Abstract
We examined the relationship between the time to clearance of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia while patients were receiving vancomycin therapy and the in vitro bactericidal activity of vancomycin. Vancomycin killing assays were performed with 34 MRSA bloodstream isolates (17 accessory gene regulator group II [agr-II] and 17 non-agr-II isolates) from 34 different patients with MRSA bacteremia for whom clinical and microbiological outcomes data were available. Vancomycin doses were prospectively adjusted to achieve peak plasma concentrations of 28 to 32 μg/ml and trough concentrations of 8 to 12 μg/ml. Bactericidal assays were performed over 24 h with ∼107 to 108 CFU/ml in broth containing 16 μg/ml vancomycin. The median time to clearance of bacteremia was 6.5 days for patients with MRSA isolates demonstrating ≥2.5 reductions in log10 CFU/ml at 24 h and >10.5 days for patients with MRSA isolates demonstrating <2.5 log10 CFU/ml by 24 h (P = 0.025). The median time to clearance was significantly longer with MRSA isolates with vancomycin MICs of 2.0 μg/ml compared to that with MRSA isolates with MICs of ≤1.0 μg/ml (P = 0.019). The bacteremia caused by MRSA isolates with absent or severely reduced delta-hemolysin expression was of a longer duration of bacteremia (10 days and 6.5 days, respectively; P = 0.27) and had a decreased probability of eradication (44% and 78%, respectively; P = 0.086). We conclude that strain-specific microbiological features of MRSA, such as increased vancomycin MICs and decreased killing by vancomycin, appear to be predictive of prolonged MRSA bacteremia while patients are receiving vancomycin therapy. Prolonged bacteremia and decreased delta-hemolysin expression may also be related. Evaluation of these properties may be useful in the consideration of antimicrobial therapies that can be used as alternatives to vancomycin for the treatment of MRSA bacteremia.
The efficacy of vancomycin against methicillin-resistant Staphylococcus aureus (MRSA) has come under increased scrutiny in recent years. This may be a result of its inferior in vitro antistaphylococcal activity compared to those of beta-lactams (1, 19), clinical data demonstrating a suboptimal clinical response of patients treated with vancomycin (7-9, 11), worse outcomes in patients with MRSA infections compared to those in patients with methicillin-susceptible S. aureus infections (3, 4, 6), and the availability of other antibiotics with activities against MRSA. MRSA strain-specific characteristics, such as a polymorphism at the accessory gene regulator (agr) locus (13), vancomycin susceptibility, and bactericidal activity (18), may also contribute to the response to vancomycin treatment.
Bactericidal antimicrobial regimens are considered to be superior to bacteriostatic regimens in the treatment of S. aureus bloodstream infections, particularly in the case of infective endocarditis (5, 14, 15). In our recent study in which we evaluated the relationship of MIC and bactericidal activity to the efficacy of vancomycin, a superior clinical response of MRSA infections to vancomycin therapy correlated with a lower vancomycin MIC and increased in vitro killing by vancomycin (18). In light of the variability in the activity of vancomycin against different MRSA strains and the continued controversy on the importance of antimicrobial bactericidal activity in the treatment of bacteremia, further evaluation of the clinical and microbiological relationship of the activity of vancomycin against MRSA is anticipated by clinicians. The primary objective of this study was to determine the relationship between the time to clearance of MRSA bacteremia while patients were receiving vancomycin therapy and the in vitro bactericidal activity of vancomycin. The secondary objectives were to evaluate the vancomycin MIC as it related to (i) agr function, (ii) time to clearance of MRSA bacteremia, (iii) the probability of MRSA eradication, and (iv) 30-day all-cause mortality.
(This study was previously presented in part at the 43rd Infectious Diseases Society of America Annual Meeting, 7 October 2005, San Francisco, CA, and at the 15th International Society of Anti-Infective Pharmacology International Symposium ([after the Interscience Conference on Antimicrobial Agents and Chemotherapy], 19 December 2005, Washington, DC.)
MATERIALS AND METHODS
Patients.
From a pool of prospectively collected MRSA isolates, 17 agr group II (agr-II) MRSA isolates were randomly selected from 17 different patients who had received vancomycin monotherapy for the treatment of MRSA bacteremia and were matched with 17 non-agr-II MRSA isolates. The analysis focused on all 34 strains from 34 unique patients. The matching was done to determine the effect of agr. The strains were matched on the basis of the date of culture isolation, patient age, and patient renal function (based on the calculated creatinine clearance). These 34 patients were previously enrolled in prospective phase III/IV trials between 1998 and 2002 from six hospitals representing six states (CT, DE, IA, LA, MA, and NY).
