Abstract
The pathogenicity of Vibrio cholerae is influenced by sodium ions which are actively extruded from the cell by the Na+-translocating NADH:quinone oxidoreductase (Na+-NQR). To study the function of the Na+-NQR in the respiratory chain of V. cholerae, we examined the formation of organic radicals and superoxide in a wild-type strain and a mutant strain lacking the Na+-NQR. Upon reduction with NADH, an organic radical was detected in native membranes by electron paramagnetic resonance spectroscopy which was assigned to ubisemiquinones generated by the Na+-NQR. The radical concentration increased from 0.2 mM at 0.08 mM Na+ to 0.4 mM at 14.7 mM Na+, indicating that the concentration of the coupling cation influences the redox state of the quinone pool in V. cholerae membranes. During respiration, V. cholerae cells produced extracellular superoxide with a specific activity of 10.2 nmol min−1 mg−1 in the wild type compared to 3.1 nmol min−1 mg−1 in the NQR deletion strain. Raising the Na+ concentration from 0.1 to 5 mM increased the rate of superoxide formation in the wild-type V. cholerae strain by at least 70%. Rates of respiratory H2O2 formation by wild-type V. cholerae cells (30.9 nmol min−1 mg−1) were threefold higher than rates observed with the mutant strain lacking the Na+-NQR (9.7 nmol min−1 mg−1). Our study shows that environmental Na+ could stimulate ubisemiquinone formation by the Na+-NQR and hereby enhance the production of reactive oxygen species formed during the autoxidation of reduced quinones.
The gram-negative bacterium Vibrio cholerae naturally inhabits aquatic ecosystems, but some strains are able to colonize the human intestine, where they can cause the severe diarrheal disease cholera (10). As an adaptation for growth at high NaCl concentrations, V. cholerae expels sodium ions from the cytoplasm during respiration and establishes a sodium motive force across its inner membrane (12). This respiratory Na+ transport is catalyzed by the Na+-translocating NADH:quinone oxidoreductase (Na+-NQR), which consists of six subunits, NqrA to -F, and contains one Fe-S center, two covalently bound flavin mononucleotides, one non-covalently bound flavin adenine dinucleotide (FAD), one riboflavin, and ubiquinone-8 as prosthetic groups (5, 17, 41). Genome comparisons reveal that a Na+-NQR is present in many pathogenic bacteria, indicating that pathogens may benefit from a sodium cycle for nutrient uptake or motility (13). The sodium motive force which is maintained by the Na+-NQR strongly influences the production of virulence factors in Vibrio cholerae (14), and environmental Na+ is likely to be an important parameter during infection both as stimulus and as respiratory coupling ion (12). Loss of the Na+-NQR, either by mutation or by chemical inhibition, results in altered virulence gene regulation in V. cholerae (14), but the putative link between sodium membrane energetics and virulence has not been identified yet.
Superoxide (O2−) is an anionic free radical produced by the oxidation of reduced cofactors of redox enzymes with O2 (21). As a charged species, it cannot cross membranes at physiological pH and therefore is constrained to the compartment where it originated, e.g., the cytoplasm or periplasm of a bacterial cell (23). Respiratory NADH dehydrogenases may directly produce superoxide from the reaction of their reduced flavin cofactors with O2 (24, 27), or they may indirectly contribute to superoxide formation in a bacterial cell by producing reduced quinones which are reoxidized by O2 (22). Here we investigate the function of the Na+-NQR in V. cholerae using native membranes and whole cells. It is shown that the Na+-NQR represents a major source for extracellular superoxide produced by respiring V. cholerae cells.
MATERIALS AND METHODS
Growth of Vibrio cholerae.
The parent strain V. cholerae O395 N1 (29) and V. cholerae O395 N1 (nqrC::Tnbla) (14) with a Tn5 transposon in the nqrC gene were cultivated aerobically in Luria-Bertani medium supplemented with 10 mM glucose in the presence of 50 μg ml−1 streptomycin at 37°C. For maintenance of the Tnbla cassette insertion in nqrC in V. cholerae O395 N1 (nqrC::Tnbla) 100 μg ml−1 ampicillin was added to the medium. V. cholerae cells were grown for 6 to 8 h to the late exponential phase and harvested by centrifugation. The cells were resuspended in 10 mM HEPES-KOH, pH 7.5, 0.2 M K2SO4, 10% glycerol; frozen in liquid nitrogen; and stored at −80°C until use.
Preparation of membranes.
Cells were disrupted under exclusion of O2. Prior to use all solutions were degassed and purged with N2 followed by equilibration in a Coy glove box (95% N2-5% H2) at least overnight (O2 concentration of <0.3 μM in buffers). Cells (8 to 11 g, wet weight) were resuspended in 25 ml buffer (10 mM HEPES-KOH, pH 7.5, 0.2 M K2SO4) containing 5 mM MgCl2 and traces of DNase I (Roche Diagnostics). The cell suspension was passed once through a French pressure cell at 83 MPa, and the eluate was collected under a stream of N2. Unbroken cells and large debris were removed by centrifugation at 35,000 × g for 20 min. To the supernatant containing the membrane vesicles, 50 mM K2-EDTA was added as a chelator for Mn2+ and Cu2+, which perturb electron paramagnetic resonance (EPR) spectra. If not indicated otherwise, all subsequent manipulations were performed in the glove box at room temperature. The reddish-brown membranes were collected by ultracentrifugation (150,000 × g, 1 h, 4°C) and were washed once with 60 ml buffer containing 10 mM K2EDTA. Two additional washing steps in 60 ml buffer without K2-EDTA were performed to further decrease the concentrations of Na+, Mn2+, and Cu2+. Membranes were thoroughly mixed with buffer to yield suspensions containing 40 ± 10 mg protein ml−1 and <0.1 mM Na+.
