Abstract
PcrA is a conserved DNA helicase present in all gram-positive bacteria. Bacteria lacking PcrA show high levels of recombination. Lethality induced by PcrA depletion can be overcome by suppressor mutations in the recombination genes recFOR. RecFOR proteins load RecA onto single-stranded DNA during recombination. Here we test whether an essential function of PcrA is to interfere with RecA-mediated DNA recombination in vitro. We demonstrate that PcrA can inhibit the RecA-mediated DNA strand exchange reaction in vitro. Furthermore, PcrA displaced RecA from RecA nucleoprotein filaments. Interestingly, helicase mutants of PcrA also displaced RecA from DNA and inhibited RecA-mediated DNA strand exchange. Employing a novel single-pair fluorescence resonance energy transfer-based assay, we demonstrate a lengthening of double-stranded DNA upon polymerization of RecA and show that PcrA and its helicase mutants can reverse this process. Our results show that the displacement of RecA from DNA by PcrA is not dependent on its translocase activity. Further, our results show that the helicase activity of PcrA, although not essential, might play a facilitatory role in the RecA displacement reaction.
DNA helicases play important roles in DNA transactions, such as replication, transcription, recombination, and repair (17, 45). While some bacterial helicases, such as the replicative DnaB helicase, are essential for cell survival, others, such as the UvrD and Rep helicases of Escherichia coli, are not absolutely necessary for cell growth (8, 24). PcrA is a conserved helicase found in gram-positive bacteria that belongs to the SF1 superfamily. It shares homology with E. coli Rep and UvrD helicases (18) and is necessary for cell survival and plasmid rolling-circle (RC) replication (20, 36). PcrA shows a strong preference for binding and unwinding substrates containing a hairpin structure and 5′ single-stranded (ss) tails (3, 35). Previous studies have shown that in the absence of PcrA, chromosomal DNA synthesis is not appreciably affected, although cells fail to undergo division (36). Furthermore, pcrA mutants are hyperrecombinogenic (37). The lethality of pcrA mutants can be overcome by suppressor mutations in the recombination genes recFOR (37). Since the function of the RecFOR proteins is to load RecA onto single-stranded DNA (ssDNA) and modulate its function during DNA recombination or repair (6, 33, 41), one function of PcrA could be to inhibit the RecA-mediated DNA strand exchange (SE) reaction. PcrA3, a mutant of PcrA that is severely impaired in its helicase activity, is unable to support plasmid RC replication (2). However, pcrA3 mutants are viable, suggesting that the helicase activity of PcrA may not be essential for cell growth.
The RecA protein plays a central role in genetic recombination by catalyzing the pairing of homologous DNA (10, 25, 38). During recombination, RecA monomers polymerize on the DNA in a 5′-to-3′ direction (see references 10 and 25 and references therein). RecA is active only as a helical, nucleoprotein filament in complex with ATP and DNA (31). Proteins that affect RecA function do so either by binding directly to RecA (16, 49), by directly competing for DNA binding (15), by modulating the dynamics of the RecA nucleoprotein filament (6, 33, 41), or by actively displacing RecA from DNA (34, 46). Here we demonstrate that PcrA inhibits RecA-mediated DNA SE. We also show that PcrA displaces RecA from both ssDNA and double-stranded DNA (dsDNA). PcrA3 and a site-directed helicase mutant of PcrA retained the above activities, demonstrating that the helicase activity of PcrA is dispensable for the displacement of RecA. Our results provide a biochemical basis for regulation of recombination by PcrA in gram-positive bacteria.
MATERIALS AND METHODS
Proteins and DNA.
E. coli RecA protein was purified as previously described (5). The PcrA helicases from Staphylococcus aureus, Bacillus anthracis, and Bacillus cereus were purified and assayed as described earlier (2, 4, 7, 35). Streptavidin magnetic particles were purchased from Stratagene. The M13mp18 RFform, M13mp18 ssDNA, and T4 gene 32 protein (gp32) were purchased from New England Biolabs. Linear dsDNA was prepared by digesting M13mp18 replicative form (RF) DNA with SmaI. ssDNA binding protein (SSB) of E. coli was purchased from United States Biochemical.
Generation of the PcrA helicase mutant.
We used the plasmid pQE-30 derivative containing the wild-type S. aureus pcrA gene as an amino-terminal His6 fusion as a template to generate the PcrA helicase mutant (PcrAH−) using the QuikChange site-directed mutagenesis kit (Stratagene). The K33A Q250R change was based on studies with Bacillus stearothermophilus PcrA (48) and was created in two steps. First, the following two overlapping PCR primers were used to make the K33A change by standard procedures described above: 5′GCTGGTTCAGGGGCGACACGTGTTTTA3′ (forward) and 5′TAAAACACGTGTCGCCCCTGAACCAGC3′ (reverse), where the underlined sequences correspond to the changed amino acid codons. The sequence of the mutant clone was verified, and the following two overlapping primers were used to make the Q250R change in PcrASau: 5′GTAGGTGACTCAGATCGGTCAATTTATGGTTGG 3′ (forward) and CCAACCATAAATTGACCGATCTGAGTCACCTAC 3′ (reverse). Ten nanograms of template DNA and 200 pM primers were used, and the amplification reaction was carried out as follows: (i) 94°C for 3 min; (ii) 15 cycles of 94°C for 1 min, 55°C for 1 min, and 68°C for 7 min; and (iii) 68°C for 10 min. The PCR product was treated with DpnI to remove the parental, methylated DNA. Subsequently, the amplified DNA was gel purified and introduced into E. coli XL-1 blue cells. Ampicillin-resistant transformants were isolated, plasmid DNA was isolated, and the sequence of the mutation was confirmed. The resulting S. aureus PcrAH− mutant (K33A Q250R) carried a His6 fusion at its amino-terminal end. The His-PcrAH− mutant proteins were overexpressed in E. coli M15 by induction with 1 mM isopropyl-β-d-thiogalactopyranoside at 37°C for 2 h and purified by nickel affinity chromatography as described previously for His-PcrA (3). The concentration of the protein preparations reached about 0.5 mg/ml in the peak fractions, and their purity was approximately 90%.
ATPase assays.
