Abstract
Heterocysts, formed when filamentous cyanobacteria, such as Anabaena sp. strain PCC 7120, are grown in the absence of combined nitrogen, are cells that are specialized in fixing atmospheric nitrogen (N2) under oxic conditions and that transfer fixed nitrogen to the vegetative cells of the filament. Anabaena sp. mutants whose sepJ gene (open reading frame alr2338 of the Anabaena sp. genome) was affected showed filament fragmentation and arrested heterocyst differentiation at an early stage. In a sepJ insertional mutant, a layer similar to a heterocyst polysaccharide layer was formed, but the heterocyst-specific glycolipids were not synthesized. The sepJ mutant did not exhibit nitrogenase activity even when assayed under anoxic conditions. In contrast to proheterocysts produced in the wild type, those produced in the sepJ mutant still divided. SepJ is a multidomain protein whose N-terminal region is predicted to be periplasmic and whose C-terminal domain resembles an export permease. Using a green fluorescent protein translationally fused to the carboxyl terminus of SepJ, we observed that in mature heterocysts and vegetative cells, the protein is localized at the intercellular septa, and when cell division starts, it is localized in a ring whose position is similar to that of a Z ring. SepJ is a novel composite protein needed for filament integrity, proper heterocyst development, and diazotrophic growth.
All cyanobacteria perform oxygenic, plant-type photosynthesis. In some filamentous cyanobacteria, heterocysts develop from vegetative cells when the filaments sense nitrogen deprivation. Heterocysts are differentiated cells that are specialized for the fixation of N2 under oxic conditions (11, 28). Two regulators necessary for the initiation of heterocyst differentiation are NtcA, the nitrogen control transcriptional regulator of cyanobacteria (10), and the development-specific regulator HetR (27). Another regulator is PatS, a small peptide produced by developing heterocysts that inhibits the differentiation of nearby vegetative cells (29). Heterocyst development includes the deposition, outside the outer membrane of the cell (8, 28), of a polysaccharide layer and of a more internal glycolipid layer. The outer membrane, which is continuous in filamentous cyanobacteria (8), may help to join the cells physiologically. However, proteins required for the integrity of intercellular junctions also appear to exist; mutations resulting in a filament fragmentation phenotype have been reported (1).
The mature heterocyst does not perform oxygenic photosynthesis but, instead, carries out nitrogen fixation catalyzed by nitrogenase, an enzyme that is inactivated by oxygen (28). Thus, the N2-fixing filament consists of two metabolically complementary cell types, photosynthetic vegetative cells and N2-fixing heterocysts, in a ratio of approximately 10 to 1 for Anabaena sp. strain PCC 7120 (hereinafter, Anabaena sp.) and some related cyanobacteria. Net fluxes of carbon (from vegetative cells to heterocysts) and nitrogen (from heterocysts to vegetative cells) take place in the filament. The mechanisms by which metabolites and regulatory molecules such as PatS are exchanged between the two cell types are unknown. One proposed hypothesis predicts transfer through an extracytoplasmic route, the periplasm, with the participation of uptake transporters and exporters in the two types of cells (8). We show here that the product of Anabaena sp. open reading frame alr2338 is located at the site of the division ring and the intercellular septa and that it is needed for filament integrity, proper heterocyst development, and diazotrophic growth.
MATERIALS AND METHODS
Bacterial strains.
Anabaena (Nostoc) sp. strain PCC 7120 was used in this work. Mutant strains 216 (2) and CSE2 (9) have previously been described. An internal fragment of open reading frame alr2338 (sepJ) was amplified by PCR using the primers 5′-AAT CTC TCC CAG CAG TTG TCT C-3′ and 5′-GAA CAG AAG CCA GGA TAA CAC C-3′, with DNA from wild-type Anabaena sp. as the template, and cloned into plasmid pGEM-T Easy (Promega Corp.). Insertion of a PstI-cat-erm-oriT(RK2)-PstI cassette from pRL2665b (12) into the unique PstI site of the resulting plasmid yielded plasmid pRL2787a. pRL2787a was transferred to Anabaena sp. by triparental mating with selection for erythromycin (Em) resistance, yielding mutant strain SR2787a. Single-crossover homologous recombination with sepJ was confirmed by Southern blotting and by PCR with primers 5′-TAA CCT GAC GAT CGC CTT TAA T-3′ and 5′-GAT GGA CTT TAG CCG CAC AT-3′.