EPR spectroscopy.
EPR samples were prepared in the anaerobe chamber. Membranes (0.27 ml in a reaction tube) were mixed with 0.03 ml substrate (NADH or succinate) in the presence of Na+ or inhibitors as indicated in the legends to the figures. The viscous suspension was transferred to an EPR quartz tube using plastic tubing fitted to a syringe. The time between mixing with substrate and complete freezing of the membranes in liquid N2 was 5 to 8 min. The EPR spectra were recorded as described elsewhere (40) and simulated using the program EPR (30). The concentration of a paramagnetic species was calculated by comparing the total intensity of its simulated EPR spectrum with the intensity of the EPR spectrum of a CuSO4 standard (31) (see also Fig. S1 in the supplemental material).
Analytical methods.
Oxidation of NADH (0.1 mM) by membrane vesicles was followed in 20 mM Tris-H2SO4, pH 7.5, containing 50 mM Na2SO4, 50 μg/ml bovine serum albumin, and 0.1 mM ubiquinone-1 as an electron acceptor (41). Protein was determined by the microbiuret method (8) using bovine serum albumin as standard. Na+ was determined by atomic absorption spectroscopy with a Shimadzu AA-646 spectrometer.
The formation of superoxide was followed according to the procedure in reference 19, modified as follows. To prepare cell suspensions, anoxic buffers with K+ salts replacing the corresponding Na+ salts were used, and cells were kept in the anaerobe chamber until the reactions were started. The residual Na+ concentration in cell suspensions was <0.1 mM. Fifty milliliters of V. cholerae O395 N1 or V. cholerae O395 N1 (nqrC::Tnbla) was grown as described above and harvested at an approximate optical density at 550 nm of 1.2 to 1.4. Cells were washed twice with phosphate-buffered saline (4.3 mM K2HPO4, 1.4 mM KH2PO4, 140 mM KCl, pH 7.5) and resuspended in ice-cold Hanks balanced salt solution (5.6 mM glucose, 142 mM KCl, 0.3 mM K2HPO4, 0.4 mM KH2PO4, 4.2 mM KHCO3, 1.3 mM CaCl2, 0.5 mM MgCl2, 0.6 mM MgSO4, pH 7.5) to an optical density at 550 nm of 0.2. The reaction was started by adding 0.99 ml anoxic, prewarmed cell suspension (25°C) to 10 μl cytochrome c (from beef heart; Sigma; final concentration, 20 μM) in a cuvette under magnetic stirring to aerate the cells. The reduction of cytochrome c by superoxide was followed with a UV-visible-light spectrometer (HP 8452A) at 550 nm. If indicated, 0.5 mg superoxide dismutase (SOD; Fluka; 3,231 U mg−1) or NADH (0.7 mM) was added. Rates of cytochrome c reduction were calculated using an extinction coefficient of 21.5 mM−1 cm−1 (28).
Hydrogen peroxide was determined using horseradish peroxidase according to the method in reference 9. The reaction was started by adding 0.97 ml anoxic, prewarmed cell suspension to 30 μl Hanks solution containing 4-aminoantipyrine (0.49 mM), phenol (1.06 mM), and 4 U horseradish peroxidase from Sigma (final concentrations in the assay). The H2O2-dependent formation of the quinoneimine dye (37) was followed at 550 nm in a cuvette under magnetic stirring to aerate the cells. The extinction coefficient (ɛ550 = 4.3 mM−1 cm−1) was determined from standards of 44 to 440 μM hydrogen peroxide in 970 μl Hanks solution in the absence of cells. Rates of H2O2 formation were corrected by subtracting the residual activity (0.28 to 0.30 nmol min−1) of the cell-free supernatant obtained by centrifugation of the cell suspension. Superoxide and H2O2 formation activities from three to four experiments are presented. The protein content of V. cholerae cell suspensions was estimated from the optical density, assuming that 1 unit of absorbance at 550 nm corresponds to 0.33 g total dry weight liter−1 (42) and that 55% of the total dry weight represents protein (32).
Inhibition studies.
Inhibitors were added in the anaerobe chamber 10 min prior to the start of the reaction. 2-n-Heptyl-4-hydroxyquinoline-N-oxide (HQNO) was added from a stock solution in ethanol to V. cholerae cells or membranes. KCN was added from a buffered stock solution to membranes (final concentration, 29 mM). Note that cyanide interferes with the detection of extracellular superoxide since it acts as a ligand to the heme iron of cytochrome c (38).
RESULTS
Respiratory chain complexes in wild-type V. cholerae and a mutant devoid of a functional Na+-NQR.