ATPase activity was measured by two different methods. In the first method, the ATPase activities of PcrA and its mutants were measured by hydrolysis of [α-32P]dATP. Reactions (20 μl) were carried out in 1× TEKEM buffer [10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 100 mM KCl, 10 mM Mg acetate, and 10% ethylene glycol (vol/vol)] containing 1 μCi of [α-32P]dATP and appropriate concentrations of PcrA or its various mutants. The reaction mixtures also contained 5 μg/ml of an ss oligonucleotide where indicated. Reaction mixtures were incubated at 37°C for 1 h. To stop the reaction, EDTA and sodium dodecyl sulfate (SDS) were added to final concentrations of 20 mM and 1%, respectively, and the reactions heated to 65°C for 2 min. Five-microliter aliquots were subjected to thin-layer chromatography (TLC) on cellulose polyethyleneimine sheets using 0.5 M KH2PO4 (pH 3.5) buffer. The TLC sheets were dried and subjected to autoradiography. In the second method, quantification of the ATPase activity was done by using the malachite green phosphate assay kit (Bioassay Systems) according to the manufacturer's instructions. ATP hydrolysis was monitored by formation of a green complex between malachite green, molybdate, and free orthophosphate by measuring the increase in absorbance at 650 nm. Reactions were carried out by incubating PcrA or its mutants at a concentration of 12 nM with 1 mM ATP at 37°C for 1 h in the presence or absence of 5 μg/ml of an ss oligonucleotide.
DNA binding assays.
Binding of PcrA or its mutants to various DNA substrates was studied by electrophoretic mobility shift assays. Various DNA substrates (8) were prepared by labeling one strand of the oligonucleotides with 32P at the 5′ end using T4 polynucleotide kinase and annealing the cold complementary strand at a threefold molar excess. Reactions were carried out in a buffer consisting of 20 mM Tris-HCl (pH 8.0), 100 mM KCl, 1 mM EDTA, 4 ng/μl poly(dI-dC), 5 mM dithiothreitol (DTT), 10% ethylene glycol, various concentrations of PcrA or its mutants, and approximately 3 nM DNA substrate. The reactions were incubated at 20°C for 15 min and the DNA-protein complexes resolved by electrophoresis on 6% native polyacrylamide gels. The gels were dried and subjected to autoradiography.
DNA helicase assays.
DNA substrates were prepared by labeling one strand of the oligonucleotides with 32P as described above. Helicase reactions were performed at 37°C for 20 min in a buffer containing 20 mM Tris-HCl (pH 7.5), 100 mM KCl, 3 mM MgCl2, 3 mM ATP, 10 mM DTT, 10% glycerol, approximately 4 nM labeled DNA substrate, and the indicated concentrations of PcrA or its mutants. Reactions were stopped by the addition of SDS-dye and the products analyzed by 10% native polyacrylamide gel electrophoresis. Gels were subsequently dried and exposed to Kodak X-ray films.
RecA DNA SE reaction.
Circular M13mp18 ssDNA (SSC) (5 μM nucleotides) was incubated at 37°C for 5 min with 6 μM RecA in RecA buffer (20 mM Tris acetate, pH 7.5, 1 mM DTT, 10 mM magnesium acetate) in the presence of an ATP regenerating system consisting of pyruvate kinase, lactate dehydrogenase, and phosphoenol pyruvate essentially as described previously (11). DNA SE reactions were carried out in the presence of 0.3 μM SSB (tetramer) and initiated by addition of linear dsDNA (15 μM nucleotides), and incubations were continued for the indicated time periods. The total volume of the reaction was 25 μl, containing 5 mM ATP. At specific time intervals, aliquots were removed and deproteinized by incubation at 37°C for 15 min using RecA stop buffer (0.1% SDS, 5 mM EDTA, 10 μg/ml proteinase K). The reaction products were analyzed on 1% agarose gels in the presence of 0.5 μg/ml ethidium bromide. The time dependence of PcrA inhibition was checked by adding PcrA at appropriate times after the addition of dsDNA. In a separate experiment, PcrA was added at various concentrations immediately prior to the addition of linear dsDNA in order to check the concentration-dependent inhibition of SE reactions. Samples were incubated further for 30 min at 37°C.
Quantification of inhibition of DNA SE.
The products obtained in DNA SE were denatured with 0.5 N NaOH, neutralized, and transferred to nylon membranes by the capillary method (40). The DNA was fixed to the membrane by UV cross-linking and probed with M13mp18 RF DNA labeled with 32P using random primers (40). Hybridization was carried out at 65°C for 3 h, followed by two washes each at 65°C for 30 min with 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1% SDS, and 0.1× SSC-0.1% SDS. The membranes were dried, and hybridization signals were imaged and quantified by phosphorimaging. The results were plotted using Microsoft Excel. The results are expressed as percentages of open circular (OC) DNA formed relative to the intensity of the OC DNA in the absence of PcrA (100%).
RecA displacement from ssDNA.
Streptavidin magnetic particles (SM) were equilibrated in RecA buffer, and 20 μl of SM slurry was used per reaction. The sequences of oligonucleotides used were 5′ C/BiodT/T GGC GAC GGC AGC GAG GC (dT)20 3′ and 5′ (dT)20GGC GAC GGC AGC GAG GC/BiodT/C 3′ (BiodT is biotinylated dT). One microliter of 20 pM biotinylated oligonucleotides (bio-oligonucleotides) was added to SM in the RecA buffer and allowed to bind for 20 min at 37°C. The length of the oligonucleotides used was based on a previous study (5). Unbound oligonucleotides were removed by magnetic separation and washing and the beads resuspended in RecA buffer supplemented with an ATP regenerating system. The reaction contained 5 mM ATP or other nucleotides as indicated. RecA protein was added to a final concentration of 6 μM and allowed to bind to the slurry in a total volume of 20 μl by incubation of the mixture at 37°C for 1 or 5 min. RecA bound to the bio-oligonucleotides was collected using magnetic capture of SM and the protein eluted with 1× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer. The eluted fractions, along with the RecA protein released spontaneously into the supernatant during the above-described incubations, were analyzed by 10% SDS-PAGE and Coomassie blue staining. To study the displacement of RecA by PcrA, 460 nM PcrA was added to the RecA-bio-oligonucleotide complexes and incubated at 37°C for the indicated time periods. Displaced RecA was obtained by collecting the supernatant after removing the remaining RecA-bio-oligonucleotide complexes by magnetic separation. Both the displaced RecA and RecA remaining bound to the oligonucleotides were subjected to SDS-PAGE and Coomassie blue staining.