The sepJ-green fluorescent protein gene (gfp) strain CSAM137 was constructed as follows. Plasmid pCSAM134 contains a PCR-amplified, 515-bp DNA fragment from the C-terminal portion of sepJ cloned into the pGEM-T Easy plasmid. The amplified fragment, generated with primers 5′-TTT TCT GTG GTG AGG TGC-3′ (alr2338-2) and 5′-GGC AGG TTT GTT GAT ATC TTG-3′ (alr2338-EcoV-2), corresponds to nucleotides −527 to −13 with respect to the translational stop site of sepJ and contains an EcoRV site (underlined in primer alr2338-EcoV-2) introduced into the sequence encoding amino acids 9 and 10 from the stop codon. A promoterless gfp gene was excised from pCSEL19 (18) as an EcoRV-ApaI fragment and introduced into EcoRV- and ApaI-digested pCSAM134, producing pCSAM135. A PstI fragment from pCSAM135, containing the translational fusion between the C-terminal region of sepJ and the gfp gene, was cloned into the polylinker of pCSV3 (23), yielding pCSAM137. Plasmid pCSAM137 was transferred to Anabaena sp. by conjugation as previously described, and colonies resistant to streptomycin and spectinomycin were selected. The entire plasmid was integrated by single-crossover homologous recombination, resulting in an altered sepJ gene, with the gfp gene substituting for the last 27 bp of the gene. The genomic structure was confirmed by PCR performed with primers 5′-CGA ATG TAT AAC CAA CAG CAG C-3′ (alr2338-3; corresponding to nucleotides 505 to 526 with respect to the translational start site of the sepJ gene) and 5′-CAA GAA TTG GGA CAA CTC C-5′ (gfp-4). Using DNA from the exconjugants as the template, these oligonucleotide primers amplify a DNA fragment of 1,774 bp only if the integration took place in the homologous sepJ region. The lack of wild-type chromosomes was confirmed with primers 5′-GAA TTC TCA CCA GTC CAA GTG-3′ (3SepJEcoRI-B) and 5′-ACT AGT CCG GAA CTT ATC TGC-3′ (3SepJSpeI-C); these oligonucleotides amplify a DNA fragment of 1,670 bp only if wild-type chromosomes are present in the template DNA.
Northern analysis.
Total RNA from Anabaena sp. and its derivatives was isolated as previously described from cultures grown or incubated with the indicated nitrogen sources and bubbled with air supplemented with 1% CO2 (20). Northern analysis was carried out as described previously (19).
Microscopy.
For standard light microscopy, Anabaena sp. and SR2787a were grown in BG11 medium (in the presence of 5 μg Em/ml for the mutant) and incubated in BG110 medium (which lacks a source of combined nitrogen) for 48 h at 30°C in the light (75 μE m−2 s−1) on a rotary shaker (90 rpm). For staining with Alcian blue, a cell suspension was mixed 1:2 with a 1% Alcian blue (Sigma) solution.
For transmission electron microscopy, strain SR2787a was grown in BG11 medium in the presence of 5 μg Em/ml and incubated in BG110 medium for 48 h in the light (75 μE m−2 s−1) on a rotary shaker (90 rpm). Filaments were harvested and fixed with glutaraldehyde and KMnO4, dehydrated with increasing concentrations of ethanol, and embedded in EPON, and ultra-thin sections were stained with uranyl acetate and lead citrate (7). The samples were examined with a Zeiss EM10C transmission electron microscope at 80 kV.
For confocal microscopy, strain CSAM137 was grown in BG110, BG110 supplemented with 4 mM NH4Cl plus 8 mM TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] buffer, pH 7.5, or BG11 medium in the presence of 2 μg streptomycin/ml and 2 μg spectinomycin/ml. The filaments were embedded in 0.25% agarose, and fluorescence was observed using a Leica HCX PLAN-APO, 63×, 1.4-numerical-aperture, oil immersion objective attached to a Leica TCS SP2 confocal laser scanning microscope. GFP was excited using 488-nm irradiation from an argon ion laser. Fluorescent emission was monitored by collection across windows of 500 to 570 nm (GFP imaging) and 630 to 700 nm (cyanobacterial autofluorescence).
Lipid and nitrogenase analyses.
Filaments of Anabaena sp. and SR2787a grown in BG11 medium (in the presence of 5 μg Em/ml for the mutant) were harvested, washed with nitrogen-free medium, and incubated in BG110 medium for 48 h. Lipids were extracted from whole filaments with chloroform-methanol (2:1, vol/vol), concentrated under N2, and chromatographed on thin layers of silica gel (22). Heterocyst glycolipids were identified as described previously (26). Nitrogenase activity was determined by the acetylene reduction technique as described previously (17). For anaerobic assays, 10 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea was added to the cell suspension, the flask containing the cells was sealed with a rubber stopper, bubbled with argon for 15 min, and further incubated, under culture conditions, for 1 h before the assay was started by the addition of acetylene.
RESULTS
Identification of the sepJ gene.