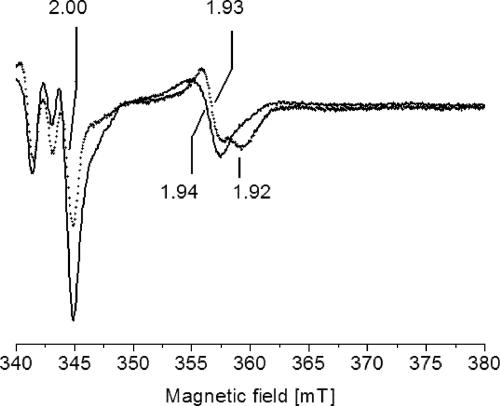
We compared the EPR spectra of membranes from wild-type V. cholerae and a mutant carrying an insertion in the nqrC gene with the aim of assigning organic radicals to the Na+-NQR in its membrane-bound state. The NqrF subunit of the Na+-NQR complex harbors a [2Fe-2S] cluster which accepts electrons from the FAD cofactor located in close proximity. Upon addition of NADH, a resonance at g = 1.94 appeared in the EPR spectrum of membranes from the wild-type V. cholerae strain but not in membranes from the mutant carrying a transposon in the nqrC gene (Fig. 1). In a previous study using the isolated NqrF subunit, the g = 1.94 resonance was assigned to the gx,y component of a nearly axial signal of the one-electron reduced [2Fe-2S] cluster in NqrF (41). Thus, the disruption of the nqrC gene encoding the membrane-bound NqrC subunit prevented the synthesis or assembly of the NqrF subunit. We could not detect the gz component of the [2Fe-2S] from NqrF in membranes from the parent strain since the g > 2 region of the EPR spectrum was dominated by unassigned resonances (Fig. 1). The NADH dehydrogenase activities of membranes (0.4 to 0.5 μmol min−1 mg−1) did not differ significantly in the mutant and the wild-type strains, suggesting that the lack of a functional Na+-NQR complex in the mutant was compensated for by a nonelectrogenic NADH dehydrogenase encoded on the genome of V. cholerae. On the two chromosomes of V. cholerae El Tor, four open reading frames encoding NADH dehydrogenases of the membrane-bound, nonelectrogenic type were identified: VC1581, VCA0155, VCA0157, and VC1890 (only the last is annotated ndh) (18). We also considered the possibility that NADH was oxidized by a subcomplex of Na+-NQR assembled in the mutant strain even in the absence of the NqrC subunit. Ag+ is a specific inhibitor of the Na+-NQR which promotes the dissociation of the non-covalently bound FAD from the NqrF subunit and thereby prevents the initial oxidation of NADH (39). In the presence of Ag+, rates of NADH oxidation by membranes from the parent V. cholerae strain decreased to 50% (0.1 μM Ag+) or 14% (1 μM Ag+) of the activity observed in the absence of the inhibitor. In contrast, no inhibition of NADH oxidation by Ag+ was observed with membranes from the mutant strain under identical conditions (data not shown). These results further corroborate our assumption that the mutant strain lacks a functional Na+-NQR and oxidizes NADH via an alternative NADH dehydrogenase.
FIG. 1.
Detection of the [2Fe-2S] cluster localized in subunit NqrF of the membrane-bound Na+-NQR by EPR spectroscopy. Membranes from the wild-type V. cholerae strain containing the Na+-NQR (solid line) and from the nqrC insertion mutant (dotted line) were mixed with 7.3 mM Na2-NADH prior to freezing. EPR conditions: microwave frequency, 9.652 GHz; modulation amplitude, 0.5 mT; microwave power, 2 mW; temperature, 40 K. Characteristic g values are indicated.
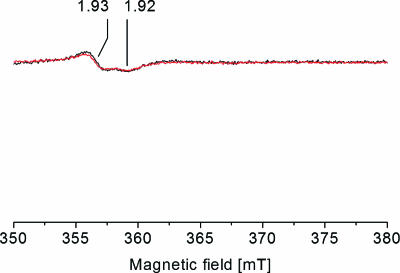
The wild-type and the mutant strains contained similar amounts of succinate dehydrogenase or fumarate reductase as judged from the intensities of the succinate-induced EPR signal at gx,y = 1.93 and 1.92 (Fig. 2). These resonances arise from the one-electron reduced [2Fe-2S] center I of succinate dehydrogenase-fumarate reductase (1). Center I was also observed in NADH-treated membranes from the mutant strain, demonstrating that electrons were delivered from NADH to the quinone pool even in the absence of a functional Na+-NQR (Fig. 1). We conclude that the disruption of the nqrC gene had no significant effect on the respiratory chain complexes located downstream of the succinate:quinone segment. By comparing the EPR spectra of membranes from the wild-type and the mutant V. cholerae strains, we could assign NADH-induced organic radicals to the Na+-NQR in its membrane-bound state, as described below.
FIG. 2.
Detection of the Fe-S center I from succinate dehydrogenase/fumarate reductase in V. cholerae membranes by EPR spectroscopy. Membranes from the wild-type V. cholerae strain containing the Na+-NQR (black trace) and from the nqrC insertion mutant (red trace) were mixed with 36.4 mM Na2-succinate prior to freezing. Characteristic g values are indicated. For EPR conditions, see the legend to Fig. 1.
Na+ stimulates radical formation by the Na+-NQR.
