Abstract
Extracts from Holostylis reniformis were tested in vivo against Plasmodium berghei and in vitro against a chloroquine-resistant strain of Plasmodium falciparum. The hexane extract of the roots was the most active, causing 67% reduction of parasitemia in vivo. From this extract, six lignans, including a new (7′R,8S,8′S)-3′,4′-methylenedioxy-4,5-dimethoxy-2,7′-cyclolignan-7-one, were isolated and tested in vitro against P. falciparum. The three most active lignans showed 50% inhibitor concentrations of ≤0.32 μM. An evaluation of minimum lethal dose (30%) values showed low toxicity for these lignans in a hepatic cell line (Hep G2A16). Therefore, these compounds are potential candidates for the development of antimalarial drugs.
Malaria is the most important parasitic disease in the world, responsible for 500 million new cases and 2 to 3 million deaths every year (http://www.who.int/malaria/epidemicsandemergencies.html, http://www.who.int/tdr/diseases/malaria/diseaseinfo.html). The number of clinical attacks due to Plasmodium falciparum seems to be 50% higher than WHO estimates (24). This situation, together with the progressive spread of chloroquine-resistant strains of P. falciparum and, more recently, Plasmodium vivax, has caused an intensive search for novel blood schizonticides to replace chloroquine, a cheap, safe, and, formerly effective therapeutic antimalarial drug (9, 20, 21). Many natural products of various structural types have shown antiparasitic potency in the laboratory, and they represent interesting lead structures for the development of new drugs (12). The molecular diversity and efficacy of antiparasitic plants, extracts, and herbal preparations have been intensively discussed in recent reviews (22, 26, 27).
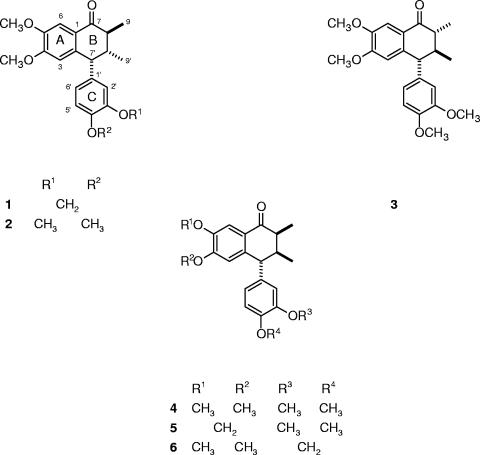
More than 60 Brazilian plant species used in traditional medicine to treat malaria and/or fever have been screened by our group, and several species are active against P. falciparum in culture and Plasmodium berghei in mice (1, 13). In the present study, we investigated the antimalarial activities and toxicities of compounds isolated from Holostylis reniformis Duch. (Aristolochiaceae), which is used in traditional Brazilian medicine as an antirheumatic, stomachic, and depurative (10). H. reniformis is a rich source of aryltetralone lignans (4-6). Several lignans (1 to 6) (Fig. 1) isolated from extracts of this species by chromatography (column, thin-layer chromatography, and high-performance liquid chromatography [HPLC]) were then bioassayed in vitro for their antiplasmodial activities and toxicities. The structures of lignans 1 to 5 had been determined by spectroscopic methods and chemical transformations (5, 6). Lignan 6 is reported here for the first time.
FIG. 1.
Bioassayed lignans from H. reniformis.
MATERIALS AND METHODS
Plant material.
The plant material was collected in Ituiutaba, MG, Brazil, in February 1998 and identified as H. reniformis Duch. by Condorcet Aranha and Lindolpho Cappellari Jr. A voucher specimen (ESA88282) was deposited at the herbarium of the Escola Superior de Agricultura “Luiz de Queiroz”, Piracicaba, SP, Brazil. The material was separated according to the plant parts, dried (∼45°C), and ground (4-6).
Extraction and isolation of the chemical constituents.