Fox− mutants are defined as those that are unable to fix N2 under oxic conditions. In a set of 1,076 random Fox− transposon mutants (6), 21 transpositions were localized in alr2338. All of seven that were tested were complemented upon recombination with a pUC18 derivative, pRL2689, that bears a partial Sau3AI fragment of Anabaena sp. chromosomal DNA from bp 2812776 to 2821689 (14). To test further whether the Fox− phenotype resulted from the inactivation of alr2338, an alr2338 mutation was constructed by single recombination using a plasmid, pRL2787a, that carries a 1,200-bp internal fragment from bp 2818917 to 2820116 (corresponding to the protein fragment indicated in Fig. 1) of alr2338 (bp 2818434 to 2820689). The resulting mutant, strain SR2787a, reproduced the Fox− phenotype and was used for further analysis. A Southern blot of wild-type and mutant DNAs digested with EcoRV and probed with the same 1,200-bp fragment showed the expected ∼5.2-kb fragment in the wild type and the replacement of this fragment by an ∼12-kb fragment in the mutant (data not shown). Insertion of pRL2787a at the expected site in the genome was further confirmed by PCR of the wild-type and the mutant DNAs as templates with primers near, but external to, the 1,200-bp fragment in the chromosome (data not shown). The mutant phenotype cannot have resulted from a polar effect on the 3′ gene hetR, because SR2787a forms proheterocysts (see below) and a hetR mutant does not (2) and because the expression of hetR is known to be separately promoted (3, 20). We designate the alr2338 gene sepJ (from septal protein [see below]).
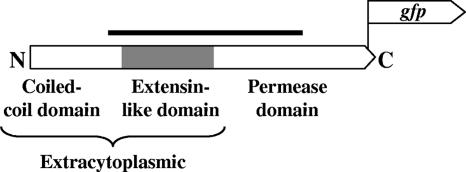
FIG. 1.
Scheme showing the different domains of SepJ and the approximate location of the fusion to GFP. The solid bar denotes the region of the protein that corresponds to the DNA fragment cloned for the generation of mutant SR2787a.
The 751-amino-acid, predicted protein SepJ comprises (i) a 200-residue N-terminal coiled-coil region such as is normally engaged in protein-protein interactions; (ii) an internal, 211-amino-acid sequence that is rich in Pro and Ser and shows similarity to plant extensins and mammalian mucins; and (iii) an ∼340-amino-acid C-terminal portion that is homologous to proteins in the drug/metabolite exporter (DME) family of Bacteria and Archaea (13), including the RhtA amino acid exporter of Escherichia coli (15) (Fig. 1). Protein topology and subcellular localization predictions, made using programs that allow predictions for gram-negative bacteria (http://www.expasy.org/tools/#topology), suggest that the 411-residue N-terminal region of SepJ is extracytoplasmic and that the C-terminal portion is a cytoplasmic membrane protein. Most predicted topologies place the carboxyl terminus of the protein in the cytoplasm, but whether the protein bears 9 or 11 α-helical transmembrane segments is uncertain.
Phenotype of a sepJ mutant.
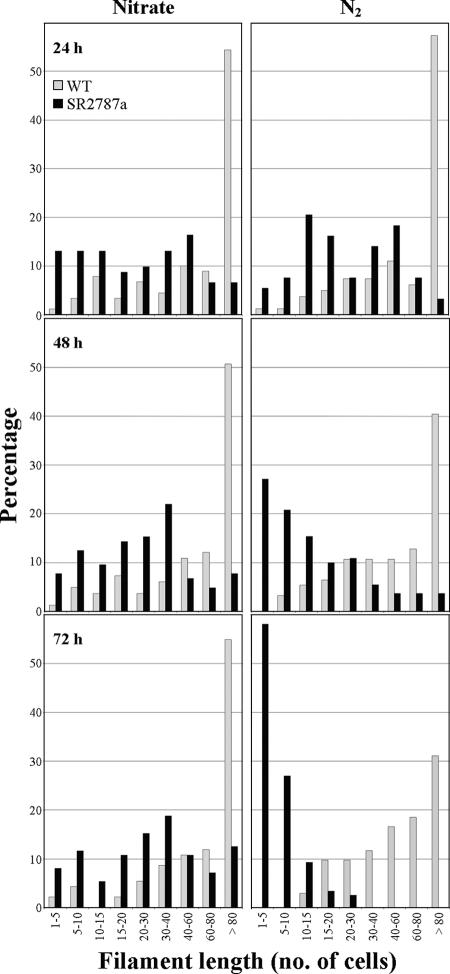
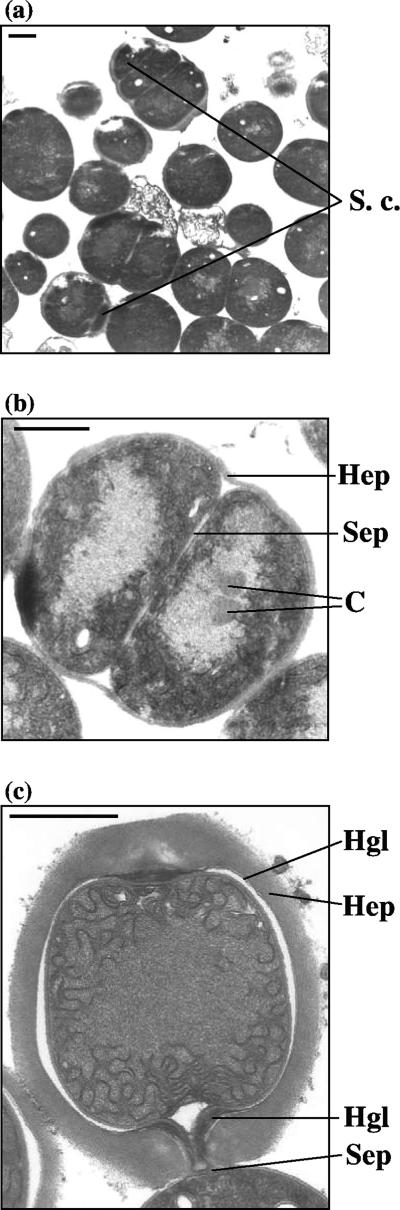