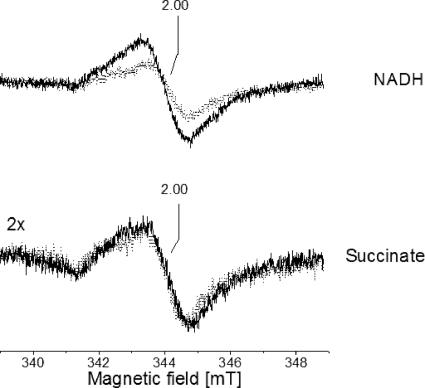
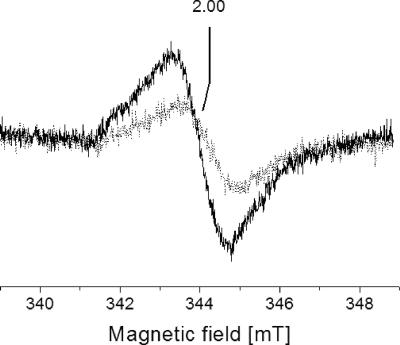
The NqrF subunit acts as a converter between the two-electron donor NADH and subsequent one-electron transfer steps in the Na+-NQR complex which could result in the formation of organic radicals like flavo- or ubisemiquinones. Membranes from the wild type and the mutant strain devoid of a functional Na+-NQR complex were inspected by EPR under conditions which are optimal for the detection of organic radicals. Essentially identical amounts of organic radicals centered at g = 2.00 were detected in the membranes after incubation with 36.4 mM succinate for at least 6 min in the absence of O2 (Fig. 3). We conclude that the two V. cholerae strains contained very similar amounts of organic radicals in the succinate:O2 segment of the respiratory chain. Using NADH (7.3 mM) as an electron donor, approximately twice the amount of organic radicals detected in the mutant strain was detected in membranes from the parent strain (Fig. 3), suggesting that the Na+-NQR promoted the one-electron reduction of organic redox carriers in V. cholerae membrane vesicles. These vesicles obtained by French press cell rupture were presumed to be predominantly oriented inside-out (35), hereby exposing the cytoplasmic aspect of the inner membrane and the NADH-oxidizing domain of the Na+-NQR complex. We asked whether NADH-induced radical formation by the Na+-NQR was influenced by the coupling cation and added Na+ to the external buffer which represents the cytoplasmic aspect of the membrane vesicles. Increasing the Na+ concentration from 0.08 to 14.7 mM led to a significant increase of NADH-induced organic radicals in membranes from the wild-type strain compared to the mutant lacking a functional Na+-NQR (Fig. 4). Spin quantification of the radical signal at low Na+ concentrations (0.08 mM) gave an approximate radical concentration of 200 μM in 0.3 ml membrane suspension, or 5 nmol mg−1 protein. In the presence of 14.7 mM Na+, the radical concentration increased to approximately 400 μM, or 10 nmol mg−1 protein (see Fig. S1 in the supplemental material). This twofold increase in radical concentration can be assigned to a Na+-dependent redox reaction catalyzed by the Na+-NQR if one takes into account that increasing the Na+ concentration from below 0.1 mM to 25 mM resulted in a threefold stimulation of NADH:quinone oxidoreduction activity of the purified enzyme (2) and that the organic radicals detected in NADH-reduced membranes from wild-type V. cholerae did not exclusively arise from the Na+-NQR. Organic radicals associated with the succinate dehydrogenase/fumarate reductase (Fig. 2) or with other respiratory complexes in the quinol:O2 segment of the respiratory chain will contribute to the EPR spectrum of NADH-reduced membranes but will not be affected by a rise in the Na+ concentration.
FIG. 3.
Respiration-induced organic radicals in V. cholerae membranes in the presence and absence of a functional Na+-NQR. Membranes from the wild-type V. cholerae strain (black trace) and from the nqrC insertion mutant (gray trace) were mixed with 7.3 mM Na2-NADH (top) or 36.4 mM Na2-succinate (bottom) prior to freezing. EPR conditions: microwave frequency, 9.652 GHz; modulation amplitude, 0.5 mT; microwave power, 4 μW; temperature, 70 K.
FIG. 4.
Effect of Na+ on the NADH-induced formation of organic radicals by the membrane-bound Na+-NQR. Membranes from the wild-type V. cholerae strain were reduced with 7.3 mM NADH in the presence of 0.08 mM Na+ (gray trace) or 14.7 mM Na+ (black trace). For EPR conditions, see the legend to Fig. 3.
The inhibition by Ag+ indicated that at least 86% of the NADH dehydrogenase activity of membranes from the parent V. cholerae strain was catalyzed by the Na+-NQR, corresponding to 0.43 μmol NADH min−1 mg−1. We estimated the content of Na+-NQR in the membranes by dividing the total Na+-NQR activity in 1 mg membrane protein (0.43 μmol NADH min−1) by the turnover number determined for purified Na+-NQR (16,000 μmol NADH μmol−1 Na+-NQR min−1) (2). Comparing the content of Na+-NQR in the membranes (0.03 nmol mg−1 protein) with the amount of NADH-induced organic radicals (5 to 10 nmol mg−1 protein) revealed that radical formation by native membrane vesicles from V. cholerae was clearly overstoichiometric compared to the Na+-NQR, with a [radical]/[Na+-NQR] ratio of 170:1 at 0.08 mM Na+ or 340:1 at 14.7 mM Na+, respectively. The NADH-induced organic radicals cannot exclusively result from the flavin cofactors of the Na+-NQR, and we conclude that a significant fraction of membrane-bound quinones in V. cholerae is converted to the one-electron reduced state during oxidation of NADH by the Na+-NQR. The isolated Na+-NQR from Vibrio alginolyticus produced ubisemiquinone radicals which were reoxidized by O2 under formation of superoxide (16, 34, 40). We asked whether the Na+-NQR could also contribute to superoxide formation in vivo and studied the formation of reactive oxygen species by intact V. cholerae cells.
Extracellular superoxide production by V. cholerae.