Displacement of SSB and gp32 by PcrA.
The ability of PcrA to displace the E. coli SSB protein was tested as described above for RecA except that the reaction mixtures (20 μl) contained 20 mM Tris-HCl, pH 8.0, 50 mM NaCl, 1 mM DTT, 3 mM MgCl2, 5% glycerol, and 5 μg of SSB (30) and supplemented with the ATP regenerating system. T4 gene 32 protein (gp32) binding was carried out in a buffer (20 μl) containing 20 mM Tris-acetate, pH 7.9, 50 mM potassium acetate, 10 mM magnesium acetate, 1 mM DTT, and 12 μg of gp32. Analysis of bound and displaced SSB or gp32 protein was carried out by 15% SDS-PAGE and Coomassie blue staining.
Synthesis of single-pair FRET (spFRET) substrate.
The DNA substrate containing four fluorescence resonance energy transfer (FRET) pairs was synthesized as follows: template oligonucleotide [5′ TA(dT)12G(dT)20G(dT)12A(dT)20A (dT)12G(dT)20G(dT)12ATTTTGATTAGGATCCGG 3′] was hybridized to biotinylated primer oligonucleotide [5′/5Bio/(dT)20CCGGATCCTAATCAA 3′] utilizing a complementary 15-nucleotide (nt) region downstream of the (dT)12 tail in the template oligonucleotide. Extension synthesis was done in the presence of 100 μM dATP, 10 μM Cy5-dCTP, and 10 μM Cy-3 dUTP using Klenow polymerase (NEB) at 37°C for 1 h. Unincorporated label was removed by ethanol precipitation and washing the nucleic acid pellet with an excess of 70% ethanol. The DNA substrate containing two FRET pairs was synthesized in a similar manner, except that the template oligonucleotide used was 5′ (dT)9A(dT)12G(dT)20G(dT)12ATTTTGATTAGGAT CCGG 3′, which yielded a shorter product.
spFRET-based assay for RecA assembly and disassembly from dsDNA.
Fluorescence intensity measurements for Cy3 and Cy5 dyes were done using total internal reflection fluorescence microscopy. Construction of total internal reflection fluorescence microscopy and assembly of the reaction chamber have been described previously (50). Briefly, the slide surface was coated with streptavidin and biotinylated DNA substrates were added at ∼2 fmol/μl. Unbound DNA was washed away, and RecA buffer containing oxygen scavenger (3% [wt/wt] glucose, 1% β-mercaptoethanol, 0.1 mg/ml glucose oxidase, and 0.02 mg/ml catalase) was added, and fluorescence intensities for Cy3 and Cy5 were measured. RecA was added at a concentration of 6 μM in a buffer containing an ATP regenerating system. The slide was preincubated in a water bath chamber for 5 min to allow RecA binding, and Cy3 and Cy5 intensities were measured. To measure RecA displacement, 500 nM of PcrA or its mutants was added. Fluorescence intensity measurements were carried out immediately. The apparent efficiency of FRET (Eapp) value was calculated using IPLab software, and the results were plotted using Origin and Excel as described previously (44). The mean values were obtained by Gaussian curve fitting of the Eapp values. The ssDNA containing the dye pairs was separated from the dsDNA substrate essentially as described previously (19) to analyze the Eapp value coming from the same molecule of DNA when it is ss.
RESULTS
PcrA inhibits RecA-mediated DNA SE.
To understand how S. aureus PcrA (PcrASau) might inhibit RecA function, we utilized the well-characterized DNA SE reaction. Since the S. aureus RecA protein is not available, we used the homologous RecA protein of E. coli in these experiments. The E. coli and S. aureus RecA proteins are 62% identical. Further, the RecA protein of E. coli and other organisms, including those of gram-positive bacteria, such as Bacillus subtilis, can complement recA mutants of each other (13, 39).
Thus, to evaluate whether PcrA could affect DNA SE by E. coli RecA, typical three-strand DNA SE reactions were carried put. As observed previously, incubation of M13mp18 ssDNA (SSC) with RecA followed by the addition of linear M13mp18 dsDNA (DSL) is expected to generate OC and linear ssDNA (Fig. 1A). Further, at early time points, joint molecule intermediates are observed (Fig. 1B), and these are converted to the OC form by branch migration (Fig. 1B). Quantification of the reaction products by Southern blot hybridization showed that conversion of the linear substrate to the OC product was complete within 20 min (Fig. 1C).
FIG. 1.
Time dependence of RecA SE reaction and its inhibition by PcrA. (A) Schematic of RecA SE reaction. SSC DNA was coated with 6 μM RecA and mixed with DSL DNA. Transfer of the complementary ssDNA strand from DSL DNA to SSC DNA results in the formation of closed OC DNA and linear ssDNA. (B) Time course of SE reaction. RecA-mediated SE reaction was carried out for the indicated time periods, samples were run on a 1% agarose gel and transferred to a nylon membrane, and Southern blots were hybridized to labeled M13 RF DNA to visualize and quantify products. (C) Quantification of gel shown in panel B. Relative levels of DSL and OC DNA are shown as percentages of intensity of DSL DNA in the absence of RecA, which was taken to be 100%. Empty bars represent DSL DNA, while filled bars represent OC DNA. (D) Time dependence of inhibition of SE reaction by PcrA. Four hundred sixty nanomolar PcrA was added at various times after addition of DSL DNA. Samples were run on a 1% agarose gel containing ethidium bromide and visualized after extensive destaining. A negative image of the ethidium bromide-stained gel is shown. (E) Inhibition of SE reaction by PcrASau (Sau), PcrABan (Ban), or PcrABce (Bce) helicase. Increasing concentrations (4.6 nM, 46 nM, and 460 nM) of the various PcrA helicases were added to the SE reaction containing RecA-SSC DNA complex immediately before addition of dsDNA. A negative image of the gel is shown. (F) Quantification of SE inhibition by PcrASau, PcrABan, and PcrABce at the above three concentrations was carried out by transferring the DNA on the gel shown in panel E to a nylon membrane and hybridizing to labeled M13 RF DNA. Results are expressed as percent inhibition of the OC DNA product formed in the presence of various concentrations of different PcrA helicases. JM, joint molecule intermediates; OC, open-circular DNA; DSL, linear double-stranded DNA; SSC, circular ssDNA.