In nitrate-containing liquid medium, the wild-type strain forms mostly long filaments whereas the sepJ mutant SR2787a forms filaments with a somewhat homogeneous distribution of lengths from a few cells to more than 80 cells (Fig. 2). When transferred to medium without combined nitrogen, filaments of the wild type remain long whereas filaments of the mutant strain fragment, within a few days, into filaments that are mostly ≤10 cells in length (Fig. 2). When prepared for transmission electron microscopy, filaments of the mutant strain fragment even more extensively (Fig. 3a). Many of its cells appear to initiate differentiation, with deposition of what may be a heterocyst polysaccharide layer (Fig. 3b). Some cells in cultures of the mutant incubated for 48 h without combined nitrogen were stained with Alcian blue, suggesting that they had produced extracellular polysaccharides, although staining was less intense than that shown by wild-type heterocysts of a 48-h induction (results not shown). However, a polysaccharide-like shell was the only morphological sign of differentiation that was evident in nitrogen-deprived SR2787a. Unlike in wild-type heterocysts from a 48-h induction (Fig. 3c), intracellular membranes were not reorganized, carboxysomes were not degraded, and no heterocyst glycolipids were deposited in the mutant cells (Fig. 3). Consistently, no heterocyst-specific glycolipids were detected in extracts of SR2787a cells incubated for 48 h in the absence of combined N (Fig. 4). Cells of SR2787a continued to divide inside the newly synthesized envelope, a phenomenon never seen in the wild type, giving rise to groups of two or three encapsulated cells (Fig. 3a and b). The shape of these cells differed from that of normal vegetative cells, with occasional production of very small cells (Fig. 3a), and the septum between the cells was broader than between vegetative cells or between heterocysts and neighboring vegetative cells in the wild type (Fig. 3 and not shown). In conclusion, SepJ enhances the integrity of the filament, especially when it is nitrogen deprived, and is necessary for heterocyst maturation.
FIG. 2.
Filament lengths of Anabaena sp. and SR2787a. Filaments grown in BG11 medium (in the presence of 5 μg Em/ml for the mutant) were harvested, washed with nitrogen-free medium, and resuspended in BG11 or BG110 medium with antibiotic for the mutant. After incubation for the indicated times at 30°C in the light on a shaker, samples, which were taken with great care to prevent disruption, were visualized by standard light microscopy. Ninety to 120 filaments were counted in each case. WT, wild type.
FIG. 3.
Ultrastructures of strain SR2787a incubated in nitrogen-free medium for 48 h (a, b) and of a heterocyst of wild-type Anabaena sp. induced for 48 h in nitrogen-free medium (c). Size markers, 1 μm. C, carboxysomes; Hep, heterocyst polysaccharide layer; Hgl, heterocyst glycolipid layer; Sep, septum; S. c., small cell.
FIG. 4.
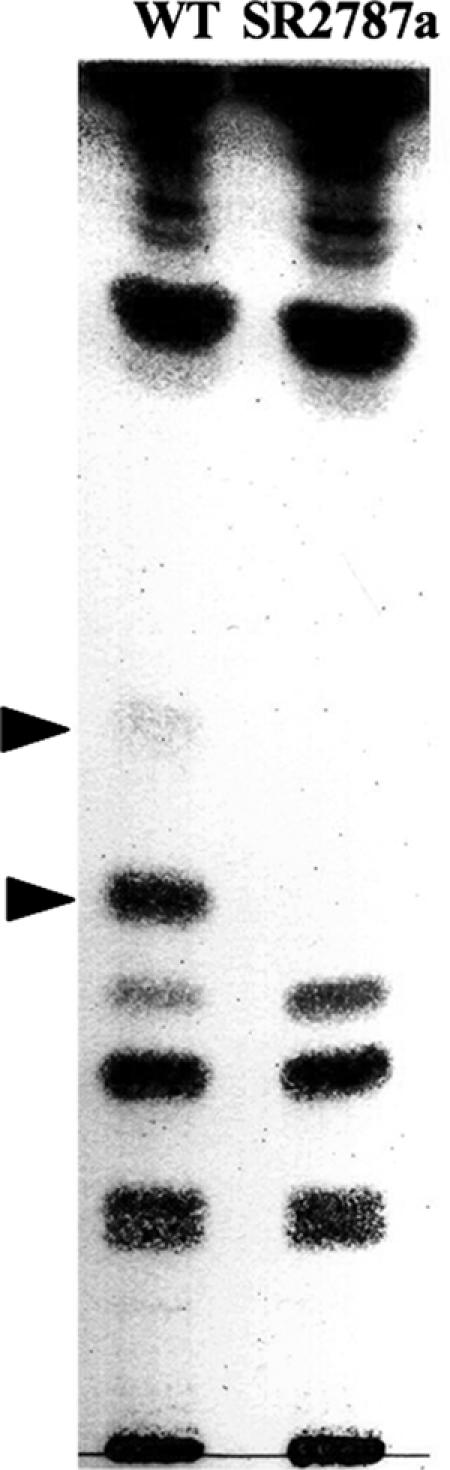
Lack of heterocyst glycolipids in mutant strain SR2787a. Filaments of Anabaena sp. (wild type [WT]) and SR2787a incubated in BG110 medium for 48 h were harvested, and lipids were extracted and analyzed by thin-layer chromatography. Arrowheads indicate heterocyst-specific glycolipids.