The plant material was extracted exhaustively at room temperature with hexane, acetone, and ethanol, successively, and the extracts were individually concentrated (4-6). The hexane extract (6.17 g) from the roots was fractionated by column chromatography (60.0 by 4.8 cm; silica gel 60 H; 151.0 g; hexane-ethyl acetate gradient, 95:5 to 100% ethyl acetate) to give 28 fractions (100 ml), as previously described (6). Several of these fractions were subjected to semipreparative HPLC (MeOH-H2O, 3:2). Fraction 10 was comprised of lignans 1, 5, and 6 (11:3:2) and gave lignans 1 (67.6 mg), 5 (18.4 mg), and 6 (12.3 mg). Fraction 11 gave lignans 1 (25.7 mg) and 5 (22.1 mg). Fraction 12, comprised of lignans 2, 3, and 4 (3:1:2), was combined with fraction 13, comprised of lignans 3 and 4 (3:8), and subjected to semipreparative HPLC (MeOH-H2O, 3:2) to give lignans 2 (255.7 mg), 3 (275.8 mg), and 4 (28.1 mg).
Instrumentation.
One-dimensional (1H, 13C, and distortionless enhancement by polarization transfer [DEPT]) and two-dimensional (1H-1H gradient-selected correlated spectroscopy [gCOSY]; gradient-selected heteronuclear multiple-quantum coherence, inverse detected 1H-13C one-bound correlation experiment [gHMQC]; gradient-selected heteronuclear multiple-bond correlation, inverse detected 1H-13C long-range correlation experiment [gHMBC]; and gradient-selected nuclear Overhauser enhancement spectroscopy [gNOESY]) nuclear magnetic resonance (NMR) experiments were recorded on a Varian INOVA 500 spectrometer (11.7 T) at 500 MHz (1H) and 126 MHz (13C), with the residual solvent CHCl3 used as an internal standard for 1H (δ 7.23) and CDCl3 for 13C (δ 77.0). Mass spectra (electrospray ionization-mass spectrometry [ESI-MS]) were obtained on a Fisons Platform II, and flow injection into the electrospray source was used for ESI-MS. Infrared (IR) spectra were obtained on a Nicolet-730 FT-IR spectrometer using KBr discs. UV absorptions were measured on a Hewlett-Packard 8452A diode array spectrophotometer. Optical rotations were measured on a Polamat A (Carl Zeiss, Jena, Switzerland). Circular dichroism (CD) spectra were recorded on a JASCO J-720 spectrometer. HPLC analyses were carried out using a Shimadzu liquid chromatograph 10Avp equipped with a UV-visible light detector. Columns were RP18 (Shimadzu; C18; 3.9 by 150 mm for analytical analysis and 250 by 20 mm for semipreparative analysis), and chromatograms were acquired at 254 nm. Melting points were recorded on a Microquimica MQAPF-301 melting point apparatus and were uncorrected.
(7′R,8S,8′S)-4,5-Dimethoxy-3′,4′-methylenedioxy-2,7′-cyclolignan-7-one [(−)-8′-epi-aristotetralone; lignan 6] was obtained as a yellow solid, m.p. 136.2 to 138.0°C; 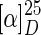 −57.0 (c 1.18, CHCl3); UV (MeOH) λmax nm (log ɛ) 239 (3.6), 278 (3.4), 323 (3.2); IR (KBr) νmax 3469, 3022, 2959, 2924, 2862, 1667 cm−1; for 1H and 13C NMR, see Table 1; ESI-MS (+35 eV) m/z 355 [M+H]+ (100); CD (MeOH, c 0,1) [θ]212 +34650, [θ]227 +1155, [θ]238 +12177, [θ]247 0, [θ]254 −5709, [θ]265 0, [θ]275 +5181, [θ]280 0, [θ]291 −17061, [θ]303 −11616, [θ]307 −11616, [θ]315 −11880; analysis C 71.2%, H 6.2%, calculated for C21H22O5, C 71.2%, H 6.3%.
−57.0 (c 1.18, CHCl3); UV (MeOH) λmax nm (log ɛ) 239 (3.6), 278 (3.4), 323 (3.2); IR (KBr) νmax 3469, 3022, 2959, 2924, 2862, 1667 cm−1; for 1H and 13C NMR, see Table 1; ESI-MS (+35 eV) m/z 355 [M+H]+ (100); CD (MeOH, c 0,1) [θ]212 +34650, [θ]227 +1155, [θ]238 +12177, [θ]247 0, [θ]254 −5709, [θ]265 0, [θ]275 +5181, [θ]280 0, [θ]291 −17061, [θ]303 −11616, [θ]307 −11616, [θ]315 −11880; analysis C 71.2%, H 6.2%, calculated for C21H22O5, C 71.2%, H 6.3%.