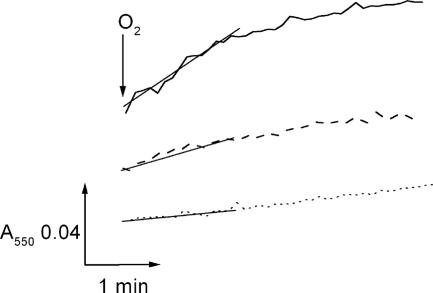
With a standard redox potential O2/O2− of −0.16 V (21), the superoxide anion acts as a one-electron donor for ferricytochrome c with E0′ = +0.24 mV. As a 12-kDa protein, cytochrome c does not diffuse across intact cellular membranes and can therefore be used to monitor extracellular superoxide production by V. cholerae cells. Using late-exponential V. cholerae cells and glucose as substrate, superoxide formation was initiated by rapid mixing of the cells with air (Fig. 5). Cytochrome c reduction rates were highest in the wild-type strain containing the Na+-NQR (15.7 nmol min−1 mg−1) compared to 8.9 nmol min−1 mg−1 in the nqrC deletion mutant, indicating that the Na+-NQR represents a major source for superoxide in respiring V. cholerae cells (Table 1). Raising the Na+ concentration from 0.1 to 5 mM increased the cytochrome c reduction rates in the wild-type V. cholerae by at least 70% but showed no significant effect in the mutant strain (data not shown). In both strains, rates of cytochrome c reduction decreased to 5 to 6 nmol min−1 mg−1 upon addition of SOD, indicating that O2− produced in the periplasm of V. cholerae represented the major electron donor for cytochrome c reduction (Table 1; Fig. 5). The residual activity observed in the presence of SOD could result from the reduction of cytochrome c by an unknown low-molecular-weight compound excreted by V. cholerae, as proposed for Escherichia coli cells which also exhibited SOD-insensitive cytochrome c reduction activity (22). By subtracting the SOD-insensitive rate, superoxide formation activities of 10.2 nmol min−1 mg−1 for the wild-type V. cholerae and 3.1 nmol min−1 mg−1 for the NQR deletion strain were calculated (Table 1). To exclude the possibility that superoxide was produced by lysed cells, we followed the reduction of cytochrome c in the presence of NADH (15). Like the superoxide anion (23), NADH does not readily permeate through the inner bacterial membrane of intact cells but will react exclusively with cytoplasmic redox enzymes from broken cells, thereby increasing the overall amount of superoxide formed. Rates of superoxide formation were essentially identical with or without added NADH (Table 1), indicating that the V. cholerae cells were intact.
FIG. 5.
Superoxide production by respiring V. cholerae cells in the presence and absence of a functional Na+-NQR. The reaction was started by mixing the cell suspension with cytochrome c in air-saturated buffer. The superoxide produced was reoxidized by cytochrome c in a nonenzymatic reaction. The reduction of cytochrome c was followed at 550 nm. Solid trace, with Na+-NQR; dashed trace, without Na+-NQR; dotted trace, with Na+-NQR and 1,615 U SOD.
TABLE 1.
Reduction of cytochrome c by respiring V. cholerae cells
| Strain | Sp acta (nmol min−1 mg−1)
|
Superoxide formationb | ||
|---|---|---|---|---|
| No addition | With 1,615 U SOD | With 0.7 mM NADH | ||
| V. cholerae O395 N1 | 15.7 ± 1.9 | 5.5 ± 0.4 | 14.7 ± 3.8 | 10.2 |
| V. cholerae O395 N1 (nqrC::Tnbla) | 8.9 ± 2.3 | 5.8 ± 0.5 | 8.0 ± 0.3 | 3.1 |
Mean values from three experiments.
The superoxide formation activity was calculated by subtracting the cytochrome c reduction rate observed in the presence of SOD from the rate observed without addition.
In E. coli, superoxide is disproportionated to H2O2 and O2 by SODs found in the cytoplasm and periplasm (22). V. cholerae possesses a periplasmic SOD (7) which could contribute to the overall formation of H2O2 by converting the superoxide generated in the periplasm. We followed H2O2 formation by respiring V. cholerae cells and observed approximately threefold-higher rates in the wild-type strain (30.9 nmol H2O2 min−1 mg−1) than in the mutant lacking the Na+-NQR (9.7 H2O2 nmol min−1 mg−1), indicating that the Na+-NQR represents a major source for reactive oxygen species in V. cholerae. Since H2O2 in contrast to the superoxide anion is membrane permeable, these rates reflect both cytoplasmic and periplasmic H2O2 production activities and therefore are expected to be higher than the rates of extracellular superoxide formation. The source for intracellular H2O2 in V. cholerae remains to be identified.
Effect of respiratory chain inhibitors on radical formation and superoxide production.
The Na+-NQR contains several redox-active flavins which participate in electron transfer from NADH to quinone (3, 41). Hence, reduced flavin(s) in the Na+-NQR rather than ubisemiquinones formed by the Na+-NQR could act as an electron donor for extracellular superoxide formation in V. cholerae. We addressed this question by studying the production of superoxide and the formation of radicals in the presence of HQNO. HQNO interacts with quinone-binding sites of respiratory complexes (36) and also effectively inhibits the Na+-NQR (2, 34). HQNO (0.3 mM) inhibited extracellular superoxide formation of wild-type V. cholerae cells by 60 to 80% (data not shown). At the same time, the NADH-induced radical signal in wild-type V. cholerae membranes was decreased by approximately 50% in the presence of 0.5 mM HQNO (Fig. 6).
FIG. 6.
