Streptomyces coelicolor is the best-developed model system for an enormous family of filamentous soil bacteria. One reason for the interest in these organisms is that they produce numerous secondary metabolites, many of which are employed clinically as antibiotics. Most streptomycetes produce several biologically active secondary metabolites; S. coelicolor produces at least four. Not surprisingly, they also possess resistance genes for the antimicrobial molecules they produce; often these are linked to and are coregulated with the antibiotic biosynthesis genes. As our understanding of secondary metabolism advances, it is becoming increasingly clear that the relationship between antibiotic production and resistance is more complicated than expected. For example, the S. coelicolor genome encodes proteins that are similar in sequence and mechanism to those that confer clinical resistance to vancomycin (7, 8). This was a surprise because S. coelicolor does not produce vancomycin or, indeed, any glycopeptide antibiotics. More recently, environmental isolates of Streptomyces spp. have been described that harbor enzymatic resistance mechanisms for antibiotics that are semisynthetic or wholly synthetic and, presumably, have never existed in nature (5, 17). Where did the selective pressure for these resistance mechanisms come from? In addition to this apparent disconnect between biosynthesis and resistance, antibiotic production appears to be controlled by a regulatory network of truly Byzantine proportions: to date at least 18 genes have been shown to influence antibiotic production in S. coelicolor—a subset of these also control sporulation (3). Clearly, bacteria have devoted a great deal of evolutionary time to developing antibiotic resistance mechanisms and the regulatory apparatus for controlling for antibiotic production. In this issue of the Journal of Bacteriology, and in a companion article published in Molecular Microbiology (15), Kenji Nishimura and coworkers in Kozo Ochi's laboratory report the elucidation of the mechanism of type II streptomycin resistance (12). Their discoveries strongly reinforce the suspicion that there is much to learn about the relationship between antibiotic resistance and biosynthesis.
Streptomycin, a secondary metabolite produced by several Streptomyces strains, was introduced as a therapeutic agent in the early 1940s and proved spectacularly successful against a number of serious infections. Sadly, however, it went on to set the pattern for clinical resistance to antibiotics. By 1946, resistant strains had been reported, and by the early 1950s, clinical resistance was so widespread that the antibiotic began to fall into disuse, supplanted as a miracle cure-all by newer drugs.
In S. coelicolor, two categories of streptomycin-resistant mutants have been characterized. Type I mutants are resistant to high concentrations of the antibiotic, and type II mutants are resistant to much lower concentrations. Both mechanisms are specific to streptomycin; neither confers resistance to other antibiotics. This pattern of distinct high and low resistance has been reported for other bacteria (6, 11). An odd effect of both types of strR mutations on S. coelicolor is that they bring about the overproduction of the secondary metabolite actinorhodin, a polyketide that is otherwise unrelated to streptomycin (9, 13, 16). Indeed, strR mutations can overcome the effects of mutations in genes such as relA, relC, and brgA that, on their own, impair actinorhodin production (16).
Type I resistance is brought about by mutations in the rpsL gene, which encodes the S12 protein of the 30S subunit of the ribosome (16). The mechanism responsible for type II resistance was first demonstrated to be genetically distinct from that of type I resistance in 1948 (6) but eluded molecular characterization until now. Nishimura and coworkers (12) have demonstrated its association with the gene rsmG in S. coelicolor and its orthologue gidB in Escherichia coli, Mycobacterium tuberculosis, and other species (15).
This work linking rsmG to streptomycin resistance is of interest for technical reasons as well as biological ones. Genetic mapping is challenging in S. coelicolor and has apparently proven to be particularly difficult in this case. Reasons for this may be that the rsmG mutant phenotype is a relatively weak one and that most bacteria throw off type II streptomycin-resistant mutants at a relatively high frequency. The authors therefore made use of chip technology (1) recently developed for S. coelicolor, in which the entire genome sequence is arrayed in overlapping oligonucleotides. The arrays are interrogated by annealing them to wild-type and mutant chromosomal DNA, and the result is the straightforward identification of point mutations, insertions, or deletions within the mutant genome. In this case, the authors were able to show that a type II mutant had a sequence change in the S. coelicolor gene SCO3885, which they went on to rename rsmG for rRNA small subunit methyltransferase (12). This technology would likely be applicable to many organisms. For example, the identification of mutations that confer resistance to the diarylquinolone drug R207910 necessitated the nearly complete sequencing of three Mycobacterium sp. genomes (2). While high-throughput DNA sequencing grows increasingly efficient and affordable, it still requires a significant computational effort that could be avoided by this array technology.