TABLE 1.
NMR spectroscopic data (500 MHz, CDCl3) for lignans 5 and 6
| Position | Lignan 5 (5)
|
Lignan 6
|
||||
|---|---|---|---|---|---|---|
| δCa | δH (J in Hz) | δCa | δH (J in Hz) | gHMBCb | NOESY (two dimensional) | |
| 1 | 127.0 | 125.6 | 3, 6, 7′ | |||
| 2 | 141.0 | 138.4 | 6 | |||
| 3 | 109.5 | 6.35 s | 108.2 | 6.35 s | 7′ | OCH3-4, 7′ |
| 4 | 152.2 | 153.8 | 3, 6, OCH3-4 | |||
| 5 | 147.2 | 148.3 | 3, 6, OCH3-5 | |||
| 6 | 105.8 | 7.45 s | 108.1 | 7.49 s | OCH3-5 | |
| 7 | 199.5 | 199.8 | 6, 9 | |||
| 8 | 43.0 | 2.71 dq (4.5, 7.0) | 42.6 | 2.70 dq (3.5, 7.0) | 9, 7′, 9′ | 9, 8′, 2′ |
| 9 | 11.7 | 1.06 d (7.0) | 11.9 | 1.05 d (7.0) | 8, 9′ | |
| 1′ | 136.0 | 137.7 | 5′ | |||
| 2′ | 111.9 | 6.55 d (2.5) | 111.8 | 6.47 d (1.5) | 7′ | |
| 3′ | 149.2 | 147.9 | OCH2O, 2′, 5′ | |||
| 4′ | 147.9 | 146.3 | 2′, 6′, OCH2O | |||
| 5′ | 111.1 | 6.72 d (8.0) | 109.0 | 6.67 d (8.0) | 6′ | |
| 6′ | 121.1 | 6.49 dd (8.0, 1.5) | 122.0 | 6.44 dd (8.0, 1.5) | 2′, 7′ | 8, 7′, 8′ |
| 7′ | 50.6 | 3.86 d (5.5) | 50.5 | 3.85 d (5.5) | 3, 6′, 9′ | 3, 2′, 8′, 9′ |
| 8′ | 42.0 | 2.35 ddq (5.5, 4.5, 6.5) | 42.5 | 2.35 ddq (5.5, 3.5, 6.5) | 9, 7′, 9′ | 8, 9, 9′, 2′, 6′, 7′ |
| 9′ | 15.9 | 0.91 d (6.5) | 15.9 | 0.92 d (6.5) | 7′ | 9, 7′, 8′ |
| OCH3-4 | 56.0 | 3.72 s | 3, 2′, 6′ | |||
| OCH3-5 | 56.0 | 3.87 s | 6 | |||
| OCH3-3′ | 55.9 | 3.76 s | ||||
| OCH3-4′ | 56.0 | 3.80 s | ||||
| OCH2O | 101.6 | 5.87 s | 101.0 | 5.87 s | ||
The 13C NMR data were assigned with the assistance of DEPT, gHMQC (optimized for 140 Hz), and gHMBC experiments.
gHMBC correlations (optimized for 7 Hz) are from the proton(s) stated to the indicated carbon.
Antimalarial tests in vivo.
The antimalarial tests were performed with adult Swiss albino mice (body weight, 20 ± 2 g), and their use was approved by the Ethical Committee for Using Animals (CEUA-P0094-01, Fundação Instituto Oswaldo Cruz). The animals received water and food ad libitum. The antimalarial suppressive test was performed as previously described (3) in mice infected with P. berghei strain NK-65, originally received from New York University Medical School. Each mouse (five mice per group) received 105 infected red blood cells (day zero), followed by daily treatment, via gavage, for 4 consecutive days. The extracts were suspended in Tween 20 (2% final concentration) immediately before use and then diluted so that doses of 100 to 500 mg/kg of body weight were delivered in 0.2 ml per animal. Three control groups were used in each test: one received chloroquine, and the others were not treated or were treated with Tween 20 (< 0.2% final concentration). Blood smears were taken on days 5 and 7 after parasite inoculation, and mortality was monitored for 3 weeks. The results are expressed as the percent reduction of parasitemia in relation to untreated mice, and a compound was considered active when this reduction was ≥30% (3). Each experiment was performed in triplicate and repeated three times.
