. 12(Suppl. 4)1158. [Google Scholar]
- 24.Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O'Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2902976-2984. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing. 9th informational supplement. Document M100-S11, vol. 21, no. 1. National Committee for Clinical Laboratory Standards, Villanova, PA.
- 26.Pace, J. L., and J. K. Judice. 2005. Telavancin (Theravance). Curr. Opin. Investig. Drugs 6216-225. [PubMed] [Google Scholar]
- 27.Pace, J. L., K. Krause, D. Johnston, D. Debabov, T. Wu, L. Farrington, C. Lane, D. L. Higgins, B. Christensen, J. K. Judice, and K. Kaniga. 2003. In vitro activity of TD-6424 against Staphylococcus aureus. Antimicrob. Agents Chemother. 473602-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeltz, R. F., and B. J. Wilkinson. 2004. The escalating challenge of vancomycin resistance in Staphylococcus aureus. Curr. Drug Targets Infect. Disord. 4273-294. [DOI] [PubMed] [Google Scholar]
- 29.Reyes, N., R. Skinner, B. M. Benton, K. M. Krause, J. Shelton, G. P. Obedencio, and S. S. Hegde. 2006. Efficacy of telavancin in a murine model of bacteraemia induced by methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58462-465. [DOI] [PubMed] [Google Scholar]
- 30.Reyes, N., R. Skinner, K. Kaniga, K. M. Krause, J. Shelton, G. P. Obedencio, A. Gough, M. Conner, and S. S. Hegde. 2005. Efficacy of telavancin (TD-6424), a rapidly bactericidal lipoglycopeptide with multiple mechanisms of action, in a murine model of pneumonia induced by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 494344-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruef, C. 2004. Epidemiology and clinical impact of glycopeptide resistance in Staphylococcus aureus. Infection 32315-327. [DOI] [PubMed] [Google Scholar]
- 32.Sakoulas, G., G. Eliopoulos, J. Alder, and C. Thauvin-Eliopoulos. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 471714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt-Ioanas, M., A. de Roux, and H. Lode. 2005. New antibiotics for the treatment of severe staphylococcal infection in the critically ill patient. Curr. Opin. Crit. Care 11481-486. [DOI] [PubMed] [Google Scholar]
- 34.Shaw, J. P., J. Seroogy, K. Kaniga, D. L. Higgins, M. Kitt, and S. Barriere. 2005. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob. Agents Chemother. 49195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sievert, D. M., M. L. Boulton, G. Stoltman, D. Johnson, M. G. Stobierski, F. P. Downes, P. A. Somsel, J. T. Rudrik, W. Brown, W. Hafeez, T. Lundstrom, E. Flanagan, R. Johnson, J. Mitchell, and S. Chang. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51565-567. [PubMed] [Google Scholar]
- 36.Small, P. M., and H. F. Chambers. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 341227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, and W. R. Jarvis for the Glycopeptide-Intermediate Staphylococcus aureus Working Group. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340493-501. [DOI] [PubMed] [Google Scholar]
- 38.Stryjewski, M. E., V. H. Chu, W. D. O'Riordan, B. L. Warren, L. M. Dunbar, D. M. Young, M. Vallée, V. G. Fowler, Jr., J. Morganroth, S. L. Barriere, M. M. Kitt, and G. R. Corey for the FAST 2 Investigator Group. 2006. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob. Agents Chemother. 50862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stryjewski, M. E., W. D. O'Riordan, W. K. Lau, F. D. Pien, L. M. Dunbar, M. Vallée, V. G. Fowler, Jr., V. H. Chu, E. Spencer, S. L. Barriere, M. M. Kitt, C. H. Cabell, and G. R. Corey for the FAST Investigator Group. 2005. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin. Infect. Dis. 401601-1607. [DOI] [PubMed] [Google Scholar]
- 40.Stucki, A., P. Gerber, F. Acosta, M. Cottagnoud, and P. Cottagnoud. 2006. Efficacy of telavancin against penicillin-resistant pneumococci and Staphylococcus aureus in a rabbit meningitis model and determination of kinetic parameters. Antimicrob. Agents Chemother. 50770-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun, H. K., K. Duchin, C. H. Nightingale, J.-P. Shaw, J. Seroogy, and D. P. Nicolau. 2006. Tissue penetration of telavancin after intravenous administration in healthy subjects. Antimicrob. Agents Chemother. 50788-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wootton, M., R. A. Howe, T. R. Walsh, P. M. Bennett, and A. P. MacGowan. 2002. In vitro activity of 21 antimicrobials against vancomycin-resistant Staphylococcus aureus (VRSA) and heteroVRSA (hVRSA). J. Antimicrob. Chemother. 50760-761. [DOI] [PubMed] [Google Scholar]