The rsmG gene encodes a highly conserved S-adenosylmethionine (SAM) binding protein and is found in all sequenced bacterial genomes. In spite of this high degree of conservation, the gene is nonessential: a deletion mutation confers type II streptomycin resistance and, in S. coelicolor, the overproduction of actinorhodin. This phenotype is associated with the loss of a specific 16S rRNA methylation at G518 in S. coelicolor (12) or G527 in E. coli (15), a residue that is found within the “530 loop” of the 16S rRNA and which interacts directly with streptomycin (4). Mutations in the M. tuberculosis orthologue of rsmG, gidB, were found to be tightly associated with type II streptomycin resistance in a large collection of clinical isolates (15).
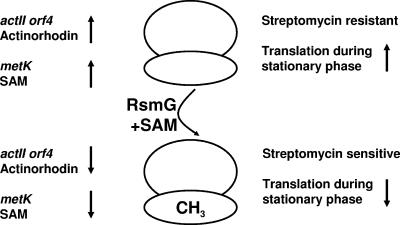
Previous work from the Ochi laboratory and others (10, 14, 16) demonstrated that type II mutants of S. coelicolor express SAM synthetase at higher levels than congenic wild-type strains (14). Consistent with this, they show here that an rsmG deletion mutant exhibits increased SAM synthetase activity late in the growth cycle and that this could be correlated with enhanced transcription of the SAM synthetase-encoding gene metK. An additional feature of this mutant is that translation was greatly enhanced in stationary phase cells relative to that a wild-type strain. This, however, was not caused by the elevated level of SAM synthetase or SAM levels as overexpression of metK from a high-copy-number plasmid did not confer enhanced translation in a wild-type strain. Enhanced translational efficiency may therefore be linked to the absence of 16S rRNA methylation. Overexpression of metK did, however, give rise to the overproduction of actinorhodin, as reported previously (10, 14). These phenomena are summarized in Fig. 1. What is most striking is the fact that in addition to changing the ribosome's sensitivity to streptomycin, modification by RsmG seems to lower actinorhodin and SAM production through transcriptional effects. Somehow, the status of the ribosome is influencing the transcription of metK and actII-ORF4, the pathway-specific activator of the actinorhodin biosynthetic genes.
FIG. 1.
Summary of the known biochemical, transcriptional, translational, and resistance effects of the RsmG methyltransferase.
In addition to addressing a 60-year-old question in antibiotic resistance, this work raises significant questions (12). It would appear that all bacteria, including the soil bacterium S. coelicolor, which may well share its habitat with streptomycin producers, encode a methyltransferase that makes them more sensitive to streptomycin. What do they gain from this? Loss of RsmG-mediated modification of the ribosome increases SAM synthetase production, translation efficiency during stationary phase, and remarkably, the production of the polyketide actinorhodin. How do the pathway-specific and pleiotropic antibiotic regulators identified in S. coelicolor contribute to this regulation? What is the significance of this chemical genetic interaction between the two types of streptomycin resistance and actinorhodin production, and does this sort of interaction extend to other antibiotics? This work is clearly an important step toward addressing these questions.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
Published ahead of print on 23 March 2007.
