Abstract
The food-borne pathogen Listeria monocytogenes attaches to environmental surfaces and forms biofilms that can be a source of food contamination, yet little is known about the molecular mechanisms of its biofilm development. We observed that nonmotile mutants were defective in biofilm formation. To investigate how flagella might function during biofilm formation, we compared the wild type with flagellum-minus and paralyzed-flagellum mutants. Both nonmotile mutants were defective in biofilm development, presumably at an early stage, as they were also defective in attachment to glass during the first few hours of surface exposure. This attachment defect could be significantly overcome by providing exogenous movement toward the surface via centrifugation. However, this centrifugation did not restore mature biofilm formation. Our results indicate that it is flagellum-mediated motility that is critical for both initial surface attachment and subsequent biofilm formation. Also, any role for L. monocytogenes flagella as adhesins on abiotic surfaces appears to be either minimal or motility dependent under the conditions we examined.
Listeria monocytogenes is a gram-positive food-borne pathogen that causes life-threatening infections in fetuses, newborns, and immunocompromised people. It also causes a severe flu-like illness in pregnant women and self-limited gastrointestinal infections in immunocompetent people (8, 15). L. monocytogenes successfully contaminates processed foods because it persists on food-processing surfaces in the form of biofilms (16). Unlike most other food-borne pathogens, L. monocytogenes grows during refrigeration. Thus, a small inoculum at the time of packaging can lead to a significant burden of organisms by the time it reaches the consumer (16). Biofilm-coated surfaces are particularly difficult to decontaminate, since bacteria in biofilms are more resistant to detergents, biocides, and antibiotics than are their planktonic counterparts (5, 9, 12). Very little is known about the molecular mechanisms of L. monocytogenes biofilm formation. Flagellum-mediated motility is important for biofilm formation by several gram-negative bacteria (18). In contrast, flagella are implicated as surface adhesins early in L. monocytogenes surface attachment, but a role for motility in biofilm formation has not been examined (29). This prompted us to investigate the role of flagella and flagellum-mediated motility in L. monocytogenes biofilm formation.
L. monocytogenes has four to six peritrichous flagella per cell, each of which consists of thousands of flagellin monomers which are modified by β-O-linked glycosylation (23). In contrast to the case with many other bacteria, in L. monocytogenes, biosynthesis of flagella is temperature dependent and regulated by a distinctly different mechanism than the well-described hierarchical regulation of gram-negative bacteria. Thus, at mammalian host physiologic temperature, 37°C, most L. monocytogenes strains do not produce flagella and are nonmotile (21). This is due to MogR repression of flagellar gene transcription at 37°C (11, 24). In contrast, at 30°C and below, L. monocytogenes is motile because MogR is inhibited by its antirepressor GmaR, thus permitting flagellar gene transcription (21, 25).
Flagella play important roles in early biofilm formation in several gram-negative bacteria (18). While it has been proposed that flagella might act in biofilm formation both as surface adhesins and as providers of force-generating motility, in at least Escherichia coli and Vibrio cholerae it is motility itself that is critical (19, 22, 30, 31). To date, the only published report on the role of L. monocytogenes flagella in attachment to abiotic surfaces suggests that, in the absence of motility, flagella have a role as adhesins in initial surface attachment to stainless steel (29). In this study, comparing wild-type bacteria to flagellum-minus and paralyzed-flagellum mutants, we demonstrate that flagellum-mediated motility is critical for L. monocytogenes biofilm formation on abiotic surfaces, and if there is any role for flagella as surface adhesins, it is either minimal or dependent upon motility.
MATERIALS AND METHODS
Strains and media.
Previous work on biofilm formation by other bacteria suggests that many of the most commonly studied, domesticated strains of bacteria are poor biofilm formers (4, 28). Preliminary experiments suggested that this might also be the case for L. monocytogenes. Therefore, we selected an environmental isolate, M35303A (also known as ZK3457), as our wild type because it had been previously identified as a robust biofilm former (3). This strain belongs to serovar 1/2a, a serovar commonly isolated from food preparation sites and one of the two serovars most commonly associated with human listeriosis (15). M35303A is a cysteine auxotroph, hence minimal medium was supplemented with 0.1 mg/ml cysteine (3, 27). The wild-type strain 10403S was grown in minimal medium supplemented with both cysteine and methionine at 0.1 mg/mg each. O'Neil and Marquis constructed and characterized the nonmotile mutant strains, HEL-304 (ΔflaA; flagellum-minus) and HEL-742 (motBD23A; paralyzed-flagella), and their respective complemented strains, HEL-447 (flaA+) and HEL-759 (motB+), in the 10403S background as previously described (17). Media used were tryptic soy broth yeast extract (TSBYE; containing 3% Bacto tryptic soy broth [Becton, Dickinson, and Co.] and 0.6% Bacto yeast extract [Becton, Dickinson, and Co.]) and Hsiang-Ning Tsai medium (HTM) with 3% glucose plus essential amino acid(s), a defined minimal medium (27). Chloramphenicol was used at 10 μg/ml for strain construction via allelic exchange in either TSBYE or Bacto brain heart infusion (BHI; Becton, Dickinson, and Co.).