To determine whether PcrASau could inhibit the function of RecA, PcrASau was added to DNA SE reactions at different times. When PcrA was added immediately after the addition of linear duplex DNA, the reaction was almost completely inhibited (Fig. 1D, 0-min time interval). When PcrA was added after 5 min or later after the start of the reaction (i.e., after the addition of linear dsDNA), PcrA-mediated inhibition was severely reduced, as seen by the formation of significant levels of both joint molecule intermediates and OC products (Fig. 1D). Since a significant level of OC product is obtained in the presence of PcrA, these results also rule out the possibility that the inhibition of DNA SE was not the result of unwinding of the product once the exchange of strands was complete. Since PcrA does not unwind blunt-ended linear dsDNA (3), these results are not due to the formation of ssDNA, which may promote the annealing reaction by the RecA protein. Therefore, PcrA inhibits DNA SE by RecA.
We next tested the ability of PcrA from various gram-positive bacteria to inhibit DNA SE. For this, we added PcrASau or the PcrA helicase from Bacillus anthracis (PcrABan) or Bacillus cereus (PcrABce) to the reaction immediately prior to the addition of linear dsDNA. These three PcrA proteins were found to be active in their ATPase, helicase, and DNA binding activities (data not shown). We observed that PcrASau, PcrABan, and PcrABce inhibited the DNA SE reaction in a concentration-dependent manner (Fig. 1E and F). Furthermore, the observed levels of inhibition of PcrABan and PcrABce were similar to that of PcrASau. The inhibition of OC product formation was almost complete at a ratio of 1 molecule of PcrA per 10 monomers of RecA in the case of PcrABce.
Inhibition of RecA-mediated DNA SE by PcrA mutants.
The inhibition of DNA SE could be mediated via protein-protein interactions, or possibly the helicase activity of PcrA could be responsible. In the latter case, the translocating PcrA protein would displace the RecA protein for the DNA. To determine whether the helicase activity of PcrA is required for the inhibition of RecA-mediated DNA SE, two different helicase mutants of PcrA were utilized. PcrA3 is a mutant of PcrASau that supports cell survival but is unable to support the RC replication of the pT181 plasmid (2, 20). We have previously shown that PcrA3 lacks detectable helicase activity and is deficient in its ability to hydrolyze ATP (2). Since the mutation in PcrA3Sau (T61I) does not involve residues directly involved in ATP hydrolysis or ssDNA contacts (48), we generated an additional ATPase/helicase mutant of PcrASau (K33A Q250R). The corresponding individual mutations in these conserved residues in the related B. stearothermophilus PcrA protein (PcrABst) have been shown to severely impair its ATPase and helicase activities (14, 48).
The K33A Q250R helicase mutant of S. aureus (PcrAH−) was purified (Fig. 2A) and tested for its ability to hydrolyze ATP and unwind a variety of DNA substrates. This mutant was found to be severely compromised in its ATPase activity (Fig. 2B). The lack of ATPase activity of this mutant was also confirmed using a sensitive malachite green-based colorimetric assay. This assay using PcrAH− failed to detect formation of any inorganic phosphate even in the presence of ssDNA (data not shown), confirming that the mutant had completely lost its ability to hydrolyze ATP. However, similar to wild-type PcrA, the PcrAH− mutant bound efficiently to a partially duplex DNA substrate in which the ss region is predicted to fold into a stem-loop structure (Fig. 2C). In contrast, both wild-type PcrA and the helicase mutant did not show detectable binding to partially duplex substrates containing either 5′ or 3′ oligo(dT) tails (Fig. 2C and 3). As reported earlier (3), the wild-type PcrASau protein unwound the stem-loop DNA substrate efficiently (Fig. 2D). As expected, the PcrAH− mutant lacked detectable helicase activity on all DNA substrates examined (Fig. 2D and E). The PcrAH− mutant bound to the folded DNA substrate, yielding a protein-DNA complex (Fig. 2D). The results of the helicase assays were consistent with the inability of this mutant to hydrolyze ATP.
FIG. 2.
(A) Purification and characterization of His-PcrAH− protein. (A) SDS-PAGE of purified His-PcrAH−. M, molecular weight markers; U and I, protein lysates from uninduced or isopropyl-β-d-thiogalactopyranoside-induced cells, respectively; P, 500 ng purified His-PcrAH− protein. (B) ATPase activity of PcrAH−. Various concentrations of wild-type (wt) PcrA or PcrAH− were tested for ATP hydrolysis in the presence or absence of ssDNA by TLC on polyethyleneimine-cellulose. (C) DNA binding activity of PcrAH−. DNA binding activities of wild-type PcrA and PcrAH− were analyzed by electrophoretic mobility shift assays using three different DNA probes. P, free probe; C, DNA-protein complex. (D) Helicase activities of PcrA and PcrAH− in the presence of a folded DNA substrate. ds, partially double-stranded probe; ss, single-stranded product; C, DNA-protein complex. (E) Helicase activities of PcrA and PcrAH− in the presence of partially duplex substrates containing either 3′ or 5′ ss dT tails. The structures of the various DNA substrates used are represented schematically at the bottom of the gel.
FIG. 3.
Inhibition of SE reaction by PcrA mutants. (A) Increasing concentrations (4.6 nM, 46 nM, and 460 nM) of PcrA or its mutants were added to the SE reaction and the products analyzed as described in the legend to Fig. 2A. (B) Quantification of SE inhibition by PcrA3Sau and PcrAH− at the above three concentrations was carried out as described in the legend to Fig. 1E.
Once we had demonstrated that these mutants were in fact helicase deficient, we used them to ascertain whether the helicase activity of PcrA is required for the inhibition of RecA-mediated DNA SE. These experiments showed that both the PcrA3 and PcrAH− mutant proteins inhibited DNA SE with efficiencies comparable to that of wild-type PcrA (Fig. 3A and B). This suggested that the helicase activity of PcrA is dispensable for this function.
PcrA displaces RecA from ssDNA.