Some Anabaena mutants that produce defective heterocysts show nitrogenase activity when assayed under micro-oxic conditions, a Fix+ phenotype (28). To check whether the proheterocysts observed in cultures of SR2787a could develop a heterocyst-like metabolism independently of their abortive morphological differentiation, we tested for nitrogenase activity under both oxic and anoxic conditions in cells incubated for 48 h without combined nitrogen. Under assay conditions in which the wild type produced about 3.9 μmol ethylene·mg chlorophyll−1·h−1 (aerobic activity) and 6.6 μmol ethylene·mg chlorophyll−1·h−1 (anaerobic activity), no production of ethylene was detected with mutant strain SR2787a, which thus exhibits a Fix− phenotype. Therefore, not only morphological heterocyst maturation but also metabolic differentiation was arrested as a result of the inactivation of sepJ.
Expression of sepJ and localization of the SepJ protein.
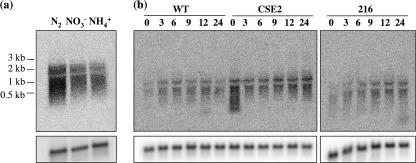
Because the SR2787a mutant shows some fragmentation even during growth on nitrate, we investigated the growth conditions under which sepJ is expressed. We performed Northern blot analysis with two different probes of sepJ and with RNA isolated from wild-type cells grown with N2, nitrate, or ammonium as the nitrogen source. Hybridization was strongest with RNA from the N2-grown cells, but it was also appreciable with RNA from ammonium- and nitrate-grown cells (data generated with the longer probe are shown in Fig. 5a). Although some signals that corresponded to degraded RNA were observed, signals corresponding to the largest mRNA molecules indicated that the gene was expressed as a monocistronic transcript of about 2.3 kb. When wild-type cells were grown with ammonium and incubated for up to 24 h in the absence of combined nitrogen, expression increased during the period of 3 to 12 h of nitrogen deprivation (data generated with the shorter probe are shown in Fig. 5b). To test whether this increase in expression requires the heterocyst development regulators NtcA and HetR, RNA isolated from mutants of the corresponding genes was used. Expression in the ntcA mutant CSE2 (9) was greater than in the wild type at all time points tested. In contrast, expression in the hetR mutant 216 (2) was similar to that in the wild type. Thus, the expression of sepJ does not require these heterocyst development regulators, although it is affected by NtcA.
FIG. 5.
Expression of sepJ. (a) RNA was isolated from bubbled cultures of Anabaena sp. grown with ammonium, nitrate, or N2 as the nitrogen source and hybridized with a probe containing nucleotides −35 to +1475 of sepJ with respect to the ATG gene start codon. (b) RNA was isolated from bubbled cultures of wild-type Anabaena sp. (WT) and its derivatives CSE2 (ntcA) and 216 (hetR). Strains were either grown with ammonium (time zero) or grown with ammonium and incubated in the absence of combined nitrogen for the indicated numbers of hours. RNA was hybridized with a probe containing nucleotides +505 through +1475 of sepJ with respect to the ATG gene start codon. In both cases, hybridization with a probe of the rnpB gene was used as a loading and transfer control (lower panels). Similar results were obtained when the hybridization shown in panel a was performed with the shorter probe.
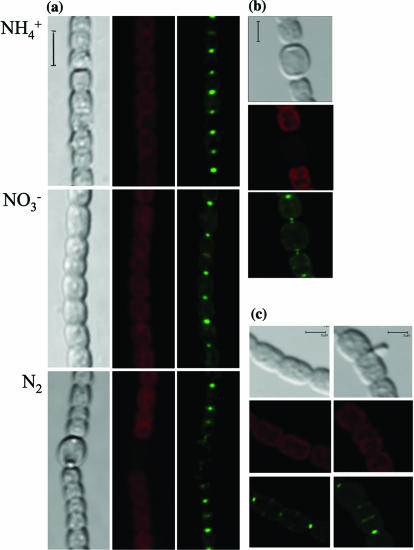
The in vivo localization of SepJ was studied using a SepJ-GFP translational fusion protein in which GFP was added at amino acid 742 of the protein, 9 amino acids from the C terminus (Fig. 1). According to the predicted topology of SepJ, this construct should place GFP in the cytoplasm adjacent to the cytoplasmic face of the membrane. In strain CSAM137 carrying the sepJ-gfp fusion, the fusion gene substituted for the sepJ gene so that no intact sepJ gene was present and sepJ-gfp would be transcribed from the sepJ promoter (see Materials and Methods for details). Strain CSAM137 developed heterocysts and grew diazotrophically, indicating that the SepJ-GFP polypeptide retains SepJ function. Fluorescence from SepJ-GFP was observed in the septa joining vegetative cells in ammonium-, nitrate-, and N2-grown filaments (Fig. 6a) and also at the apposed ends of vegetative cells and heterocysts in N2-grown filaments (Fig. 6b). Only one spot of increased fluorescence was observed between each pair of vegetative cells, suggesting that the proteins from separate cells are closely clustered in those septa. However, two separate spots of increased fluorescence could be distinguished in the junctions between vegetative cells and heterocysts (Fig. 6b), suggesting that there are separate foci of the protein in the vegetative-cell cytoplasmic membrane and within the neck of the heterocyst. Perhaps the poles of adjacent cells contribute to the cluster of that protein, with the clusters closely appressed in the case of the vegetative cells but more disjointed when the two types of cells are juxtaposed.