Mutant strain construction.
Mutants were constructed in the M35303A background using allelic exchange with plasmid (1) pKSV7ΔflaA for the flagellum-minus mutant (ΔflaA) (26), (2) pKSV7-motBD23A for the paralyzed-flagellum mutant (motBD23A) (17), and (3) pCON1-688* for the nonglycosylated flagellum mutant (gmaRD83N,D85N) (25). The ΔflaA mutant (ZK3602) was nonmotile, independent of temperature, and did not have visible flagella by crystal violet (CV) staining (13). The motBD23A mutant (ZK3603) was confirmed by colony PCR of the region with primers oKL57 (TATATGATGATTGAGGGAGTCGTC) and oKL58 (TCTTTTGATACATATGCTTGGTTG) and subsequent sequencing using these same primers. The motBD23N mutant was nonmotile, independent of temperature, and had visible flagella by CV staining. The gmaRD83N,D85N mutant (ZK3604) was confirmed by colony PCR of the region with primers oKL053 (CACCGTGCGAGTAGCGATAA) and oKL054 (GCCATTTGGTTTGTTGTTGTCACT), followed by sequencing with the same primers. The gmaRD83N,D85N mutant had motility comparable to that of the wild-type strain and had wild-type-appearing flagella by CV staining. The lack of flagellar glycosylation was also confirmed by immunoblotting of surface-extracted protein preparation with anti-β-O-linked N-acetylglucosamine (GlcNAc)-specific antibody (monoclonal antibody CTD110.6; Pierce) as described by Shen and colleagues (25). All three mutants displayed exponential-phase growth rates that were indistinguishable from those of wild-type M35303A in HTM with 3% glucose and cysteine at 30°C in shaking culture and reached the same optical density after 24 h of incubation.
Motility assay.
Using a sterile toothpick, bacteria were inoculated into 0.3% BHI agar and incubated at room temperature (∼24°C), 30°C, or 37°C. The diameter of the bacterial swarm in the agar was measured 24 to 48 h later. The wild type and the nonglycosylated-flagellum mutant were comparably motile at ∼24°C and 30°C, and both were nonmotile at 37°C. The flagellum-minus and paralyzed-flagellum mutants were nonmotile at all temperatures tested.
Biofilm assay.
Surface-adhered biofilm formation was assayed in a 96-well format using a modified version of previously published protocols (6, 20). Overnight (15 to 20 h) rolling cultures grown in TSBYE at ∼20°C (optical density at 600 nm [OD600], ∼2.5 to 3.5) were diluted into freshly made HTM with 3% glucose plus cysteine to an initial OD600 of 0.05 to 0.1. One hundred fifty microliters of each strain was aliquoted into each of eight wells. Sterile medium was aliquoted into any empty wells, and a lid was placed and then secured circumferentially with parafilm to minimize evaporation. Biofilm cultures were grown in standing culture for 1 to 5 days at 30°C. Planktonic cells were then removed using a multiwell pipettor after pipetting up and down three to four times to remove any loosely adhering and sedimented cells, leaving behind firmly adhered cells. The OD600 of the planktonic cell supernatant from each well was measured using a SPECTRAmax M2 plate reader equipped with SOFTmax Pro software (Molecular Devices). The biofilms were washed three times with 150 μl of sterile double-distilled H2O, and each wash was pipetted up and down four times per well before removal. Biofilms were stained for 30 min with a filtered 0.1% aqueous solution of CV. Wells were washed four times by pouring tap water over the plate. Plates were inverted and dried overnight and then stored in the dark until pictures and quantification were done. To quantify biofilm formation, 150 μl of 33% acetic acid was added to each well, pipetted up and down, and transferred to a fresh polystyrene 96-well microtiter plate; the OD590 was then measured as described above.
Surfaces.