Parasite culture and in vitro antimalarial tests.
The P. falciparum used for the in vitro tests, a chloroquine-resistant isolate (BHz 26/86), was from an imported case of malaria from the Amazon region (3). Parasites were maintained in continuous culture on human erythrocytes (blood group AB+ or A+, using RPMI medium supplemented with 10% human serum), as previously described (25). The antiparasitic effects of extracts, purified compounds (lignans 1 to 6), and fractions were measured by the percent inhibition of parasite growth in relation to the control (parasites cultivated in drug-free medium), as previously described (3). Briefly, the drugs tested were diluted with Tween 20 at a final concentration of 0.02% in culture medium (RPMI 1640). These stock solutions were further diluted in complete medium (RPMI 1640 plus 10% human serum) to give each of the concentrations used (0.02 to 20 μM for purified compounds and fractions and 0.2 to 50 μg/ml for extracts). The cultures, with trophozoites in sorbitol-synchronized blood (14) at 1 to 2% parasitemia and 2.5% hematocrit, were then incubated with extracts, fractions, or isolated compounds for a total of 72 h at 37°C. A positive control with chloroquine (the reference antimalarial drug) and a control with medium and the Tween 20 solution were used in each experiment. The 50% and 90% inhibitory concentrations (IC50 and IC90, respectively), compared to the drug-free control responses, were estimated by linear interpolation (11). Each experiment was performed in triplicate and repeated three times. The blood smears were read in a double-blind manner.
Cytotoxicity test.
An in vitro culture of Hep G2 A16 hepatic cells (7) was mixed with William's E culture medium in 96-well microtiter plates and incubated at 37°C in an enriched CO2 environment for 24 h (17). The compounds were diluted with a 0.02% final concentration of Tween 20 solution in culture medium to obtain six concentrations: 500, 250, 100, 50, 10, and 5 μg/ml. After incubation periods of 24 and 48 h, the culture medium was replaced with 200 μl fresh medium with or without the drugs. At the end of the incubation periods, 20 μl of MTT solution (5 mg of thiazolyl blue salt in RPMI 1640) without phenol red was added to each well, and the plates were incubated for three more hours. The supernatant was then removed, and 200 μl of acidified isopropanol was added to the wells. The culture plates were read by spectrophotometer with a filter of 570 nm and a background of 630 nm. The minimum lethal dose that killed 30% of the cells was determined (17). The assays were performed in three independent experiments.
Statistical analysis.
The average parasitemias in vivo were compared using analysis of variance and Student t tests. Differences between IC50 values were evaluated by the Mann-Whitney U test performed with Biostat 1.0 MCT-CNPq. A P value of ≤0.05 was considered to be statistically significant.
RESULTS
The crude hexane, acetone, and ethanol extracts of the roots, stems, and leaves of H. reniformis partially reduced the malaria parasitemia and mortality of mice infected with P. berghei. The hexane extracts were the most active, especially the root and leaf extracts, which caused 67% and 48% reduction of parasitemia, respectively, at doses of 500 mg/kg (day 5; P ≤ 0.05). Lower doses tested were inactive. The extracts were also screened in vitro against P. falciparum parasites (isolate BHz 26/86; chloroquine resistant). The apolar extracts (hexane and acetone) exhibited the best antiplasmodial activities, and they exhibited the lowest IC50 values (∼0.70 μg/ml), whereas the positive control (chloroquine) showed an IC50 of 0.09 μg/ml.
All of the isolated lignans were tested for antiplasmodial activity in vitro; their IC50 and IC90 values, as well as the values for the standard antimalarial chloroquine, obtained in three sets of experiments are shown in Table 2. Lignans 1 to 3 exhibited IC50 values of ≤0.32 μM (≤0.12 μg/ml). The lowest IC50 value obtained was for lignan 3 (0.20 μM), whereas the lowest IC90 value was for lignan 4 (2.61 μM), which showed that these lignans are active and that they are the major active principles in the extracts. Lignan 5 exhibited low activity, with the highest IC50 (8.00 μM) and IC90 (19.7 μM) values, whereas lignan 6 did not exhibit any activity under the same experimental conditions at the maximal dose tested (140.0 μM = 50 μg/ml). Mixtures of these lignans, which were not previously subjected to semipreparative HPLC (3 and 4; 2, 3, and 4; and 1, 5, and 6), also showed some activity and exhibited significant IC50 (from 1.9 to 6.0 μM) and IC90 (from 8.4 to 18.2 μM) values (Table 2).