Patients with endocarditis, osteomyelitis, and/or a central nervous system infection were excluded. Therapeutic drug monitoring was performed prospectively to obtain target 1-h peak serum concentrations of 28 to 32 mg/liter and trough serum concentrations of 8 to 12 mg/liter. Only patients for whom blood for culture was taken daily were included. The isolates were stored at −70°C and were grown and maintained on Trypticase soy agar for subsequent testing.
Microbiological testing.
Blinded from the clinical data, we determined the reduction in the log10 CFU/ml at 24 h in Mueller-Hinton broth containing vancomycin at 16 μg/ml using an initial bacterial inoculum of approximately 107 to 108 CFU/ml. Bacterial colony counts were determined by serial dilution, as described previously (18). Susceptibility to vancomycin was determined by the broth microdilution methods of the Clinical and Laboratory Standards Institute (formerly NCCLS) (2).
A semiquantitative assessment of the function of agr of the clinical MRSA isolates was performed by determination of the expression of delta-hemolysin on sheep blood agar plates, as described previously (17). Each isolate's delta-hemolysin expression was scored by one investigator (G.S.), who was blinded to the clinical data, as follows: 0, absent; 1, severely depressed/barely detectable 2, easily detectable; 3, increased; 4, exceptionally high.
Definitions.
The probability of MRSA eradication from the blood was determined at the end of therapy. The bacteriological response was classified as eradication of the baseline MRSA pathogen if blood cultures at the end of therapy were negative for MRSA. The time to bacterial clearance (i.e., the number of days to eradication) was defined as the time from the initiation of vancomycin therapy until the first day after the last positive culture on which the blood culture was negative.
Thirty-day all-cause mortality was defined as the patient's status 30 days following the initiation of vancomycin therapy.
Statistics.
Continuous variables were compared by using Kruskal-Wallis analysis of variance. Categorical variables were compared by the chi-square or Fisher exact test, where appropriate. The median time to eradication was compared by using Kaplan-Meier survival analysis and the log rank test. Tree-based modeling was used to determine the breakpoints in the log10 decrease in 24 h (LogDec) as a predictor of bacterial eradication. Iterative nonlinear regression was used to fit a mathematical function to the relationship between the probability of bacteriological success (dependent variable) and the log10 decrease at 24 h (independent variable). Model discrimination was performed by using Akaike's information criterion. We used the following Hill-type model:
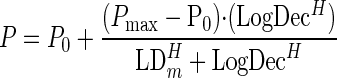 |
where P is the probability of coming to the event (eradication), P0 is the probability of success as LogDec approaches 0, Pmax is the asymptotic maximum probability of success, H is Hill's constant (reflects steepness), LogDec is the log10 decrease at 24 h, and LDm is the LogDec giving a half-maximal effect. Multivariable logistic regression analysis was performed on bacterial eradication by using forward stepping, with a P value of ≤0.10 required for inclusion in the model. Variables identified as being borderline significant in our univariate analysis (P < 0.10) were used for the analysis. All statistical procedures were performed with Systat 11 software (Systat Software Inc., Point Richmond, CA).
RESULTS
We evaluated 34 MRSA bacteremia isolates from 34 patients consisting of 17 agr-II MRSA isolates matched for date of isolation, patient age, and patient renal function to 17 non-agr-II strains. The median age of the 34 patients investigated was 70 years (range, 30 to 87 years), 68% were male, and 50% were in an intensive care unit at the time of onset of the infection (Table 1). The median duration of vancomycin therapy was 16 days. The vancomycin MICs of the 34 strains ranged from 0.5 to 2.0 μg/ml (Table 2), and the killing observed in vitro ranged from 0.82 log10 CFU/ml to 4.92 log10 CFU/ml. The results of analysis of the clinical and the in vitro data are displayed in Table 3 and demonstrate a relationship between the reduction in log10 CFU/ml in vancomycin killing assays, the median day to clearance of bacteremia, and the clearance rate at the end of treatment.
TABLE 1.