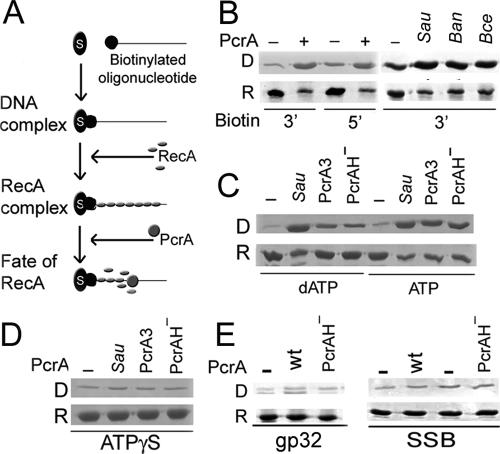
To determine if PcrA inhibits DNA SE by displacing RecA bound to the ssDNA substrate, we developed an assay using streptavidin-coated magnetic beads and biotinylated ss oligonucleotides (schematic in Fig. 4A). The 40-nt-long ssDNA contained a 19-base region of mixed composition which is predicted not to form hairpins and an oligo(dT)21 tail. These features have been shown to be optimal for formation of stable RecA complexes on short ssDNA (5). In the assay, RecA filaments were formed on ssDNA bound to the beads in the presence of ATP and an ATP regenerating system. Unbound RecA was removed, and PcrA was then added to the RecA-DNA complexes in the presence of an ATP regenerating system. PcrA was unable to disrupt the biotin-SA complex (data not shown). RecA displaced from ssDNA by PcrA and RecA that remained bound to the DNA were analyzed by SDS-PAGE. The RecA-DNA complex was stable for at least 15 min in the absence of PcrA (data not shown). In contrast, RecA was displaced by PcrA within 5 min from oligonucleotides labeled with biotin at their 3′ or 5′ ends with almost the same efficiency (Fig. 4B). The PcrABan and PcrABce helicases also had RecA displacement activity (Fig. 4B).
FIG. 4.
Displacement of RecA from ssDNA by PcrA. (A) Schematic of the assay. Bio-oligonucleotides were bound to streptavidin-coated magnetic beads. RecA was added to form the RecA-DNA-bead complex. PcrA was then added, and the fate of RecA was followed. (B) RecA displacement by PcrASau, PcrABan, and PcrABce. Row D indicates displaced RecA, and row R indicates RecA remaining on the beads. (C) RecA displacement by PcrASau from RecA-DNA complexes assembled in the presence of dATP or ATP. (D) Lack of RecA displacement by PcrASau when γ-S-ATP is substituted for ATP. (E) PcrASau does not displace the gp32 protein or SSB from ssDNA. wt, wild type.
The helicase activity of PcrA is not required to displace RecA from ssDNA.
To determine if the helicase activity of PcrA is necessary for the displacement of RecA from ssDNA, the helicase mutants PcrA3Sau and PcrAH− were used. As for DNA SE, these mutants were as capable of displacing RecA bound to the DNA as wild-type PcrA in the presence of ATP (Fig. 4C). It is known that RecA filaments assembled in the presence of dATP are more stable than those assembled in the presence of ATP (32). Therefore, we repeated the above experiments but replaced ATP with dATP. The results showed that RecA filaments assembled in the presence of dATP were more resistant to disassembly by PcrA helicase mutants than wild-type PcrA (Fig. 4C). These results suggest that the inherent stability of RecA nucleoprotein filaments affects its displacement by PcrA. If this is true, then a more stable RecA nucleoprotein filament should be resistant to displacement by either wild-type or mutant helicases. RecA-DNA complexes assembled in the presence of γ-S-ATP are known to be highly stable (10, 25). Wild-type PcrA, as well as PcrA3 and PcrAH− mutants, failed to displace RecA when ATP was replaced with γ-S-ATP (Fig. 4D). These results showed that the ATP hydrolysis function of RecA is an important component of its displacement from DNA by PcrA. RecA displacement activity of PcrA was specific to RecA, since PcrA did not displace other ssDNA binding proteins, such as E. coli SSB or the phage T4 gene 32 protein, from ssDNA (Fig. 4E).
Demonstration of RecA displacement from dsDNA by PcrA using a spFRET-based assay.
spFRET allows the measurement of the relative distance between donor and acceptor fluorescent dyes attached to DNA. Excitation of a donor dye at its specific wavelength leads to transfer of energy to an acceptor dye located within Forster distance of the donor, thereby exciting the acceptor dye. This energy transfer is reduced as the distance between the two dyes is increased. We developed a novel spFRET-based assay to study RecA displacement from dsDNA by PcrA (Fig. 5A). We used a synthetic tailed duplex substrate (134 bp with a dT20 ss tail) containing four equally placed Cy3/Cy5 FRET pairs (Fig. 5A). The optimal distance (Forster distance [R0]) between the Cy3 donor and the Cy5 acceptor dyes for yielding maximum FRET is 4.4 nm. The individual FRET pairs were kept 33 bp (∼11 nm) apart to prevent FRET between the pairs. The schematic in Fig. 5A shows one FRET pair (distance 1× = 4.4 nm), and the tailed duplex substrate carries four such pairs. Using this substrate, FRET would be observed only between a donor dye and an acceptor dye which are ∼4.4 nm apart (distance “1×”), thereby forming one FRET pair, but not between a donor within one pair and an acceptor of a neighboring pair, since they would be separated by ∼11 nm. Binding of RecA increases the length of dsDNA 1.5-fold (43). This is indicated by an increase in the length of the DNA at all four FRET pairs from “1×” to “1.5×” (Fig. 5A). Therefore, RecA binding to the substrate was expected to decrease the Eapp value due to lengthening of the DNA and the concomitant increase in distance separating the individual FRET pairs (from ∼4.4 nm to ∼6.6 nm). The 20-nt ssDNA tail served as the major site for nucleation of RecA, after which it could extend into the neighboring double-stranded region as the polymerization of RecA proceeds in a 5′-to-3′ direction (28).
FIG. 5.
PcrA displaces RecA from dsDNA. (A) Schematic of FRET-based assay for studying RecA displacement. Binding of RecA lengthens dsDNA 1.5-fold and decreases Eapp. Addition of PcrA displaces RecA from the DNA and increases the Eapp value. Distance 1× = 4.4 nm; 4n, four FRET pairs. (B to F) Histograms of Eapp of reactions as indicated. Means of Eapp values are indicated. Counts indicate numbers of individual DNA molecules. (G) PcrASau, PcrA3, and PcrAH− display similar efficiencies of RecA displacement from dsDNA. The y axis represents the number of molecules displaying low Eapp values divided by that of those displaying high Eapp values.