TABLE 2.
IC50s and IC90s of lignans, alone or in mixtures, tested against P. falciparum isolate BHz26/86
| Lignan(s) | Compound | IC50 (μM)a | IC90 (μM)a |
|---|---|---|---|
| 1 | (7′R,8S,8′R)-4,5-Dimethoxy-3′,4′-methylenodioxy-2,7′-cyclolignan-7-one | 0.26 ± 0.08 | 3.35 ± 0.12 |
| 2 | (7′R,8S,8′R)-3′,4,4′,5-Tetramethoxy-2,7′-cyclolignan-7-one | 0.32 ± 0.11 | 4.60 ± 0.30 |
| 3 | (7′R,8R,8′S)-3′,4,4′,5-Tetramethoxy-2,7′-cyclolignan-7-one | 0.20 ± 0.09 | 3.00 ± 0.15 |
| 4 | (7′R,8S,8′S)-3′,4,4′,5-Tetramethoxy-2,7′-cyclolignan-7-one | 0.63 ± 0.20 | 2.61 ± 0.06 |
| 5 | (7′R,8S,8′S)-3′,4′-Dimethoxy-4,5-methylenodioxy-2,7′-cyclolignan-7-one | 8.00 ± 0.65 | 19.70 ± 0.42 |
| 6 | (7′R,8S,8′S)-4,5-Dimethoxy-3′,4′-methylenodioxy-2,7′-cyclolignan-7-one | >140.00 | >140.00 |
| 3 + 4 | Combination (3:8) | 2.80 ± 0.34 | 9.13 ± 0.30 |
| 2 + 3 + 4 | Combination (3:1:2) | 6.00 ± 0.50 | 18.20 ± 0.40 |
| 1 + 5 + 6 | Combination (11:3:2) | 1.90 ± 0.09 | 8.40 ± 0.15 |
| Chloroquineb | 0.19 ± 0.02 | 0.70 ± 0.13 |
Values are means ± standard deviations in triplicate.
Antimalarial reference drug.
The cytotoxicities of the active lignans evaluated in vitro were considered low, since the mean minimum lethal dose that killed 30% of the cells (450 μg/ml) was at least 5,000 times higher than the mean IC50 value obtained for them.
Compound 6 has not yet been described in the literature. It was isolated from the active fraction 1, 5, and 6 (11:3:2) by semipreparative HPLC. The 1H and 13C NMR, UV, IR, and ESI-MS data for lignan 6 were similar to those reported for lignan 5 (5).
DISCUSSION
Lignoids with different structural types (up to 60) have been previously isolated from the family Aristolochiaceae (4-6, 8, 16, 19). The biosynthesis, functions, and pharmacological and physiological effects of lignans have been studied, and these compounds have been shown to possess a wide range of biological activities (15, 18, 23). Lignans have been used as lead compounds for the development of new drugs, mainly due to their low cytotoxicity and their antiangiogenic, antiviral, antileishmanial, antifungal, hypolipidemic, and antirheumatic activities (2). Here, we show that they also have an antiplasmodial activity, as well as rather low cytotoxicity, as tested for one cell line so far.
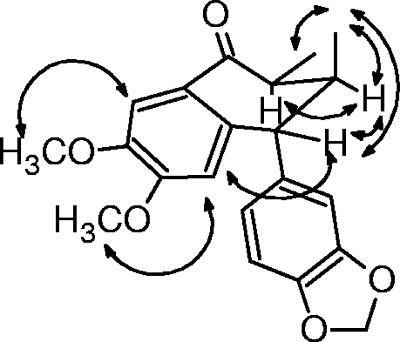
Compound 6 was suggested to be an aryltetralone lignan, since it showed quasi-molecular ions at m/z 355 [M+H]+, which were consistent with the molecular formula C21H22O5, and its IR, 1H, and 13C NMR spectra were very similar to those of lignan 5 (5). A detailed analysis of 1H and 13C NMR, 1H-1H COSY, DEPT, gHMQC, and gHMBC experiments enabled the precise assignment of all hydrogens and carbons in the basic structure of lignan 6 (Table 1). 1H-1H COSY and 1H selective-irradiation NMR experiments with lignan 6 allowed us to establish the same conformations and relative configuration for the B ring as in lignan 5 (Fig. 2) (5). Therefore, the main difference between lignans 6 and 5 is due to the interchange of substituents on the A and C rings. This deduction was further confirmed by NOESY experiments (Table 1 and Fig. 2). Moreover, the similarity between the CD curves of these lignans allowed us to determine the same absolute configuration for stereocenters on the B ring (5, 6). Thus, the absolute configuration 7′R,8S,8′S was determined for lignan 6.