Unless otherwise noted, surface-adhered biofilm assays were done using non-tissue culture-treated polyvinyl chloride (PVC) 96-well plates (Falcon #353912). Plates are marketed as nonsterile, but we tested multiple plates by incubating them with medium alone at 30°C and never observed any growth. Also, for each plate used we included at least eight wells of medium alone and, again, did not observe any contamination. Biofilm assays were also performed in the following types of 96-well plates: (i) polystyrene that was tissue culture treated by vacuum gas plasma, and sterile (Falcon 353072) and (ii) polypropylene plates (Costar). Borosilicate glass culture tubes (13 by 100 mm; VWR) were sterilized by autoclaving prior to use. Stainless steel coupons (type 304, 2B, 20 gauge; 2 by 2 cm; Atlantic Stainless Co., Inc., North Attleboro, MA) were prepared essentially as previously described (1).
Adhesion to glass.
A rolling culture of each strain grown for 16 to 18 h at ∼20°C to an OD600 of 0.4 to 1.3 in TSBYE was diluted into freshly made HTM with 3% glucose and cysteine to a starting OD600 of ∼0.01. We confirmed that the overnight culture of the wild type and nonglycosylated-flagellum mutant were motile by microscopy. Dishes (P35G-1.5-14-C; MatTek Co., Ashland, MA) were inoculated with 1.5 ml of each culture at time zero. Prior to imaging, the MatTek dish coverslip was gently washed once by adding 1 ml of fresh medium to the edge of the dish. Digital images of five high-powered fields (hpf) per dish were captured using a 100× Plan Apo objective with differential interference contrast optics on an inverted Nikon TE2000-U microscope equipped with a cooled charge-coupled device (CCD) camera (ORCA-ER; Hamamatsu) and MetaMorph imaging software (version 6.1; Molecular Devices). Images were converted to 8 bit in MetaMorph and opened using ImageJ (NIH), and then the cells/hpf were counted manually.
Adhesion to stainless steel.
An overnight culture, as described for the biofilm assay, was diluted into freshly made HTM with 3% glucose and cysteine to a starting OD600 of ∼0.05. A sterile stainless steel coupon (described above) was placed in the bottom of a well of a 6-well polystyrene plate, inoculated with 2.5 ml of culture, and left at room temperature for 3 h. Following this, DAPI (4′,6′-diamidino-2-phenylindole) was added to a final concentration of 2 μg/ml for 10 min. Each coupon was washed three times with 2.5 ml of minimal medium and then removed, and a coverslip was placed on top. Digital images of 5 hpf per coupon were captured using an Axioskop 2 microscope (Carl Zeiss, Inc.) equipped with a 100× plan-Apochromat oil immersion objective, Orca-100 CCD camera (Hamamatsu Photonics), and Openlab software (Improvision). Images were processed using ImageJ (NIH), and cells/hpf were counted manually.
Centrifugation experiments.
Biofilm assays were done as described above, with the additional step that after inoculation the 96-well plates were centrifuged at ∼1,900 × g for 60 min in a Beckman Coulter Allegra 6KR centrifuge equipped with a swinging bucket rotor and microplus carriers. To assay the effect of centrifugation on initial surface attachment, a glass coverslip (22 by 22 by 1 mm; Fisherfinest Premium coverglass) was placed, using sterile techniques, in each well of a polystyrene 6-well plate (BD Falcon). Bacteria from an overnight rolling culture grown at ∼20°C for 11 to 16 h in TSBYE (OD600, ∼1.8 to 3.2) were inoculated into freshly made HTM with 3% glucose and cysteine to an initial OD600 of 0.005, and then 3 ml of each strain was aliquoted into two separate wells. After centrifugation, coverslips were washed in the well three times with 2.5 ml of HTM. Microscopy was preformed with an Axioskop 2 Plus (Carl Zeiss, Inc.) upright scope equipped with a 40× phase-contrast lens and 1.6× optivar. Images were captured with an AxioCam CCD and AxioVision 4.4 (Carl Zeiss, Inc.). At least 5 hpf were manually counted for each strain to determine the average number of cells/hpf.
Statistical analyses.
Statistical analyses were performed using MINITAB software. Comparisons were done using one-way analysis of variance (ANOVA), followed by Tukey's multiple comparison test (set at 5%).