FIG. 6.
Subcellular localization of SepJ-GFP. (a) Light transmission (left panels), cyanobacterial autofluorescence (middle panels), and GFP fluorescence (right panels) micrographs of ammonium-grown, nitrate-grown, and N2-grown filaments of strain CSAM137. Size marker, 6 μm. Heterocysts do not autofluoresce. (b, c) Light transmission (top panels), cyanobacterial autofluorescence (middle panels), and GFP fluorescence (bottom panels) micrographs of cells of N2-grown (b) or nitrate-grown (c) filaments of strain CSAM137. Size markers, 3 μm.
In cells in the process of division, fluorescence was also observed in what appeared to be a ring at the site of division. Figure 6c shows examples of dividing cells in two stages: in the left panels, two dividing cells show the GFP fluorescence at the periphery, midway between the ends of the cells; in the right panels, the GFP fluorescence appears to originate at a division plane as the dividing cell constricts.
DISCUSSION
Inactivation of Anabaena sp. open reading frame alr2338 (gene sepJ) results in a complex phenotype that includes filament fragmentation, arrested heterocyst development (with lack of heterocyst glycolipid production), and the lack of nitrogenase activity even under anoxic conditions (Fix− phenotype). This gene (and an identical mutant phenotype) has also recently been identified in an independent work, in which the authors denoted alr2338 fraG (21). We prefer the name sepJ because it better reflects our findings on the subcellular localization of the product of alr2338.
We have shown that a SepJ-GFP fusion protein is localized at the poles of mature Anabaena sp. cells in the intercellular septa. Because the SepJ-GFP protein retains SepJ function, we think that the observed localization of the fusion protein corresponds to the physiological position of SepJ. The fragmentation phenotype of the sepJ inactivation mutants and the localization of SepJ in the intercellular septa suggest that this protein plays a role in linking cells of the Anabaena sp. filament. In bacteria, high fluorescence of GFP fused to the C-terminal region of cytoplasmic membrane proteins is observed only if the GFP is located in the cytoplasm as a result of the particular topology of the investigated protein (5). Therefore, the fact that GFP fluorescence was observed with the construct used here is consistent with predictions of a SepJ topology in which the carboxyl terminus of the protein is cytoplasmic and the GFP is in the cytoplasm, anchored to the cytoplasmic face of the cytoplasmic membrane by the C-terminal region of SepJ. The N-terminal extracytoplasmic domains of SepJ may join cells by means of protein-protein interactions, since coiled-coil domains can bind proteins. Whether SepJ monomers produced by neighboring cells interact and whether other proteins participate with SepJ in a cell-to-cell joining complex are questions that deserve future research. Direct SepJ interactions are more likely between vegetative cells than between vegetative cells and heterocysts, since separate loci of GFP fluorescence from the SepJ-GFP fusion protein are observed in the latter case.
Consistent with the requirement of its protein product for diazotrophic growth, the sepJ gene is expressed at higher levels in N2-grown filaments than in filaments grown in the presence of combined nitrogen (Fig. 5). However, expression under the latter conditions is substantial and apparently permits sufficient accumulation of SepJ-GFP that the resulting fluorescence can be observed in ammonium- and nitrate-grown cells as well as in N2-grown cells (Fig. 6). Although Nayar et al. have shown a possible moderate increase in expression in mature heterocysts (21), increased expression in the absence of combined nitrogen is dependent on neither NtcA nor HetR. Why NtcA appears, rather, to have a negative effect on expression is unknown but may be related to the presence of a canonical NtcA binding site (10) within the coding sequence of sepJ. The expression data and the evident fragmentation of the sepJ mutant SR2787a in cultures with combined nitrogen are consistent with SepJ having a role also in nitrogen-replete vegetative cells. The question then arises whether the fragmentation and developmental phenotypes are related or are independent effects of the inactivation of sepJ.