FIG. 2.
Selected nuclear Overhauser enhancement interactions and conformation for lignan 6.
Based on an analysis of the structure-activity relationships for these lignans, we could infer that the activity was affected by the configurations of the stereocenters on the B ring and by the substituents (methylenedioxy or dimethoxy groups) on the A and C rings. The best activity was achieved for lignan with dimethoxy substituents on the A ring and with the substituents on the B ring (CH3-9 and CH3-9′ and veratryl) in a trans-trans orientation (lignan 3).
Aryltetralone lignans from H. reniformis showed antimalarial activities and low toxicity on hepatic cells, and the three most active lignans (1 to 3) showed IC50 values of ≤0.32 μM. Although mixtures of the lignans were at least 10 times less active than lignan 3 and the standard chloroquine, their IC50 values were still low, i.e., in the micromolar range. However, a lower antimalarial activity than one would expect for fractions comprising mixtures of these lignans was observed. Whether this reflects an antagonist effect is unclear, and further work must be undertaken to elucidate this finding. These lignans are worthy of further investigation, including chemical transformations, to optimize the activity and to study structure-activity relationships of this class of antiplasmodial compounds. As toxicity is a very important parameter for a suitable lead candidate in the development of antimalarial drugs, it also has to be further investigated for the active lignans using other cell lines, as well as animal models.
Acknowledgments
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) for financial support and Conselho Nacional de Desenvolvimento Científico (CNPq) for fellowships for A.U.K., T.D.S., and V.F.D.A.N.
We also thank Condorcet Aranha and Lindolpho Cappellari Jr. for plant identification.
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Andrade-Neto, V. F., M. G. L. Brandão, F. Q. Oliveira, V. W. D. Casali, B. Njaine, M. G. Zalis, L. A. Oliveira, and A. U. Krettli. 2004. Antimalarial activity of Bidens pilosa L. (Asteraceae) ethanol extracts from wild plants collected in various localities of plants cultivated in humus soil. Phytother. Res. 18634-639. [DOI] [PubMed] [Google Scholar]
- 2.Apers, S., A. Vlietinck, and L. Pieters. 2003. Lignans and neolignans as lead compounds. Phytochem. Rev. 2201-217. [Google Scholar]
- 3.Carvalho, L. H., M. G. L. Brandão, D. Santos-Filho, J. L. C. Lopes, and A. U. Krettli. 1991. Antimalarial activity of crude extracts from Brazilian plants. Studied in vivo in Plasmodium berghei-infected mice and in vitro against Plasmodium falciparum in culture. Braz. J. Med. Biol. Res. 241113-1123. [PubMed] [Google Scholar]
- 4.da Silva, T., A. U. Krettli, V. F. de Andrade-Neto, and L. M. X. Lopes. 2005. Lignans, and particularly aryltetralone lignans, extracts containing them, processes for obtaining the extracts and the lignans, and use of the lignans or the extracts in pharmaceutical compositions for treating or preventing malaria. Rev. Prop. Ind. 17951774-1786. [Google Scholar]
- 5.da Silva, T., and L. M. X. Lopes. 2004. Aryltetralone lignans and 7,8-seco-lignans from Holostylis reniformis. Phytochemistry 65751-759. [DOI] [PubMed] [Google Scholar]
- 6.da Silva, T., and L. M. X. Lopes. 2006. Aryltetralol and aryltetralone from Holostylis reniformis. Phytochemistry 67929-937. [DOI] [PubMed] [Google Scholar]
- 7.Denizot, F., and R. Lang. 1986. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 89271-277. [DOI] [PubMed] [Google Scholar]