Patient and organism characteristicsa
| Characteristic | All patients (n = 34) | Patients infected with:
|
P value | |
|---|---|---|---|---|
| agr-II isolates (n = 17) | Non-agr-II isolates (n = 17) | |||
| Age (yr) | 66 ± 17 (70) | 64 ± 18 (70) | 68 ± 16 (70) | 0.605 |
| Male sex (no. [%] of patients) | 23 (68) | 11 (65) | 12 (71) | 0.714 |
| ICUb (no. [%] of patients) | 17 (50) | 10 (59) | 7 (41) | 0.303 |
| Creatinine clearance (ml/min) | 62 ± 36 (58) | 61 ± 39 (57) | 63 ± 33 (61) | 0.524 |
| No. (%) of isolates for which the vancomycin MIC was: | 0.777 | |||
| 0.5 μg/ml | 13 (38) | 6 (35) | 7 (41) | |
| 1.0 μg/ml | 7 (21) | 3 (14) | 4 (23) | |
| 2.0 μg/ml | 14 (41) | 8 (47) | 6 (35) | |
| No. of days of vancomycin therapy | 19 ± 10 (16) | 21 ± 10 (17) | 17 ± 9 (14) | 0.184 |
The data represent means ± standard deviations (medians), unless stated otherwise.
ICU, the patient was in an intensive care unit at the time of onset of the infection.
TABLE 2.
Vancomycin MIC versus eradication rates
| Vancomycin MIC (μg/ml) | No. of isolates | Median DTEa | Median duration of vancomycin therapy (days) | Eradication rate by EOTb | Median reduction in log10 CFU/ml |
|---|---|---|---|---|---|
| 0.5 | 13 | 6.0 | 13.0 | 10/13 (77) | 3.06 |
| 1.0 | 7 | 9.5 | 17.0 | 5/7 (71) | 3.09 |
| 2.0 | 14 | >15.0c | 18.5 | 3/14 (21) | 2.75 |
DTE, day to eradication.
EOT, end of treatment. The eradication rate data represent the number of patients from the organism was eradicated/total number of patients in the group (percent).
The median time to eradication is greater than 15 days, as only 21% of patients showed clearance of bacteremia.
TABLE 3.
Outcomes by vancomycin bactericidal activity
| Reduction in log10 CFU/ml | No. of isolates | Median DTEa | Median duration of vancomycin therapy (days) | Eradication rate by EOTb |
|---|---|---|---|---|
| <2.00 | 6 | NAc | 16.0 | 0/6 (0) |
| 2.00-2.74 | 10 | 7.5 | 14.0 | 5/10 (50) |
| 2.75-3.50 | 12 | 6.5 | 22.0 | 8/12 (67) |
| >3.50 | 6 | 5.5 | 15.5 | 5/6 (83) |
DTE, day to eradication.
EOT, end of treatment. The eradication rate data represent the number of patients from the organism was eradicated/total number of patients in the group (percent).
NA, not applicable as no patients showed clearance of bacteremia.
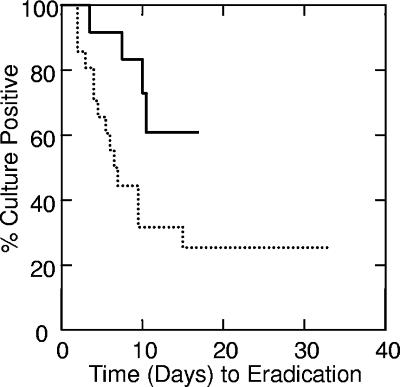
By tree-based modeling, we determined a vancomycin log10 CFU of killing/ml at 24 h of 2.5 to be a categorical breakpoint for both of these outcomes. At a vancomycin log10 CFU of killing/ml at 24 h of <2.5, 4 of 13 patients (31%) had bacterial eradication; at a vancomycin log10 CFU of killing/ml at 24 h of ≥2.5, 14 of 21 (67%) did (P = 0.042). In addition, the median time to clearance of bacteremia for MRSA isolates demonstrating a ≥2.5-log10 reduction in CFU/ml at 24 h (n = 21) was 6.5 days and was in excess of 10.5 days for MRSA isolates demonstrating a <2.5-log10 reduction in CFU/ml at 24 h (n = 13) (P = 0.025) (Fig. 1). In these patients, for whom the vancomycin peak and trough concentrations (and, thus, a steady-state area under the concentration-time curve at 24 h) were prospectively adjusted, there was also an association between vancomycin treatment success, the time to eradication, and the vancomycin MIC (Table 2). We found only a 21% treatment success rate of vancomycin treatment of MRSA bacteremia caused by isolates showing a vancomycin MIC of 2 μg/ml. In addition, the median time to clearance of bacteremia was significantly longer for patients infected with isolates for which the MICs were 2.0 μg/ml than for patients infected with isolates for which the MICs were ≤1.0 μg/ml (P = 0.019).