Mutations in other genes are known to lead to the fragmentation of filaments of Anabaena sp. (1), and therefore other proteins may have a related role in filament integrity. The phenotype of a fraC mutant is similar to that of the sepJ mutant in that the mutant fragments and exhibits cell division after the synthesis of a putative heterocyst polysaccharide layer, but whereas a sepJ mutant is Fox− and Fix−, a fraC mutant is Fox− but Fix+, i.e., it exhibits anaerobic nitrogenase activity (1). Filaments of a fraH mutant also fragment, but mature heterocysts are observed in combined nitrogen-free cultures of this mutant (1). Therefore, fragmentation does not necessarily lead to the severe heterocyst development arrest observed in the Fix− sepJ mutant.
The similarity of the C-terminal domain of SepJ to transporters, and the localization of this protein at the junction between cells, raises the possibility that it is involved in the movement of metabolites or regulatory compounds between neighboring cells. This movement could take place directly from cell to cell, mediated by a junction complex that includes SepJ, or could involve a periplasmic route (8). Using a hetR promoter-gfp fusion construct as a reporter for the start of heterocyst differentiation, Nayar et al. (21) showed that SepJ is not needed for pattern formation. However, their and our results show that development is arrested between the developmental steps of the production of heterocyst cell wall polysaccharides and of heterocyst-specific glycolipids. Perhaps the multidomain SepJ has two differentiated functions: (i) a function in joining cells in both combined nitrogen-replete and -free media, possibly related to the extracytoplasmic region of the protein, and (ii) a function in heterocyst differentiation, possibly related to the permease portion of the protein.
Heterocyst-forming cyanobacteria are truly multicellular organisms in which different cells, namely, heterocysts and vegetative cells, contribute complementary functions to the performance of the organism as a whole. The results presented in this work illustrate the novel finding of the subcellular localization of the SepJ protein that has a role in filament integrity and heterocyst development. Our results suggest that SepJ has a specific localization in the cytoplasmic membrane, where it forms a ring during cell division; when cell division has been completed, it is localized at the center of the septum, occupying a position corresponding to a cell pole (Fig. 6). The apparently ring-like distribution of SepJ during division suggests that SepJ may interact with the divisional Z ring observed in cyanobacteria (16, 25). The actin-related bacterial cytoskeletal protein MreB is involved in subcellular protein localization (4), and the MinCDE system regulates septation in diverse bacteria (24). The Anabaena sp. genome (14) contains genes encoding homologues to these proteins, and their role, if any, in the subcellular localization of SepJ would be worth studying. More generally, how SepJ is localized during cell division remains to be investigated.
Acknowledgments
We thank Enrique Martínez-Force for help with lipid analysis and Alicia Orea for help with the confocal microscope.
Our work was supported by grants BFU2005-07672 (E.F.) and BFU2004-00872 (A.H.) from the Ministerio de Educación y Ciencia, Spain, grants MCB0090232 from the U.S. NSF and DOE-FG02-91ER20021 from the U.S. DOE (C.P.W.), and Germany-Spain joint travel grant HA2003-0159 (A.M.M.-P. and I.M.).
Footnotes
Published ahead of print on 16 March 2007.
REFERENCES
- 1.Bauer, C. C., W. J. Buikema, K. Black, and R. Haselkorn. 1995. A short-filament mutant of Anabaena sp. strain PCC 7120 that fragments in nitrogen-deficient medium. J. Bacteriol. 177:1520-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buikema, W. J., and R. Haselkorn. 1991. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 5:321-330. [DOI] [PubMed] [Google Scholar]
- 3.Buikema, W. J., and R. Haselkorn. 2001. Expression of the Anabaena hetR gene from a copper-regulated promoter leads to heterocyst differentiation under repressing conditions. Proc. Natl. Acad. Sci. USA 98:2729-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carballido-López, R. 2006. Orchestrating bacterial cell morphogenesis. Mol. Microbiol. 60:815-819. [DOI] [PubMed] [Google Scholar]
- 5.Drew, D., D. Sjostrand, J. Nilsson, T. Urbig, C. N. Chin, J. W. de Gier, and G. von Heijne. 2002. Rapid topology mapping of Escherichia coli inner-membrane proteins by prediction and PhoA/GFP fusion analysis. Proc. Natl. Acad. Sci. USA 99:2690-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan, Q., S. Lechno-Yossef, S. Ehira, T. Kaneko, M. Ohmori, N. Sato, S. Tabata, and C. P. Wolk. 2006. Signal transduction genes required for heterocyst maturation in Anabaena sp. strain PCC 7120. J. Bacteriol. 188:6688-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiedler, G., M. Arnold, S. Hannus, and I. Maldener. 1998. The DevBCA exporter is essential for envelope formation in heterocysts of the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 27:1193-1202. [DOI] [PubMed] [Google Scholar]