RESULTS
L. monocytogenes flagellum-minus mutants are defective in biofilm formation.
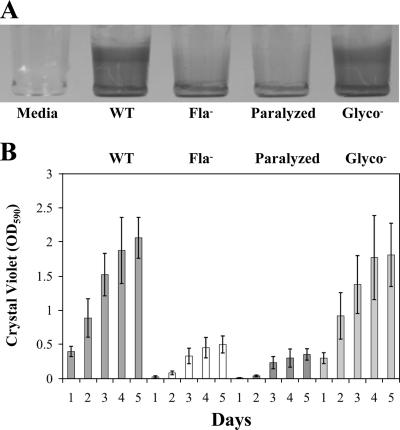
Compared to the wild-type strain, an isogenic flagellum-minus mutant containing an in-frame deletion in the flagellin gene was defective in surfaced-adhered biofilm formation (Fig. 1). The bacteria were grown in a defined minimal medium (HTM with 3% glucose and cysteine) at 30°C in 96-well PVC microtiter plates. After removal of planktonic cells and washing, surface-adhered biofilms were stained with CV (Fig. 1A). We observed that the flagellum-minus mutant was defective in biofilm formation compared to the wild type over each of five days (Fig. 1B). The difference in biofilm-forming ability is unlikely to be secondary to a difference in growth, as both strains grew comparably. We were unable to assess biofilm formation for more than 5 days, because after 5 to 6 days in standing culture, biofilm levels of the wild type began to deteriorate. This deterioration might be the result of either cell death or biofilm dispersion. These data indicate that flagella play a role in biofilm formation but do not distinguish whether this role is in motility, adhesion, or both.
FIG. 1.
Motile strains form greater amounts of surface-adhered biofilm than nonmotile strains. (A) CV-stained L. monocytogenes biofilms formed on wells of a 96-well PVC plate. The first well has medium alone (Media), followed by the wild type (WT), flagellum-minus mutant (Fla−), paralyzed-flagellum mutant (Paralyzed), and nonglycosylated-flagellum mutant (Glyco−). (B) Graph of surface-adhered biofilm (quantified by measuring the OD590 of solubilized CV after biofilm staining) from four independent experiments with seven replicates of each strain per experiment. Error bars represent standard errors of the means. Analysis using one-way ANOVA followed by Tukey's multiple comparison test (set at 5%) indicated that there was a statistically significant difference between the amounts of biofilm formed by motile and nonmotile strains on each of five days.
Flagellum-mediated motility is critically important for L. monocytogenes biofilm formation.
To determine whether motility, or simply the presence of flagella, is critical for biofilm formation, we compared biofilms formed by the wild-type strain and a flagellum-minus mutant with that of a paralyzed-flagellum mutant (motBD23A). The paralyzed-flagellum mutant had a defect in biofilm formation comparable to that of the flagellum-minus mutant (Fig. 1). MotB is postulated to be part of the flagellar stator (the stationary part of the flagellar motor within which the rotor turns), and the corresponding aspartic acid in E. coli MotB is required for torque generation (32). In the original L. monocytogenes strain background (10403S), the paralyzed-flagellum mutant (motBD23A) is nonmotile, has normal numbers of peritrichous flagella, expresses normal amounts of flagellin, and can be complemented by allelic exchange of wild-type motB back onto the chromosome (17). In our wild-type background, the motBD23A mutant was similarly nonmotile and by CV-based staining had flagella indistinguishable from the wild type. Both the flagellum-minus and paralyzed-flagellum mutants had growth rates indistinguishable from the wild type in shaking culture in HTM with 3% glucose and cysteine at 30°C. The biofilm-defective phenotype of the paralyzed-flagellum mutant indicates that motility plays a critical role in surface-adhered biofilm formation by L. monocytogenes. These data do not, however, exclude some role for flagella as adhesins once cells reach the surface.
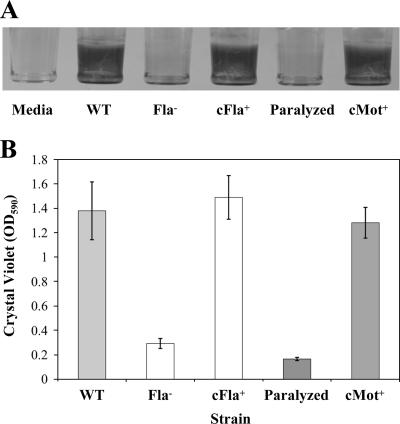
M35303A is not a commonly studied strain of L. monocytogenes (it was selected for its robust biofilm forming ability), therefore, we subsequently examined the biofilm phenotype of flagellum-minus and paralyzed-flagellum mutants in the commonly used 10403S background using mutants generated and published by O'Neil and Marquis (17). We observed that, in this background, both the flagellum-minus and paralyzed-flagellum mutants were defective in biofilm formation compared to wild-type 10403S and that complementation of the respective mutations with a copy of the wild-type gene resulted in strains that formed wild-type levels of biofilm (Fig. 2A). O'Neil and Marquis have previously demonstrated that complementation of both the flaA and motBD23A mutation with a copy of the respective wild-type gene restores wild-type levels of motility. Analysis using one-way ANOVA followed by Tukey's multiple comparison test (set at 5%) indicated that there was a statistically significant difference between the amounts of biofilm formed by the motile and nonmotile strains (Fig. 2B). In contrast, there was no statistical difference between the amounts of biofilm formed by the two nonmotile mutants. Likewise, there was no statistical difference in biofilm formation by the two complemented strains and the wild-type strain. Based on this, we propose that our findings for M35303A are generalizable to a wide variety of L. monocytogenes strains.