FIG. 1.
Time to bacterial eradication by reduction in log10 CFU/ml. Upper line, 13 patients with a log10 decrease at 24 h of <2.5; lower dotted line, 21 patients with a log10 decrease at 24 h of ≥2.5 (P = 0.025).
As can be seen in Table 3, it appears that there may be a continuous relationship between vancomycin log10 CFU of killing/ml at 24 h and the probability of eradication. Nonlinear regression found the relationship between the percent probability of bacteriologic eradication (%P) and LogDec to be
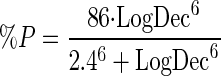 |
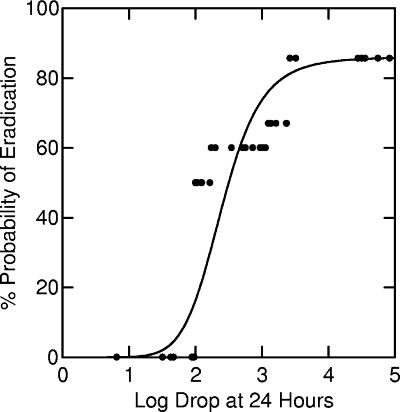
This Hill-type model fit the experimental data well (r2 = 0.85; P < 0.001) (Fig. 2).
FIG. 2.
Nonlinear relationship between percent probability of bacterial eradication and log decrease at 24 h (r2 = 85%; P < 0.001). The datum points represent the proportions of successes in empirically selected cells: <2.0-log decrease, 0% success (n = 6); 2.0- to 2.2-log decrease, 50% success (n = 6); 2.3- to 3.0-log decrease, 60% success (n = 10); 3.1- to 3.4-log decrease, 67% success (n = 6); 3.5- to 4.9-log decrease, 83% success (n = 6).
The 30-day all-cause mortality was also related to in vitro bactericidal activity: 6 of 13 (46%) patients whose isolates showed <2.5-log10 CFU of killing/ml at 24 h died within 30 days, whereas 3 of 21 (14%) patients whose isolates showed ≥2.5-log10 CFU of killing/ml at 24 h died within 30 days (odds ratio = 5.15; P = 0.041).
Although the difference was not statistically significant, patients whose isolates had delta-hemolysin scores of 0 or 1, corresponding to undetectable or barely detectable expression, had more prolonged bacteremia and a decreased probability of clearance of bacteremia. Patients with infections caused by MRSA isolates whose delta-hemolysin scores were 0 or 1 had a median time to eradication of >15 days, whereas the median time to eradication was 6.5 days for patients with infections caused by MRSA isolates whose delta-hemolysin scores were at least 2 (P = 0.28). In addition, 11 of 25 (44%) patients whose isolates had delta-hemolysin scores of 0 or 1 achieved clearance of the bacteremia, whereas 7 of 9 (78%) patients whose isolates had delta-hemolysin scores of at least 2 achieved clearance of the bacteremia (P = 0.086).
Multivariate logistic regression identified both a higher vancomycin MIC and a lower level of bactericidal activity to be associated with a decreased probability of bacterial eradication in patients with MRSA bacteremia (Table 4). These are not the results from the univariate analysis. The odds that vancomycin failed to eradicate MRSA for isolates with a vancomycin MIC of 2 μg/ml were approximately 14 times higher than that for MRSA isolates with vancomycin MICs of ≤1 μg/ml. In addition, the odds that vancomycin failed to eradicate MRSA bacteremia for isolates with a vancomycin log10 CFU of killing/ml at 24 h of <2.5 was approximately seven times more likely than that for isolates with log10 reduction in CFU/ml of at 24 h ≥2.5. Delta-hemolysin expression and the agr group were also entered into the logistic regression model. This data set found a lack of or severely depressed delta-hemolysin expression to be highly associated with higher vancomycin MICs (P = 0.015).
TABLE 4.
Odds ratios for failure of vancomycin to eradicate MRSA
| Characteristic | Odds ratio (95% CIa) | P value |
|---|---|---|
| Vancomycin MIC = 2.0 μg/ml | 14.7 (2.26-100) | 0.005 |
| Reduction in log10 CFU/ml <2.5 | 6.7 (1.03-43.5) | 0.047 |
CI, confidence interval.