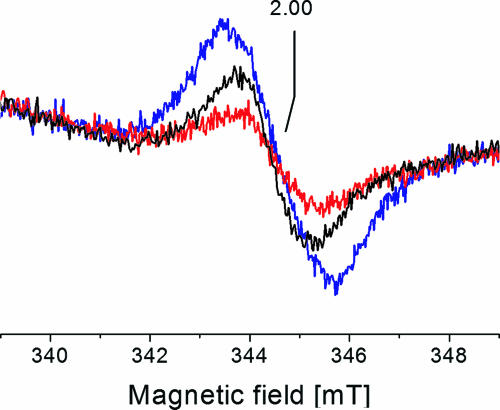
Effect of respiratory chain inhibitors on the NADH-induced formation of organic radicals by the membrane-bound Na+-NQR. Membranes from the wild-type V. cholerae strain were mixed with 29 mM KCN (blue trace) or 0.5 mM HQNO (red trace). After 10 min, 9 mM Na2NADH was added, and the samples were frozen in liquid N2. Black trace, membranes reduced with NADH in the absence of inhibitor. EPR conditions: microwave frequency, 9.674 GHz; modulation amplitude, 0.5 mT; microwave power, 1 mW; temperature, 70 K.
The genome of V. cholerae encodes heme-containing respiratory complexes like the bc1 complex (VC0573 to VC0575) and the quinol oxidase (VC1570 and VC1571) which could accept electrons from reduced quinones formed during NADH oxidation (18). Cyanide inhibits electron transfer to these complexes, and as a consequence, the ratio of reduced to oxidized quinones in the Q pool will increase. The signal intensity of the organic radical detected in NADH-reduced membranes from wild-type V. cholerae increased by approximately 60% upon addition of KCN (Fig. 6). These results further corroborate our notion that the organic radicals produced during NADH oxidation by the Na+-NQR represent ubisemiquinones which act as electron donors for superoxide formation.
DISCUSSION
The rates of extracellular superoxide production of the gram-negative V. cholerae (10 nmol min−1 mg−1 protein) were lower than activities observed with the gram-positive Enterococcus faecalis (26 nmol min−1 mg−1 protein) (19) but significantly exceeded the rates observed with the gram-negative E. coli (1 nmol min−1 mg−1 protein) (22). Like E. coli, V. cholerae possesses a periplasmic SOD (7) which should scavenge superoxide formed in the periplasm. A possible explanation for the increased production of superoxide in V. cholerae compared to E. coli is that disproportionation to H2O2 is less efficient in V. cholerae or that V. cholerae produces significantly higher amounts of superoxide than does E. coli. In support of the latter hypothesis, our study identifies the Na+-NQR which is not present in E. coli as a major source for extracellular superoxide.
The electron transfer pathway in the Na+-NQR starts by a hydride transfer from the substrate NADH to the non-covalently bound FAD on the flavin domain of the NqrF subunit (41), followed by one-electron transfer to the vertebrate-type [2Fe-2S] cluster in the N-terminal domain of NqrF (25). How electron transport from this FeS center to other flavin and ubiquinone cofactors in the complex and to the substrate quinone proceeds and how this overall exergonic reaction is coupled to Na+ transport are still enigmatic. Compared to other respiratory NADH dehydrogenases, the Na+-NQR is unique as it stabilizes the one-electron reduced state of flavin cofactors which are covalently attached to the membrane-bound NqrB and NqrC subunits, respectively (3). These flavins in their one- or two-electron reduced state could reduce O2 (24, 27). Alternatively, superoxide could be formed during the autoxidation of reduced ubiquinones generated by the Na+-NQR. In E. faecalis and E. coli, extracellular superoxide was proposed to result from the reaction of molecular oxygen with reduced quinones produced by the respiratory chain. Both two-electron reduced quinones (quinols) and one-electron reduced quinones (semiquinones) were considered as possible electron donors (20, 22). Similarly, quinones reduced by the Na+-NQR could act as electron donors for O2 reduction in V. cholerae.
We favor ubisemiquinones generated by the Na+-NQR as a source for in vivo superoxide formation, since this reaction was also observed with the isolated Na+-NQR (16, 34). Moreover, Na+ which stimulated quinone reduction by the isolated Na+-NQR (2) also promoted ubisemiquinone formation and extracellular superoxide production by the Na+-NQR in its native membrane environment. Further support comes from the finding that superoxide formation by V. cholerae cells was greatly diminished in the presence of the quinone-type inhibitor HQNO. Reduced flavins are unlikely electron donors for extracellular superoxide production since all flavin cofactors of the Na+-NQR with known locations are bound to cytoplasmic domains of the complex (6).
The question arises whether superoxide formation by the Na+-NQR takes place in the general membrane milieu or whether it occurs at a quinone-binding site of the enzyme. EPR studies of ubisemiquinones associated with the proton-pumping mitochondrial NADH dehydrogenase (complex I) revealed two distinct ubiquinone-binding sites within the membrane-embedded part of the complex (26). One of these ubisemiquinones is stabilized by applying an electrochemical proton potential and is proposed to play a central role in the coupling of electron transfer and proton transport by complex I (33). At least 1 mole of ubiquinone-8 per mole is found in the Na+-NQR from V. cholerae (2), suggesting that like complex I, the Na+-NQR comprises one or several binding sites which may accommodate quinone(s) in its different redox states. Although we cannot exclude the possibility that O2 reduction by a protein-bound ubisemiquinone contributes to NADH-dependent superoxide formation by the Na+-NQR, the overstoichiometric amount of ubisemiquinones in membranes compared to the Na+-NQR suggests that ubisemiquinones in the lipid bilayer represent the major reductant for superoxide formation by V. cholerae cells.
The superoxide anion and secondary reactive oxygen species like H2O2 and the hydroxyl radical are highly toxic compounds which may severely damage proteins, lipids, and DNA (21). In E. faecalis, extracellular O2− formation was associated with invasiveness, and superoxide was considered to be a virulence factor (19). The Na+ concentration in the small intestine and in stool from cholera patients is in the range from 90 to 150 mM (4, 11). In the human host, respiratory electron transfer by the Na+-NQR in V. cholerae, therefore, is not expected to be limited by Na+. Our study opens the possibility that the NQR-dependent production of superoxide might augment the pathogenicity of V. cholerae.