FIG. 2.
In a 10403S background, motile strains form greater amounts of surface-adhered biofilm than nonmotile strains. (A) CV stained 3-day-old L. monocytogenes biofilms formed on wells of a 96-well PVC plate. The wild-type strain is 10403S. The first well has medium alone (Media), followed by wild-type 10403S (WT), flagellum-minus mutant (Fla−), the flagellum-minus mutant complemented with the wild-type flaA allele (cFla+), paralyzed-flagellum mutant (Paralyzed), and the paralyzed mutant complemented with the wild-type motB allele (cMot+). Biofilm assays were performed as described in Materials and Methods, except that standing cultures were incubated at 36°C, where the WT, cFla+, and cMot+ strains are still motile. (B) Graph of surface-adhered biofilm (quantified by measuring the OD590 of solubilized CV after biofilm staining) from three independent experiments with seven replicates of each strain per experiment. Error bars represent standard errors of the means.
A mutant lacking flagellar glycosylation has no defect in biofilm formation.
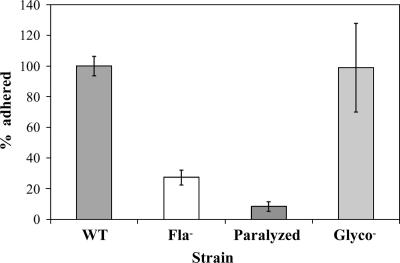
A nonglycosylated-flagellum mutant (gmaRD83N,D85N) that has wild-type motility formed biofilms generally as well as the wild-type strain (Fig. 1). We had postulated that if flagella have a role as surface adhesins, then there might be a difference in biofilm formation between the wild type and a motile mutant with an altered flagellar surface. L. monocytogenes flagellin is posttranslationally modified with β-O-linked GlcNAc at three to six sites per flagellin monomer (23). A single flagellum contains thousands of monomers, so it is conceivable that removing all glycosylation might drastically change the surface characteristics of flagella. The L. monocytogenes glycosyltransferase responsible for flagellin glycosylation, GmaR, is a uniquely bifunctional protein (25). It both glycosylates flagellin and acts as an antirepressor for MogR, which itself represses transcription of flagellar biosynthetic operons (11, 24, 25). GmaR's antirepression function is independent of its glycosyltransferase function (25). In the original strain background (EGDe), gmaRD83N,D85N has wild-type motility with nonglycosylated flagella (25). We also observed wild-type motility when we moved the gmaRD83N,D85N mutation into our strain background. Biofilm formation by the wild-type strain and the nonglycosylated-flagellum mutant were statistically indistinguishable, except for the minor difference observed on day 1 (Fig. 1B). Likewise, there was no statistical difference in initial surface adhesion by the two strains (Fig. 3). We do not know what might account for the day 1 observation (Fig. 1B). That the motile nonglycosylated-flagellum mutant attaches and forms biofilms similar to the wild-type strain again points to the critical role of flagellum-mediated motility in biofilm formation.
FIG. 3.