- 8.de Pascoli, I. C., I. R. Nascimento, and L. M. X. Lopes. 2006. Configurational analysis of cubebins and bicubebin from Aristolochia lagesiana and Aristolochia pubescens. Phytochemistry 67735-742. [DOI] [PubMed] [Google Scholar]
- 9.Guerin, P. J., P. Olliaro, F. Nosten, P. Druilhe, R. Laxminarayan, F. Binka, W. L. Kilama, N. Ford, and N. J. White. 2002. Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect. Dis. 2564-573. [DOI] [PubMed] [Google Scholar]
- 10.Hoehne, F. C. 1942. Aristolochiaceas, p. 1-265. In F. C. Hoehne (ed.), Flora Brasílica, vol. 17. Graphicars, São Paulo, Brazil. [Google Scholar]
- 11.Huber, W., and J. C. Koella. 1993. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 55257-261. [DOI] [PubMed] [Google Scholar]
- 12.Kayser, O., A. F. Kiderlen, and S. L. Croft. 2003. Natural products as antiparasitic drugs. Parasitol. Res. 90S55-S62. [DOI] [PubMed] [Google Scholar]
- 13.Krettli, A. U., V. F. Andrade-Neto, M. G. L. Brandão, and W. M. Ferrari. 2001. The search for new antimalarial drugs from plants used to treat fever and malaria or plants randomly selected: a review. Mem. Inst. Oswaldo Cruz 961033-1042. [DOI] [PubMed] [Google Scholar]
- 14.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65418-420. [PubMed] [Google Scholar]
- 15.Lewis, N. G., and L. B. Davin. 1999. Lignans: biosynthesis and function, p. 639-712. In D. H. R. Barton, K. Nakanishi, and O. Meth-Cohn (ed.), Comprehensive natural products chemistry, vol 1. Elsevier, London, United Kingdom. [Google Scholar]
- 16.Lopes, L. M. X., I. R. Nascimento, and T. da Silva. 2001. Phytochemistry of the Aristolochiaceae family, p. 19-108. In R. M. M. Mohan (ed.), Research advances in phytochemistry, vol. 2. Global Research Network, Kerala, India. [Google Scholar]
- 17.Madureira, A. M., A. P. Martins, M. Gomes, J. Paiva, M. J. U. Ferreira, A. P. Cunha, and V. E. Rosário. 2002. Antimalarial activity of medicinal plants used in traditional medicine in S. Tomé and Príncipe Island. J. Ethnopharmacol. 8123-29. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento, I. R., A. T. Murata, S. A. Bortoli, and L. M. X. Lopes. 2004. Insecticidal activity of chemical constituents from Aristolochia pubescens against Anticarsia gemmatalis larvae. Pest Manage. Sci. 60413-416. [DOI] [PubMed] [Google Scholar]
- 19.Nascimento, I. R., and L. M. X. Lopes. 1999. 2,3-Dihydrobenzofuran neolignans from Aristolochia pubescens. Phytochemistry 52345-350. (Erratum, 53:621.) [Google Scholar]
- 20.Peters, W., L. B. Stewart, and B. L. Robinson. 2003. The chemotherapy of rodent malaria. LXI. Drug combinations to impede the selection of drug resistance, part 4: the potential role of 8-aminoquinolines. Ann. Trop. Med. Parasitol. 97221-236. [DOI] [PubMed] [Google Scholar]
- 21.Ridley, R. G. 2002. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415686-693. [DOI] [PubMed] [Google Scholar]
- 22.Schwikkard, S., and F. R. Van Heerden. 2002. Antimalarial activity of plant metabolites. Nat. Prod. Rep. 19675-692. [DOI] [PubMed] [Google Scholar]
- 23.Skytte, D. M., S. F. Nielsen, M. Chen, L. Zhai, C. E. Olsen, and S. B. Christensen. 2006. Antimalarial and antiplasmodial activities of norneolignans. Syntheses and SAR. J. Med. Chem. 49436-440. [DOI] [PubMed] [Google Scholar]
- 24.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trager, W. M., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193673-675. [DOI] [PubMed] [Google Scholar]
- 26.Willcox, M. L., and G. Bodeker. 2004. Traditional herbal medicines for malaria. Br. Med. J. 3291156-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright, C. W. 2005. Plant derived antimalarial agents: new leads and challenges. Phytochem. Rev. 455-61. [Google Scholar]