Supplementary Material
Acknowledgments
This work was supported by the Roche Research Foundation (K.T.); Deutsche Forschungsgemeinschaft (G.F.); the Ellison Medical Foundation (C.C.H.); and grants from the Swiss National Science Foundation, Vontobel Stiftung, and Parkinson Schweiz (J.S.).
Footnotes
Published ahead of print on 23 February 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ackrell, B. A. C., M. K. Johnson, R. P. Gunsalus, and G. Cecchini. 1992. Structure and function of succinate dehydrogenase and fumarate reductase, p. 229-297. In F. Müller (ed.), Chemistry and biochemistry of flavoenzymes, vol. III. CRC Press, Boca Raton, FL. [Google Scholar]
- 2.Barquera, B., P. Hellwig, W. Zhou, J. E. Morgan, C. C. Häse, K. K. Gosink, M. Nilges, P. J. Bruesehoff, A. Roth, C. R. Lancaster, and R. B. Gennis. 2002. Purification and characterization of the recombinant Na(+)-translocating NADH:quinone oxidoreductase from Vibrio cholerae. Biochemistry 41:3781-3789. [DOI] [PubMed] [Google Scholar]
- 3.Barquera, B., L. Ramirez-Silva, J. E. Morgan, and M. J. Nilges. 2006. A new flavin radical signal in the Na+-pumping NADH:quinone oxidoreductase from Vibrio cholerae: an EPR/ENDOR investigation of the role of the covalently bound flavins in subunits B and C. J. Biol. Chem. 281:36482-36491. [DOI] [PubMed] [Google Scholar]
- 4.Bennish, M. L. 1994. Pathophysiology, clinical features, and treatment, p. 239-255. In I. K. Wachsmuth, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera. Molecular to global perspectives. ASM Press, Washington, DC.
- 5.Bogachev, A. V., and M. I. Verkhovsky. 2005. Na+-translocating NADH:quinone oxidoreductase: progress achieved and prospects of investigations. Biochemistry (Moscow) 70:143-149. [DOI] [PubMed] [Google Scholar]
- 6.Duffy, E. B., and B. Barquera. 2006. Membrane topology mapping of the Na+-pumping NADH:quinone oxidoreductase from Vibrio cholerae by PhoA-green fluorescent protein fusion analysis. J. Bacteriol. 188:8343-8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabbianelli, R., C. Signoretti, I. Marta, A. Battistoni, and L. Nicolini. 2004. Vibrio cholerae periplasmic superoxide dismutase: isolation of the gene and overexpression of the protein. J. Biotechnol. 109:123-130. [DOI] [PubMed] [Google Scholar]
- 8.Goa, J. 1953. A micro-biuret method for protein determination: determination of total protein in cerebrospinal fluid. Scand. J. Clin. Lab. Investig. 5:219-222. [DOI] [PubMed] [Google Scholar]
- 9.Green, M. J., and H. A. O. Hill. 1984. Chemistry of dioxygen. Methods Enzymol. 105:3-22. [DOI] [PubMed] [Google Scholar]
- 10.Guerrant, R. L., B. A. Carneiro-Filho, and R. A. Dillingham. 2003. Cholera, diarrhea, and oral rehydration therapy: triumph and indictment. Clin. Infect. Dis. 37:398-405. [DOI] [PubMed] [Google Scholar]
- 11.Guyton, A. C., and J. E. Hall. 2000. Textbook of medical physiology. W. B. Saunders Company, Philadelphia, PA.
- 12.Häse, C. C., and B. Barquera. 2001. Role of sodium bioenergetics in Vibrio cholerae. Biochim. Biophys. Acta 1505:169-178. [DOI] [PubMed] [Google Scholar]
- 13.Häse, C. C., N. D. Fedorova, M. Y. Galperin, and P. A. Dibrov. 2001. Sodium ion cycle in bacterial pathogens: evidence from cross-genome comparisons. Microbiol. Mol. Biol. Rev. 65:353-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Häse, C. C., and J. J. Mekalanos. 1999. Effects of changes in membrane sodium flux on virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 96:3183-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan, H. M., and I. Fridovich. 1979. Paraquat and Escherichia coli: mechanism of production of extracellular superoxide radical. J. Biol. Chem. 254:10846-10852. [PubMed] [Google Scholar]
- 16.Hayashi, M., and T. Unemoto. 1984. Characterization of the Na+-dependent respiratory chain NADH:quinone oxidoreductase of the marine bacterium, Vibrio alginolyticus, in relation to the primary Na+ pump. Biochim. Biophys. Acta 767:470-478. [Google Scholar]
- 17.Hayashi, M., and T. Unemoto. 2004. The Na+-translocating NADH-quinone reductase of marine and moderately halophilic bacteria, p. 15-174. In D. Zannoni (ed.), Respiration in archaea and bacteria: diversity of prokaryotic electron transport carriers, vol. 15. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- 18.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huycke, M. M., W. Joyce, and M. F. Wack. 1996. Augmented production of extracellular superoxide by blood isolates of Enterococcus faecalis. J. Infect. Dis. 173:743-746. [DOI] [PubMed] [Google Scholar]
- 20.Huycke, M. M., D. Moore, W. Joyce, P. Wise, L. Shepard, Y. Kotake, and M. S. Gilmore. 2001. Extracellular superoxide production by Enterococcus faecalis requires demethylmenaquinone and is attenuated by functional terminal quinol oxidases. Mol. Microbiol. 42:729-740. [DOI] [PubMed] [Google Scholar]