Motile strains of L. monocytogenes attached better to glass than nonmotile mutants. The number of cells attached per hpf to glass coverslips (MatTek dish) between 30 to 200 min after surface exposure was normalized such that the average number of cells/hpf for the wild-type strain for each experiment was set at 100%. Strains are the wild type (WT), flagellum-minus mutant (Fla−), paralyzed-flagellum mutant (Paralyzed), and nonglycosylated-flagellum mutant (Glyco−). Using one-way ANOVA followed by Tukey's multiple comparison (set at 5%), the difference between the motile and nonmotile strains was statistically significant, but the possible difference between the flagellum-minus and paralyzed-flagellum mutant was not statistically significant. Five microscope experiments from three independent sets of room-temperature overnight cultures (see Materials and Methods) were done on three different days, and 5 hpf of each strain were captured per experiment, such that 25 hpf were examined for each strain. Error bars represent standard errors of the means. Each strain was inoculated at an OD600 of ∼0.01, and for two of the three experiments we confirmed that at OD600 there was a comparable number of CFU per milliliter in the initial inoculum of each strain. All strains were inoculated into MatTek dishes within minutes of each other, and we performed microscopy on the wild type first followed by flagellum-minus mutant, paralyzed-flagellum mutant, and, finally, the nonglycosylated-flagellum mutant. Most adhered cells were either single cells or part of a pair of cells. As we could not determine if adherence had occurred before or after septation in these pairs, we chose to count each pair as a single cell.
The relative biofilm-forming abilities of mutants are similar on various abiotic surfaces.
The nonglycosylated-flagellum mutant also formed biofilms comparable to those of the wild type on each of four different abiotic surfaces tested: PVC, polystyrene, polypropylene, and borosilicate glass (data not shown). Likewise, the flagellum-minus and paralyzed-flagellum mutants were both defective in biofilm formation on these four different surfaces (data not shown). The four different surfaces do not constitute an exhaustive search for a surface on which the absence of flagellum glycosylation might effect some difference in biofilm formation. However, the consistency of the biofilm defect that the flagellum-minus and paralyzed-flagellum mutants have on various surfaces further supports our conclusion that the primary role for flagella in biofilm formation by L. monocytogenes is in motility.
Nonmotile mutants are defective in surface attachment during very early biofilm formation.
To further elucidate the role of motility in biofilm formation, we examined bacterial adherence to glass coverslips at the earliest stages of biofilm formation using light microscopy. Both flagellum-minus and paralyzed-flagellum mutants had a statistically significant deficit in the number of cells adhered per hpf between 30 and 200 min after inoculation compared to motile bacteria, both the wild-type strain and the nonglycosylated-flagellum mutant. The numbers of cells/hpf for the strains were was compared by one-way ANOVA followed by Tukey's multiple comparison test (set at 5%) (Fig. 3). While there was a statistically significant difference in adherence between motile and nonmotile strains, there was not a statistically significant difference in adherence between the flagellum-minus and paralyzed-flagellum mutants. Nor was there a statistically significant difference in adherence between the two motile strains (Fig. 3). We observed a similar pattern of adherence to stainless steel (type 304, finish 2B) after 3 h of surface exposure (Fig. 4). The difference in the percentage of cells adhered to stainless steel between the motile and nonmotile strains was statistically significant at the 95% confidence interval as determined by one-way ANOVA followed by Tukey's multiple comparison test (set at 5%). In contrast, there was no statistical difference between the two motile strains or between the two nonmotile strains. The defect in adhesion to stainless steel by the nonmotile mutants was less pronounced than their defect in adhesion to glass, suggesting the possibility that the role of flagellum-mediated motility might be more prominent on some surfaces than others. Nonetheless, the comparable defect in the adhesion of paralyzed-flagellum and flagellum-minus mutants to stainless steel indicates that L. monocytogenes flagella do not function as stainless steel adhesins.
FIG. 4.
Paralyzed-flagellum mutants and flagellum-minus mutants are comparably defective in adherence to stainless steel. The number of cells attached per hpf to stainless steel coupons after 3 h of surface exposure was visualized using DAPI staining of nucleoids, and each nucleoid was counted as one cell. The data were normalized such that the average number of cells/hpf for the wild-type strain for each experiment was set at 100%. Strains are the wild type (WT), flagellum-minus mutant (Fla−), paralyzed-flagellum mutant (Paralyzed), and nonglycosylated-flagellum mutant (Glyco−). The difference in the percentage of cells adhered to stainless steel between the motile and nonmotile strains was statistically significant at the 95% confidence interval as determined by one-way ANOVA followed by Tukey's multiple comparison test (set at 5%). In contrast, there was no statistical difference between the two motile strains or between the two nonmotile strains. Data are from three independent experiments, in each of which two steel coupons were analyzed per strain, with 5 hpf captured per coupon.
Exogenously supplied surface-directed force restores the initial surface attachment of nonmotile mutants.