DISCUSSION
Until 2000, vancomycin served as the main antimicrobial agent for the treatment of MRSA infections, a role that has increased dramatically since the mid-1980s as a result of the marked increase in the prevalence of this pathogen. This role is now being challenged with the emergence of several new antimicrobial agents with activities against MRSA and with the increased awareness that MRSA infections that were previously deemed susceptible to vancomycin may not respond to therapy (10-13, 18). The latter point has been highlighted by the recent change in the vancomycin MIC breakpoint for susceptibility from 4 μg/ml to 2 μg/ml. These factors have given rise to controversies in the management of MRSA infections, with some clinicians ready to replace vancomycin in favor of newer antimicrobials, while others are not convinced that the expense of the newer antibiotics is justified. Whether the vancomycin MIC susceptibility breakpoint was lowered sufficiently is also seriously questioned in light of prior data showing that bacteremias caused by MRSA demonstrating vancomycin MICs of 1 to 2 μg/ml were treated successfully in less than 10% of cases (18), as well as the findings of this study, in which we found only a 21% treatment success rate for cases of MRSA bacteremia caused by isolates showing a vancomycin MIC of 2 μg/ml.
In this study we attempted to offer additional data on the microbiological phenotype of MRSA with respect to vancomycin susceptibility as it relates to vancomycin treatment efficacy in MRSA bacteremia. We found that increased vancomycin killing of MRSA in vitro was significantly associated with a more rapid clearance of bacteremia with vancomycin therapy and a lower all-cause patient mortality. We defined a nonlinear mathematic relationship between killing by vancomycin in vitro and vancomycin treatment success in MRSA bacteremia.
With respect to MICs, we found that MRSA strains with MICs of 2 μg/ml, which are still considered susceptible by the new MIC susceptibility breakpoint, were associated with a success rate of 21% for vancomycin treatment of MRSA bacteremia. Infections with MRSA strains with MICs of 0.5 μg/ml showed a 77% treatment success rate with vancomycin. From these findings, we support the performance by clinical microbiology laboratories of additional evaluations of vancomycin MICs, for instance, by microdilution or Etest, for MRSA bloodstream isolates beyond the reporting of the categorical results (e.g., susceptible, intermediate, or resistant) offered by certain automated systems (e.g., the MicroScan system). Individual centers using qualitative susceptibility testing systems may wish to survey the vancomycin MIC distribution of their MRSA clinical isolates, and if a high percentage are found to have MICs of ≥2 mg/ml, it may be easier to move to alternative or combination empirical and directed therapy for MRSA bacteremia.
While this was not a prospective study, data from this investigation are consistent with those presented in other reports showing a microbiological correlation with vancomycin treatment success. Such data are providing increasing support for the fact that S. aureus may develop physiological changes, such as tolerance to glycopeptides, that influence vancomycin therapy in patients with serious infections, such as bacteremia, with minimal changes in susceptibility detected by MIC determination. Furthermore, by the time that the organism develops increases in vancomycin MICs that approach 2 μg/ml, the upper limit of the current range of susceptibility, the efficacy of vancomycin for the treatment of MRSA bacteremia appears to be severely compromised.
With the recent approval of daptomycin by the Food and Drug Administration, this agent may be considered an alternative for the treatment of MRSA bacteremia due to organisms with vancomycin MICs of 2 μg/ml. In fact, the recent findings of increased daptomycin MICs in S. aureus isolates exposed to vancomycin in vitro and in vivo, coupled with the possible cross-resistance to platelet-derived microbicidal proteins induced by vancomycin, draw consideration for daptomycin as a first-line agent in favor of vancomycin for some cases of MRSA bacteremia (16).
It is possible that MIC and delta-hemolysin expression are both independent predictors of the efficacy of vancomycin, but it would take a larger study to state that both are independently associated with the outcome. Since the patients in this study were matched by agr group, it is not surprising that agr grouping did not come out of the model.
In summary, this study provides additional evidence in favor of important prognostic information on the efficacy of vancomycin treatment for MRSA bacteremia that can be obtained by vancomycin MIC determination, the in vitro killing activity of vancomycin, and the semiquantitative assessment of delta-hemolysin expression. We provide a nonlinear mathematical relationship of vancomycin killing in vitro and the success of treatment with vancomycin in MRSA bacteremia. More data are necessary to guide clinicians with the optimal incorporation of newer antibiotics in place of vancomycin for the treatment of serious MRSA infections.
Acknowledgments
There were no funding sources for this project.