Using Gaussian curve fitting, we observed a high Eapp value for the naked DNA substrate, with a mean corresponding to 0.8 (Fig. 5B). Upon addition of RecA, the mean Eapp value decreased to 0.35 (Fig. 5C), indicating RecA binding and the concomitant lengthening of the DNA, leading to the separation of the dye pairs. This value was stable for the period of the experiment (at least up to 30 min). Upon addition of PcrA, there was a reversal in the mean Eapp value to ∼0.8 (Fig. 5E), indicating removal of RecA by PcrA. Compared to the Eapp value of the population of free dsDNA molecules (Fig. 5B), there was increased heterogeneity in the population of molecules upon addition of PcrA to the RecA/DNA complex (Fig. 5E). This could indicate the dynamic nature of the displacement reaction. When PcrA was added to naked dsDNA, there was no change in the mean Eapp value (Fig. 5D). We observed that naked ssDNA has an intermediate Eapp value of 0.55 (Fig. 5F), suggesting that PcrA did not fully unwind the tailed dsDNA during displacement of RecA in the above experiments. These results are consistent with the low processivity of PcrA (42).
PcrA and its helicase mutants are comparable in their efficiencies of RecA displacement from dsDNA.
Since PcrA is not very processive (42), we used a shorter substrate (74 bp with a dT20 tail) containing two FRET pairs to compare the efficiencies of RecA displacement by PcrA and its helicase mutants. We counted the total number of molecules showing high FRET (0.6 to 1.0) and low FRET (0 to 0.4) and expressed the DNA/RecA state as the ratio of the total number of low-FRET molecules to the total number of high-FRET molecules (Fig. 5G). A low FRET ratio indicates a naked (not bound by RecA) DNA molecule, whereas a high ratio indicates stretching of DNA upon binding of RecA. Addition of PcrA or its helicase mutants to the RecA-DNA complexes reversed the change in the FRET ratio (Fig. 5G). Moreover, we observed no noticeable differences in the RecA displacement activity of PcrASau, PcrA3Sau, or PcrAH−(Fig. 5G). These results further support the conclusion that the helicase activity of PcrA is dispensable for its ability to displace RecA from the DNA.
DISCUSSION
PcrA is an essential helicase present in all gram-positive bacteria (37). Genetic studies have suggested that PcrA and RecF, -O, and -R interact to sustain normal levels of recombination in bacteria (37). In this paper, we demonstrate that S. aureus PcrA displaces RecA from both ss- and dsDNA and inhibits RecA-mediated DNA SE, thereby providing a biochemical basis for the essential nature of this helicase. PcrA from B. anthracis and B. cereus also exhibited similar properties, suggesting the likely conservation of this feature across gram-positive bacterial genera.
The helicase-defective mutants PcrA3Sau and PcrAH− inhibited the RecA-mediated DNA SE and displaced RecA from DNA (Fig. 3, 4, and 5), suggesting that the helicase activity of PcrA is not essential for these functions. Since the pcrA knockout in S. aureus is not viable (21), it is unlikely that another redundant helicase substitutes for the function of PcrA in the absence of its helicase activity in vivo. Since we observed no noticeable differences in the efficiencies of RecA displacement between PcrA and its helicase mutants (PcrA3Sau and PcrAH−), our results suggest that PcrA can displace RecA without unwinding the DNA. The helicase and translocation activities of PcrA are dependent upon its ATPase activity (14). Since the K34A Q250R mutant of PcrA lacks detectable ATPase activity (Fig. 2B), our results suggest that RecA displacement may occur in the absence of translocation of PcrA on the DNA. Thus, the crucial molecular events leading to RecA displacement by helicase mutants of PcrA may occur during DNA binding of the helicase proteins adjacent to the RecA nucleoprotein filament.
An emerging theme in helicase biology is the “roadblock-clearing” role of helicases (1). Here the translocating helicases simply displace or remove DNA-bound obstacles in their path. This is an important function for DNA helicases, since proteins bound to DNA could act as barriers to DNA polymerase during DNA replication (1). Two members of the SFI family of helicases, UvrD and Rep, have been shown to displace RecA bound to the DNA (34, 46). The eukaryotic ortholog of these helicases, Srs2, displaces the RecA homolog Rad51 from DNA (27, 47). The mechanism by which helicases displace RecA homologs from DNA is thought to involve ATP hydrolysis-coupled translocation on DNA (27, 34, 46, 47). For example, an Srs2 ATPase mutant was shown to make yeast cells hyperrecombinogenic, suggesting that the ATP hydrolysis function and the resultant translocase or helicase activity of this protein were required for its essential cellular role (26). On the other hand, both wild-type Sgs1 and its helicase mutant can block DNA crossovers in yeast (29). Therefore, it is possible that Sgs1 may perform its antirecombination function independently of its helicase activity. Although a helicase mutant of Srs2 does not displace Rad51 from DNA (47), it remains to be seen if a helicase mutant of Sgs1 can displace Rad51 from DNA. The mechanism used by PcrA therefore appears to be distinct from that observed previously for other DNA helicases. Although not essential, the helicase activity of PcrA could still play a facilitatory role in RecA displacement, as seen from the inability of the helicase mutants of PcrA to displace RecA assembled on ssDNA in the presence of dATP (Fig. 4C).
A high degree of cooperativity in the RecA filament has been unambiguously demonstrated (9, 10). Moreover, there have been studies showing the transmission of allosteric information along the RecA filament (9, 10). The key residues and interfaces involved in such activities have also been mapped (22, 23). Regions of RecA involved in monomer contacts are conserved (12) and might suggest the importance of these key elements in maintaining cooperativity of the RecA filament and its regulation in vivo. It is possible that DNA binding by PcrA3Sau and PcrAH− mutants destabilizes RecA/DNA complexes by disrupting the cooperativity that exists between RecA monomers in RecA nucleoprotein filaments.