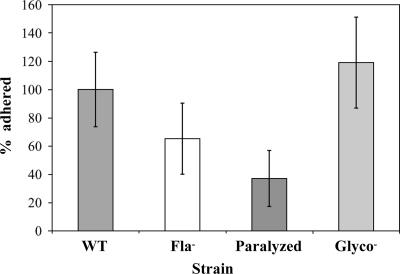
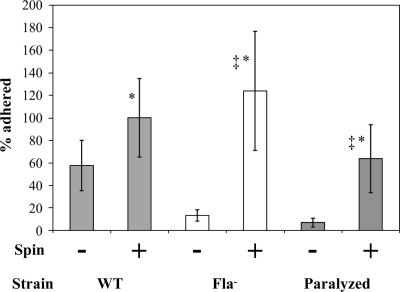
To test if exogenously supplied movement directed toward the surface might overcome the surface attachment defect of the nonmotile mutants, we compared the numbers of cells adhered to glass coverslips with and without centrifugation (∼1,900 × g for 60 min). For both the flagellum-minus and paralyzed-flagellum mutants, centrifugation increased the number of cells adhered to levels comparable to that of the wild-type strain (Fig. 5). When the numbers of cells/hpf for the strains were compared by one-way ANOVA followed by Tukey's multiple comparison test (set at 5%), the increase in cell adherence after centrifugation was statistically significant for both nonmotile strains, though not for the wild-type strain. While we cannot exclude the possibility that centrifugation for 60 min might have promoted some nonspecific interactions between the bacteria and the surface, we think that this is unlikely given that there was not a statistically significant increase in adherence of the spun wild-type strain compared to the nonspun wild-type strain. At the 95% confidence interval, there was no significant difference between the spun nonmotile strains and the spun wild type; however, when the two nonmotile strains were compared to each other, the higher number of flagellum-minus cells adhered postspin compared to the paralyzed mutant did reach statistical significance (Fig. 5). We speculate that perhaps the presence of paralyzed flagella interferes slightly with contact between putative bacterial surface adhesin(s) and abiotic surfaces when motility is supplied exogenously.
FIG. 5.
Centrifugation restored surface attachment of nonmotile mutants. Initial surface attachment of the flagellum-minus (Fla−) and paralyzed-flagellum (Paralyzed) mutants was restored to wild-type levels by exogenously supplying surface-directed motion via centrifugation (∼1,900 × g) for 1 h. Also shown are the data for the adherence of the different strains when left in standing culture for 1 h. When the level of adherence by the spun wild-type strain is compared to that of either the spun flagellum-minus mutant or the spun paralyzed-flagellum mutant, it is not significantly different at the 95% confidence interval as determined by one-way ANOVA followed by Tukey's multiple comparison test (set at 5%), as denoted by the asterisk. In contrast, when the adherence of the spun flagella-minus mutant and the spun paralyzed-flagella mutant are compared using the same statistical analysis, the level of adherence is statistically different at the 95% confidence interval, as denoted by the double dagger. Cells/hpf were counted after glass coverslips were washed. The number of cells attached per hpf to glass coverslips was normalized such that the average number of cells/hpf for the wild type for each experiment was set at 100%. Data are from four independent experiments, each using one coverslip per strain and condition, and 5 hpf were analyzed per coverslip. Error bars represent standard errors of the means.
In our experiments, centrifuging the nonmotile mutants in 96-well microtiter plates for up to 60 min at 1,900 × g did not alleviate the biofilm formation defect of either flagellum-minus or paralyzed-flagellum mutants (data not shown). Based on this, we speculate that surface-associated biofilm formation by L. monocytogenes involves both growth of the initially attached cells and ongoing recruitment of planktonic cells into the biofilm. This recruitment appears to be dependent on flagellum-mediated motility, and without it wild-type levels of biofilm development cannot occur.
DISCUSSION
We have found that flagellum-mediated motility is critical for wild-type levels of L. monocytogenes biofilm development. Compared to the wild type, both flagellum-minus and paralyzed-flagellum mutants had comparable defects in initial surface attachment and in subsequent biofilm formation. Supplying surface-directed motility exogenously via centrifugation restored wild-type levels of attachment to both nonmotile mutants. These data indicate that the primary role of flagella in L. monocytogenes biofilm formation is in generating motion, and that if there is any role for L. monocytogenes flagella as surface-adhesins in biofilm formation it is either minimal or is dependent upon motility.