REFERENCES
- 1.Albert, T. J., D. Dailidiene, G. Dailide, J. E. Norton, A. Kalia, T. A. Richmond, M. Molla, J. Singh, R. D. Green, and D. E. Berg. 2005. Mutation discovery in bacterial genomes: metronidazole resistance in Helicobacter pylori. Nat. Methods 2:951-953. [DOI] [PubMed] [Google Scholar]
- 2.Andries, K., P. Verhasselt, J. Guillemont, H. W. Gohlmann, J. M. Neefs, H. Winkler, J. Van Gestel, P. Timmerman, M. Zhu, E. Lee, P. Williams, D. de Chaffoy, E. Huitric, S. Hoffner, E. Cambau, C. Truffot-Pernot, N. Lounis, and V. Jarlier. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223-227. [DOI] [PubMed] [Google Scholar]
- 3.Bibb, M. J. 2005. Regulation of secondary metabolism in streptomycetes. Curr. Opin. Microbiol. 8:208-215. [DOI] [PubMed] [Google Scholar]
- 4.Carter, A. P., W. M. Clemons, D. E. Brodersen, R. J. Morgan-Warren, B. T. Wimberly, and V. Ramakrishnan. 2000. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407:340-348. [DOI] [PubMed] [Google Scholar]
- 5.D'Costa, V. M., K. M. McGrann, D. W. Hughes, and G. D. Wright. 2006. Sampling the antibiotic resistome. Science 311:374-377. [DOI] [PubMed] [Google Scholar]
- 6.Demerec, M. 1948. Origin of bacterial resistance to antibiotics. J. Bacteriol. 56:63-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong, H. J., M. I. Hutchings, J. M. Neu, G. D. Wright, M. S. Paget, and M. J. Buttner. 2004. Characterization of an inducible vancomycin resistance system in Streptomyces coelicolor reveals a novel gene (vanK) required for drug resistance. Mol. Microbiol. 52:1107-1121. [DOI] [PubMed] [Google Scholar]
- 8.Hong, H. J., M. S. Paget, and M. J. Buttner. 2002. A signal transduction system in Streptomyces coelicolor that activates the expression of a putative cell wall glycan operon in response to vancomycin and other cell wall-specific antibiotics. Mol. Microbiol. 44:1199-1211. [DOI] [PubMed] [Google Scholar]
- 9.Hosaka, T., J. Xu, and K. Ochi. 2006. Increased expression of ribosome recycling factor is responsible for the enhanced protein synthesis during the late growth phase in an antibiotic-overproducing Streptomyces coelicolor ribosomal rpsL mutant. Mol. Microbiol. 61:883-897. [DOI] [PubMed] [Google Scholar]
- 10.Kim, D. J., J. H. Huh, Y. Y. Yang, C. M. Kang, I. H. Lee, C. G. Hyun, S. K. Hong, and J. W. Suh. 2003. Accumulation of S-adenosyl-l-methionine enhances production of actinorhodin but inhibits sporulation in Streptomyces lividans TK23. J. Bacteriol. 185:592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein, M., and L. J. Kimmelman. 1946. The role of spontaneous variants in the acquisition of streptomycin resistance by the shigellae. J. Bacteriol. 52:471-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura, K., T. Hosaka, S. Tokuyama, S. Okamoto, and K. Ochi. 2007. Mutations in rsmG, encoding a 16S rRNA methyltransferase, result in low-level streptomycin resistance and antibiotic overproduction in Streptomyces coelicolor A3(2). J. Bacteriol. 189:3876-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto-Hosoya, Y., T. Hosaka, and K. Ochi. 2003. An aberrant protein synthesis activity is linked with antibiotic overproduction in rpsL mutants of Streptomyces coelicolor A3(2). Microbiology 149:3299-3309. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto, S., A. Lezhava, T. Hosaka, Y. Okamoto-Hosoya, and K. Ochi. 2003. Enhanced expression of S-adenosylmethionine synthetase causes overproduction of actinorhodin in Streptomyces coelicolor A3(2). J. Bacteriol. 185:601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto, S., A. Tamaru, C. Nakajima, K. Nishimura, Y. Tanaka, S. Tokuyama, Y. Suzuki, and K. Ochi. 2007. Loss of a conserved 7-methylguanosine modification in 16S rRNA confers low-level streptomycin resistance in bacteria. Mol. Microbiol. 63:1096-1106. [DOI] [PubMed] [Google Scholar]
- 16.Shima, J., A. Hesketh, S. Okamoto, S. Kawamoto, and K. Ochi. 1996. Induction of actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2). J. Bacteriol. 178:7276-7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright, G. D. 2007. The antibiotic resistome: the nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 5:175-186. [DOI] [PubMed] [Google Scholar]