Despite lacking detectable helicase activity, the PcrA3 mutant is able to support cell survival (2), suggesting that displacement of RecA by a helicase-independent mechanism is sufficient for this function. The wild-type helicase displaced RecA from the DNA more efficiently than the PcrA3Sau and PcrAH− mutants when RecA was assembled on ssDNA in the presence of dATP (Fig. 4C). Assembly in the presence of dATP is known to increase the stability of RecA filaments (32). Therefore, it is possible that wild-type PcrA may utilize both helicase-dependent and helicase-independent mechanisms during the displacement of RecA from the DNA. Thus, displacement of RecA from the DNA may be less efficient in the presence of helicase mutants of PcrA. Nevertheless, the displacement of RecA from the DNA appears to be sufficient to allow cell survival in the case of the S. aureus pcrA3 mutant, which lacks helicase activity. Current studies are directed towards gaining more insights into the relative contributions of helicase-dependent and helicase-independent mechanisms in the displacement of RecA from DNA by PcrA.
Acknowledgments
This work was supported by NIH grants GM031685 and AI064941 (S.A.K.), GM077872 (S.H.L.), and GM66831 (P.R.B.).
We thank Steve Kowalczykowski for helpful suggestions.
Footnotes
Published ahead of print on 20 April 2007.
REFERENCES
- 1.Aguilera, A. 2001. Double-strand break repair: are Rad51/RecA-DNA joints barriers to DNA replication? Trends Genet. 17:318-321. [DOI] [PubMed] [Google Scholar]
- 2.Anand, S. P., A. Chattopadhyay, and S. A. Khan. 2005. The PcrA3 mutant binds DNA and interacts with the RepC initiator protein of plasmid pT181 but is defective in its DNA helicase and unwinding activities. Plasmid 54:104-113. [DOI] [PubMed] [Google Scholar]
- 3.Anand, S. P., and S. A. Khan. 2004. Structure-specific DNA binding and bipolar helicase activities of PcrA. Nucleic Acids Res. 32:3190-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand, S. P., P. Mitra, A. Naqvi, and S. A. Khan. 2004. Bacillus anthracis and Bacillus cereus PcrA helicases can support DNA unwinding and in vitro rolling-circle replication of plasmid pT181 of Staphylococcus aureus. J. Bacteriol. 186:2195-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianco, P. R., and G. M. Weinstock. 1996. Interaction of the RecA protein of Escherichia coli with single-stranded oligodeoxyribonucleotides. Nucleic Acids Res. 24:4933-4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bork, J. M., M. M. Cox, and R. B. Inman. 2001. The RecOR proteins modulate RecA protein function at 5′ ends of single-stranded DNA. EMBO J. 20:7313-7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, T. L., A. Naqvi, S. P. Anand, M. G. Kramer, R. Munshi, and S. A. Khan. 2002. Biochemical characterization of the Staphylococcus aureus PcrA helicase and its role in plasmid rolling circle replication. J. Biol. Chem. 277:45880-45886. [DOI] [PubMed] [Google Scholar]
- 8.Colasanti, J., and D. T. Denhardt. 1987. The Escherichia coli rep mutation. X. Consequences of increased and decreased Rep protein levels. Mol. Gen. Genet. 209:382-390. [DOI] [PubMed] [Google Scholar]
- 9.Cox, J. M., O. V. Tsodikov, and M. M. Cox. 2005. Organized unidirectional waves of ATP hydrolysis within a RecA filament. PLoS Biol. 3:231-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox, M. M. 2003. The bacterial RecA protein as a motor protein. Annu. Rev. Microbiol. 57:551-577. [DOI] [PubMed] [Google Scholar]
- 11.Cox, M. M., and I. R. Lehman. 1981. recA protein of Escherichia coli promotes branch migration, a kinetically distinct phase of DNA strand exchange. Proc. Natl. Acad. Sci. USA 78:3433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta, S., N. Ganesh, N. R. Chandra, K. Muniyappa, and M. Vijayan. 2003. Structural studies on MtRecA-nucleotide complexes: insights into DNA and nucleotide binding and the structural signature of NTP recognition. Proteins 50:474-485. [DOI] [PubMed] [Google Scholar]
- 13.de Vos, W. M., S. C. de Vries, and G. Venema. 1983. Cloning and expression of the Escherichia coli recA gene in Bacillus subtilis. Gene 25:301-308. [DOI] [PubMed] [Google Scholar]
- 14.Dillingham, M. S., P. Soultanas, and D. B. Wigley. 1999. Site-directed mutagenesis of motif III in PcrA helicase reveals a role in coupling ATP hydrolysis to strand separation. Nucleic Acids Res. 27:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drees, J. C., S. Chitteni-Pattu, D. R. McCaslin, R. B. Inman, and M. M. Cox. 2006. Inhibition of RecA protein function by the RdgC protein from Escherichia coli. J. Biol. Chem. 281:4708-4717. [DOI] [PubMed] [Google Scholar]
- 16.Drees, J. C., S. L. Lusetti, S. Chitteni-Pattu, R. B. Inman, and M. M. Cox. 2004. A RecA filament capping mechanism for RecX protein. Mol. Cell 15:789-798. [DOI] [PubMed] [Google Scholar]
- 17.Egelman, E. H. 1998. Bacterial helicases. J. Struct. Biol. 124:123-128. [DOI] [PubMed] [Google Scholar]
- 18.Hall, M. C., and S. W. Matson. 1999. Helicase motifs: the engine that powers DNA unwinding. Mol. Microbiol. 34:867-877. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg, A., A. Blomstergren, O. Nord, M. Lukacs, J. Lundeberg, and M. Uhlen. 2005. The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis 26:501-510. [DOI] [PubMed] [Google Scholar]
- 20.Iordanescu, S. 1993. Characterization of the Staphylococcus aureus chromosomal gene pcrA, identified by mutations affecting plasmid pT181 replication. Mol. Gen. Genet. 241:185-192. [DOI] [PubMed] [Google Scholar]
- 21.Ji, Y., B. Zhang, S. F. Van Horn, P. Warren, G. Woodnutt, M. K. R. Burnham, and M. Rosenberg. 2001. Identification of critical staphylococcal genes using conditional phenotypes generated by antisense RNA. Science 293:2266-2269. [DOI] [PubMed] [Google Scholar]
- 22.Kelley De Zutter, J., A. L. Forget, K. M. Logan, and K. L. Knight. 2001. Phe217 regulates the transfer of allosteric information across the subunit interface of the RecA protein filament. Structure 9:47-55. [DOI] [PubMed] [Google Scholar]
- 23.Kelley, J. A., and K. L. Knight. 1997. Allosteric regulation of RecA protein function is mediated by Gln194. J. Biol. Chem. 272:25778-25782. [DOI] [PubMed] [Google Scholar]
- 24.Kornberg, A., and T. Baker. 1992. DNA replication, 2nd ed. W. H. Freeman & Co., New York, NY.