Author disclosures are as follows: P. A. Moise is a member of the speakers bureau of Cubist, G. Sakoulas is a member of the speakers bureaus of Cubist, Pfizer, and Wyeth, and J. J. Schentag received a research grant from Wyeth.
Footnotes
Published ahead of print on 23 April 2007.
REFERENCES
- 1.Cantoni, L., M. P. Glauser, and J. Bille. 1990. Comparative efficacy of daptomycin, vancomycin, and cloxacillin for the treatment of Staphylococcus aureus endocarditis in rats and role of test conditions in this determination. Antimicrob. Agents Chemother. 342348-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. CLSI document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Cosgrove, S. E., Y. Qi, K. S. Kaye, S. Harbarth, A. W. Karchmer, and Y. Carmeli. 2005. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect. Control Hosp. Epidemiol. 26166-174. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove, S. E., G. Sakoulas, E. N. Perencevich, M. J. Schwaber, A. W. Karchmer, and Y. Carmeli. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 3653-59. [DOI] [PubMed] [Google Scholar]
- 5.Denny, A. E., L. R. Peterson, D. N. Gerding, and W. H. Hall. 1979. Serious staphylococcal infections with strains tolerant to bactericidal antibiotics. Arch. Intern. Med. 1391026-1031. [PubMed] [Google Scholar]
- 6.Engemann, J. J., Y. Carmeli, S. E. Cosgrove, V. G. Fowler, M. Z. Bronstein, S. L. Trivette, J. P. Briggs, D. J. Sexton, and K. S. Kaye. 2003. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 36592-598. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez, C., M. Rubio, J. Romero-Vivas, M. Gonzalez, and J. J. Picazo. 1999. Bacteremic pneumonia due to Staphylococcus aureus: a comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin. Infect. Dis. 291171-1177. [DOI] [PubMed] [Google Scholar]
- 8.Levine, D. P., L. R. Crane, and M. J. Zervos. 1986. Bacteremia in narcotic addicts at the Detroit Medical Center. II. Infectious endocarditis: a prospective comparative study. Rev. Infect. Dis. 8374-396. [DOI] [PubMed] [Google Scholar]
- 9.Levine, D. P., B. S. Fromm, and B. R. Reddy. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann. Intern. Med. 115674-680. [DOI] [PubMed] [Google Scholar]
- 10.Moise, P. A., A. Forrest, M. C. Birmingham, and J. J. Schentag. 2002. The efficacy and safety of linezolid as treatment for Staphylococcus aureus infections in compassionate use patients who are intolerant of, or who have failed to respond to, vancomycin. J. Antimicrob. Chemother. 501017-1026. [DOI] [PubMed] [Google Scholar]
- 11.Moise, P. A., and J. J. Schentag. 2000. Vancomycin treatment failures in Staphylococcus aureus lower respiratory tract infections. Int. J. Antimicrob. Agents 16(Suppl. 1)S31-S34. [DOI] [PubMed] [Google Scholar]
- 12.Moise-Broder, P. A., A. Forrest, M. C. Birmingham, and J. J. Schentag. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43925-942. [DOI] [PubMed] [Google Scholar]
- 13.Moise-Broder, P. A., G. Sakoulas, G. M. Eliopoulos, J. J. Schentag, A. Forrest, and R. C. Moellering, Jr. 2004. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin. Infect. Dis. 381700-1705. [DOI] [PubMed] [Google Scholar]
- 14.Peterson, L. R., A. E. Denny, D. N. Gerding, and W. H. Hall. 1980. Determination of tolerance to antibiotic bactericidal activity on Kirby-Bauer susceptibility plates. Am. J. Clin. Pathol. 74645-650. [DOI] [PubMed] [Google Scholar]
- 15.Rahal, J. J., Jr., Y. K. Chan, and G. Johnson. 1986. Relationship of staphylococcal tolerance, teichoic acid antibody, and serum bactericidal activity to therapeutic outcome in Staphylococcus aureus bacteremia. Am. J. Med. 8143-52. [DOI] [PubMed] [Google Scholar]
- 16.Sakoulas, G., G. M. Eliopoulos, V. G. Fowler, Jr., R. C. Moellering, Jr., R. P. Novick, N. Lucindo, M. R. Yeaman, and A. S. Bayer. 2005. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlate with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob. Agents Chemother. 492687-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakoulas, G., G. M. Eliopoulos, R. C. Moellering, Jr., C. Wennersten, L. Venkataraman, R. P. Novick, and H. S. Gold. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 461492-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 422398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small, P. M., and H. F. Chambers. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 341227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]