- 21.Imlay, J. A. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395-418. [DOI] [PubMed] [Google Scholar]
- 22.Korshunov, S., and J. A. Imlay. 2006. Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J. Bacteriol. 188:6326-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korshunov, S. S., and J. A. Imlay. 2002. A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of Gram-negative bacteria. Mol. Microbiol. 43:95-106. [DOI] [PubMed] [Google Scholar]
- 24.Kussmaul, L., and J. Hirst. 2006. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. USA 16:7607-7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, P.-C., A. Puhar, K. Türk, S. Piligkos, E. Bill, F. Neese, and J. Steuber. 2005. A vertebrate-type ferredoxin domain in the Na+-translocating NADH dehydrogenase from Vibrio cholerae. J. Biol. Chem. 280:22560-22563. [DOI] [PubMed] [Google Scholar]
- 26.Magnitsky, S., L. Toulokhonova, T. Yano, V. D. Sled, C. Hägerhäll, V. G. Grivennikova, D. S. Burbaev, A. D. Vinogradov, and T. Ohnishi. 2002. EPR characterization of ubisemiquinones and iron-sulfur cluster N2, central components of the energy coupling in the NADH-ubiquinone oxidoreductase (complex I) in situ. J. Bioenerg. Biomembr. 34:193-208. [DOI] [PubMed] [Google Scholar]
- 27.Massey, V. 1994. Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem. 269:22459-22462. [PubMed] [Google Scholar]
- 28.Massey, V. 1959. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim. Biophys. Acta 34:255-256. [DOI] [PubMed] [Google Scholar]
- 29.Mekalanos, J. J., Swartz, D. J., and G. D. N. Pearson. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551-557. [DOI] [PubMed] [Google Scholar]
- 30.Neese, F. 1995. The program EPR. Quant. Chem. Program Exchange Bull. 136:5. [Google Scholar]
- 31.Neese, F., W. G. Zumft, W. E. Antholine, and P. M. H. Kroneck. 1996. The purple mixed-valence CuA center in nitrous oxide reductase: EPR of the copper-63-, copper-65-, and both copper-65 and [N-15]histidine-enriched enzyme and a molecular orbital interpretation. J. Am. Chem. Soc. 118:8692-8699. [Google Scholar]
- 32.Neidhardt, F. C., J. L. Ingraham, R. Curtiss III, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger. 1996. Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, DC.
- 33.Ohnishi, T., and J. C. Salerno. 2005. Conformation-driven and semiquinone-gated proton-pump mechanism in the NADH-ubiquinone oxidoreductase (complex I). FEBS Lett. 579:4555-4561. [DOI] [PubMed] [Google Scholar]
- 34.Pfenninger-Li, X. D., S. P. J. Albracht, R. van Belzen, and P. Dimroth. 1996. The NADH:ubiquinone oxidoreductase of Vibrio alginolyticus: purification, properties and reconstitution of the Na+ pump. Biochemistry 35:6233-6242. [DOI] [PubMed] [Google Scholar]
- 35.Reenstra, W. W., L. Patel, H. Rottenberg, and H. R. Kaback. 1980. Electrochemical proton gradient in inverted membrane vesicles from Escherichia coli. Biochemistry 19:1-9. [DOI] [PubMed] [Google Scholar]
- 36.Rich, P. R. 1984. Electron and proton transfers through quinones and cytochrome bc complexes. Biochim. Biophys. Acta 768:53-79. [DOI] [PubMed] [Google Scholar]
- 37.Saito, Y., M. Mifune, S. Nakashima, J. Odo, and Y. Tanaka. 1987. Determination of hydrogen peroxide with N,N-diethylaniline and 4-aminoantipyrine by use of an anion-exchange resin modified with manganese-tetrakis(sulphonphenyl)porphine, as a substitute for peroxidase. Talanta 34:667-669. [DOI] [PubMed] [Google Scholar]
- 38.Schejter, A., M. D. Ryan, E. R. Blizzard, C. Zhang, E. Margoliash, and B. A. Feinberg. 2006. The redox couple of the cytochrome c cyanide complex: the contribution of heme iron ligation to the structural stability, chemical reactivity, and physiological behaviour of horse cytochrome c. Protein Sci. 15:234-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steuber, J., W. Krebs, and P. Dimroth. 1997. The Na+-translocating NADH:ubiquinone oxidoreductase from Vibrio alginolyticus: redox states of the FAD prosthetic group and mechanism of Ag+ inhibition. Eur. J. Biochem. 249:770-776. [DOI] [PubMed] [Google Scholar]
- 40.Steuber, J., M. Rufibach, G. Fritz, F. Neese, and P. Dimroth. 2002. Inactivation of the Na+-translocating NADH:ubiquinone oxidoreductase from Vibrio alginolyticus by reactive oxygen species. Eur. J. Biochem. 269:1287-1292. [DOI] [PubMed] [Google Scholar]
- 41.Türk, K., A. Puhar, F. Neese, E. Bill, G. Fritz, and J. Steuber. 2004. NADH oxidation by the Na+-translocating NADH:quinone oxidoreductase from Vibrio cholerae: functional role of the NqrF subunit. J. Biol. Chem. 279:21349-21355. [DOI] [PubMed] [Google Scholar]
- 42.Underwood, S. A., M. L. Buszko, K. T. Shanmugam, and L. O. Ingram. 2004. Lack of protective osmolytes limits final cell density and volumetric productivity of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl. Environ. Microbiol. 70:2734-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.