- 25.Kowalczykowski, S. C. 1991. Biochemistry of genetic recombination: energetics and mechanism of DNA strand exchange. Annu. Rev. Biophys. Biophys. Chem. 20:539-575. [DOI] [PubMed] [Google Scholar]
- 26.Krejci, L., M. Macris, Y. Li, S. Van Komen, J. Villemain, T. Ellenberger, H. Klein, and P. Sung. 2004. Role of ATP hydrolysis in the antirecombinase function of Saccharomyces cerevisiae Srs2 protein. J. Biol. Chem. 279:23193-23199. [DOI] [PubMed] [Google Scholar]
- 27.Krejci, L., S. Van Komen, Y. Li, J. Villemain, M. S. Reddy, H. Klein, T. Ellenberger, and P. Sung. 2003. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature 423:305-309. [DOI] [PubMed] [Google Scholar]
- 28.Lindsley, J. E., and M. M. Cox. 1990. Assembly and disassembly of RecA protein filaments occur at opposite filament ends. Relationship to DNA strand exchange. J. Biol. Chem. 265:9043-9054. [PubMed] [Google Scholar]
- 29.Lo, Y. C., K. S. Paffett, O. Amit, J. A. Clikeman, R. Sterk, M. A. Brenneman, and J. A. Nickoloff. 2006. Sgs1 regulates gene conversion tract lengths and crossovers independently of its helicase activity. Mol. Cell. Biol. 26:4086-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohmann, T. M., and M. E. Ferrari. 1994. Escherichia coli single-stranded DNA binding protein: multiple DNA-binding modes and cooperativities. Annu. Rev. Biochem. 63:527-570. [DOI] [PubMed] [Google Scholar]
- 31.McGrew, D. A., and K. L. Knight. 2003. Molecular design and functional organization of the RecA protein. Crit. Rev. Biochem. Mol. Biol. 38:385-432. [DOI] [PubMed] [Google Scholar]
- 32.Menetski, J. P., and S. C. Kowalczykowski. 1989. Enhancement of Escherichia coli RecA protein enzymatic function by dATP. Biochemistry 28:5871-5881. [DOI] [PubMed] [Google Scholar]
- 33.Morimatsu, K., and S. C. Kowalczykowski. 2003. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol. Cell 11:1337-1347. [DOI] [PubMed] [Google Scholar]
- 34.Myong, S., I. Rasnik, C. Joo, T. M. Lohman, and T. Ha. 2005. Repetitive shuttling of a motor protein on DNA. Nature 437:1321-1325. [DOI] [PubMed] [Google Scholar]
- 35.Naqvi, A., E. Tinsley, and S. A. Khan. 2003. Purification and characterization of the PcrA helicase of Bacillus anthracis. J. Bacteriol. 185:6633-6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petit, M. A., E. Dervyn, M. Rose, K. D. Entian, S. McGovern, S. D. Ehrlich, and C. Bruand. 1998. PcrA is an essential DNA helicase of Bacillus subtilis fulfilling functions both in repair and rolling-circle replication. Mol. Microbiol. 29:261-273. [DOI] [PubMed] [Google Scholar]
- 37.Petit, M. A., and D. Ehrlich. 2002. Essential bacterial helicases that counteract the toxicity of recombination proteins. EMBO J. 21:3137-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radding, C. M. 1989. Helical RecA nucleoprotein filaments mediate homologous pairing and strand exchange. Biochim. Biophys. Acta 1008:131-145. [DOI] [PubMed] [Google Scholar]
- 39.Roca, A. I., and M. M. Cox. 1990. The RecA protein: structure and function. Crit. Rev. Biochem. Mol. Biol. 25:415-456. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 41.Shan, Q., J. M. Bork, B. L. Webb, R. B. Inman, and M. M. Cox. 1997. RecA protein filaments: end-dependent dissociation from ssDNA and stabilization by RecO and RecR proteins. J. Mol. Biol. 265:519-540. [DOI] [PubMed] [Google Scholar]
- 42.Soultanas, P., M. S. Dillingham, F. Papadopoulos, S. E. Phillips, C. D. Thomas, and D. B. Wigley. 1999. Plasmid replication initiator protein RepD increases the processivity of PcrA DNA helicase. Nucleic Acids Res. 27:1421-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stasiak, A., E. Di Capua, and T. Koller. 1981. Elongation of duplex DNA by recA protein. J. Mol. Biol. 151:557-564. [DOI] [PubMed] [Google Scholar]
- 44.Tomschik, M., H. Zheng, K. van Holde, J. Zlatanova, and S. H. Leuba. 2005. Fast, long-range, reversible conformational fluctuations in nucleosomes revealed by single-pair fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. USA 102:3278-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuteja, N., and R. Tuteja. 2004. Prokaryotic and eukaryotic DNA helicases. Essential molecular motor proteins for cellular machinery. Eur. J. Biochem. 271:1835-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veaute, X., S. Delmas, M. Selva, J. Jeusset, E. Le Cam, I. Matic, F. Fabre, and M. A. Petit. 2005. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 24:180-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veaute, X., J. Jeusset, C. Soustelle, S. C. Kowalczykowski, E. Le Cam, and F. Fabre. 2003. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 423:309-312. [DOI] [PubMed] [Google Scholar]
- 48.Velankar, S. S., P. Soultanas, M. S. Dillingham, H. S. Subramanya, and D. B. Wigley. 1999. Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell 97:75-84. [DOI] [PubMed] [Google Scholar]
- 49.Venkatesh, R., N. Ganesh, N. Guhan, M. S. Reddy, T. Chandrasekhar, and K. Muniyappa. 2002. RecX protein abrogates ATP hydrolysis and strand exchange promoted by RecA: insights into negative regulation of homologous recombination. Proc. Natl. Acad. Sci. USA 99:12091-12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng, H., M. Tomschik, J. Zlatanova, and S. H. Leuba. 2005. Evanescent field fluorescence microscopy for analysis of protein/DNA interactions at the single-molecule level, p. 429-444. In E. Golemis and P. Adams (ed.), Protein-protein interactions, a molecular cloning manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.