- 8.Flores, E., A. Herrero, C. P. Wolk, and I. Maldener. 2006. Is the periplasm continuous in filamentous multicellular cyanobacteria? Trends Microbiol. 14:439-443. [DOI] [PubMed] [Google Scholar]
- 9.Frías, J. E., E. Flores, and A. Herrero. 1994. Requirement of the regulatory protein NtcA for the expression of nitrogen assimilation and heterocyst development genes in the cyanobacterium Anabaena sp. PCC 7120. Mol. Microbiol. 14:823-832. [DOI] [PubMed] [Google Scholar]
- 10.Herrero, A., A. M. Muro-Pastor, and E. Flores. 2001. Nitrogen control in cyanobacteria. J. Bacteriol. 183:411-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero, A., A. M. Muro-Pastor, A. Valladares, and E. Flores. 2004. Cellular differentiation and the NtcA transcription factor in filamentous cyanobacteria. FEMS Microbiol. Rev. 28:469-487. [DOI] [PubMed] [Google Scholar]
- 12.Huang, G., Q. Fan, S. Lechno-Yossef, E. Wojciuch, C. P. Wolk, T. Kaneko, and S. Tabata. 2005. Clustered genes required for the synthesis of heterocyst envelope polysaccharide in Anabaena sp. strain PCC 7120. J. Bacteriol. 187:1114-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack, D. L., N. M. Yang, and M. H. Saier, Jr. 2001. The drug/metabolite transporter superfamily. Eur. J. Biochem. 268:3620-3639. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko, T., Y. Nakamura, C. P. Wolk, T. Kuritz, S. Sasamoto, A. Watanabe, M. Iriguchi, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, M. Kohara, M. Matsumoto, A. Matsuno, A. Muraki, N. Nakazaki, S. Shimpo, M. Sugimoto, M. Takazawa, M. Yamada, M. Yasuda, and S. Tabata. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:227-253. [DOI] [PubMed] [Google Scholar]
- 15.Livshits, V. A., N. P. Zakataeva, V. V. Aleshin, and M. V. Vitushkina. 2003. Identification and characterization of the new gene rhtA involved in threonine and homoserine efflux in Escherichia coli. Res. Microbiol. 154:123-135. [DOI] [PubMed] [Google Scholar]
- 16.Miyagishima, S.-Y., C. P. Wolk, and K. W. Osteryoung. 2005. Identification of cyanobacterial cell division genes by comparative and mutational analyses. Mol. Microbiol. 56:126-143. [DOI] [PubMed] [Google Scholar]
- 17.Montesinos, M. L., A. Herrero, and E. Flores. 1995. Amino acid transport systems required for diazotrophic growth in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 177:3150-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muro-Pastor, A. M., E. Olmedo-Verd, and E. Flores. 2006. All4312, an NtcA-regulated two-component response regulator in Anabaena sp. strain PCC 7120. FEMS Microbiol. Lett. 256:171-177. [DOI] [PubMed] [Google Scholar]
- 19.Muro-Pastor, A. M., A. Valladares, E. Flores, and A. Herrero. 1999. The hetC gene is a direct target of the NtcA transcriptional regulator in cyanobacterial heterocyst development. J. Bacteriol. 181:6664-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muro-Pastor, A. M., A. Valladares, E. Flores, and A. Herrero. 2002. Mutual dependence of the expression of the cell differentiation regulatory protein HetR and the global nitrogen regulator NtcA during heterocyst development. Mol. Microbiol. 44:1377-1385. [DOI] [PubMed] [Google Scholar]
- 21.Nayar, A. S., H. Yamaura, R. Rajagopalan, D. D. Risser, and S. M. Callahan. 2007. FraG is necessary for filament integrity and heterocyst maturation in the cyanobacterium Anabaena sp. strain PCC 7120. Microbiology 153:601-607. [DOI] [PubMed] [Google Scholar]
- 22.Nichols, B. W., and B. J. B. Wood. 1968. New glycolipid specific to nitrogen-fixing blue-green algae. Nature 217:767-768. [Google Scholar]
- 23.Olmedo-Verd, E., A. M. Muro-Pastor, E. Flores, and A. Herrero. 2006. Localized induction of the ntcA regulatory gene in developing heterocysts of Anabaena sp. strain PCC 7120. J. Bacteriol. 188:6694-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothfield, L., A. Taghbalout, and Y.-L. Shih. 2005. Spatial control of bacterial division-site placement. Nat. Rev. Microbiol. 3:959-968. [DOI] [PubMed] [Google Scholar]
- 25.Sakr, S., R. Jeanjean, C.-C. Zhang, and T. Arcondéguy. 2006. Inhibition of cell division suppresses heterocyst development in Anabaena sp. strain PCC 7120. J. Bacteriol. 188:1396-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkenbach, F., C. P. Wolk, and M. Jost. 1972. Lipids of membranes and of the cell envelope in heterocysts of a blue-green alga. Planta 107:69-80. [DOI] [PubMed] [Google Scholar]
- 27.Wolk, C. P. 2000. Heterocyst formation in cyanobacteria, p. 83-104. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. ASM Press, Washington, DC.
- 28.Wolk, C. P., A. Ernst, and J. Elhai. 1994. Heterocyst metabolism and development, p. 769-823. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 29.Yoon, H. S., and J. W. Golden. 1998. Heterocyst pattern formation controlled by a diffusible peptide. Science 282:935-938. [DOI] [PubMed] [Google Scholar]