Previously published data suggested that flagellated L. monocytogenes cells attach more rapidly to a stainless steel surface in the absence of motility than flagellum-minus cells (29). Based on this report, we initially hypothesized that the paralyzed-flagellum mutant might form a better biofilm than the flagellum-minus mutant, though in the gram-negative bacteria E. coli and V. cholerae, flagellum-minus mutants and paralyzed-flagellum mutants are similarly defective in biofilm formation (22, 30, 31). In the experiments of Vatanyoopaisarn and colleagues, wild-type, flagellated L. monocytogenes cells (NCTC 7973) were rendered nonmotile by washing and resuspending in phosphate-buffered saline (PBS), a condition of complete nutrient deprivation in which cells are unable to move but remain viable (29). In PBS, lacking motility, the flagellated wild type attach 10-fold more to stainless steel in the first 4 h than do the flagellum-minus mutant. By 24 h, however, attachment levels are comparable. This prior study did not examine mature biofilm formation per se, so our observation that there was no statistical difference in biofilm-forming ability between the paralyzed-flagellum mutant and a flagellum-minus mutant at 24 h and beyond is compatible (Fig. 1B). However, when we compared bacterial attachment to glass coverslips and stainless steel coupons during the first 4 h of surface exposure, the paralyzed-flagellum and flagellum-minus mutants also showed comparably defective attachments, arguing against a role for flagella as surface adhesins. Differences in experimental protocols might account for the dissimilar results. Our experiments were done in a different strain background and under nutrient-replete conditions directly comparing a paralyzed-flagellum mutant with a flagellum-minus mutant and an isogenic wild-type strain.
We are struck by the parallel between our results for the role of L. monocytogenes flagella in attachment and biofilm formation on abiotic surfaces and those of O'Neil and Marquis for the role of flagella in epithelial cell adherence and invasion (17). L. monocytogenes flagella contribute to epithelial host cell surface adhesion and invasion (2, 7). Recently, O'Neil and Marquis have shown that the function of flagella in these processes is in motility and not as adhesins (17). This is in contrast to the case for other bacteria, such as enteropathogenic E. coli, for which flagella function as cell-surface adhesins in the absence of motility (10). While in L. monocytogenes both flagellum-minus and paralyzed-flagellum mutants are defective in epithelial cell adhesion and invasion, low-speed centrifugation (40 to 1,000 × g) restores flagellum-minus mutant adhesion to epithelial cells to nearly wild-type levels (7, 17). In contrast, even after centrifugation the paralyzed flagella do not adhere to epithelial cells as well as the flagellum-minus mutant, suggesting that paralyzed flagella might actually interfere with contact between putative bacterial adhesins and the epithelial cell surface (17). With regard to abiotic surface attachment, we observed that centrifugation fully restored flagellum-minus mutant attachment, suggesting that motility is the only role for flagella in this process. In comparing spun wild-type bacteria with either the spun flagellum-minus mutant or the spun paralyzed-flagellum mutant, there was no statistically significant difference between their levels of attachment at the 95% confidence interval (Fig. 5). However, the increased level of attachment of the spun flagellum-minus mutant compared to the spun paralyzed-flagellum mutant did reach statistical significance at the 95% confidence interval (Fig. 5), hinting at the possibility that paralyzed flagella might also interfere with abiotic surface attachment.
A limited centrifugation of 1 h restored initial surface attachment, yet it did not restore mature biofilm formation to the nonmotile mutants. This suggests that biofilm development by L. monocytogenes proceeds via both growth of initially surface-adhered cells and ongoing recruitment of motile cells from the planktonic phase. In thinking about how flagellum-mediated motility is critical for L. monocytogenes biofilm formation, we favor a model, previously proposed for other bacteria, that the primary role of flagellum in surface-associated biofilm formation is to provide the force necessary to overcome repulsive forces that might exist between the bacteria and the surface (22, 30). Incorporated in this idea is also the concept that the contribution of flagellum-mediated motility is simply to increase the probability of encountering a surface. It appears that during biofilm development in many other motile bacteria, motility and extracellular matrix production are inversely regulated, such that once motile cells contact a surface they switch to producing matrix (14). We are curious to determine whether a similar situation exists in L. monocytogenes and how it might be regulated.
Acknowledgments
We thank Heather O'Neil and Hélène Marquis for providing the motBD23A construct and sharing their results prior to publication and for providing their 10403S motility mutants and complemented strains, Aimee Shen for providing the gmaRD83N,D85N construct and sharing results prior to publication, Aimee Shen and Heather Kamp for reagents and protocols used for anti-GlcNac immunoblot, Monica Borucki for providing M35303A, Jon Beckwith for use of his microscope, Hera Vlamakis and Vanja Klepac-Ceraj for critical reading of the manuscript, and the members of the Kolter and Higgins laboratories for suggestions and discussions.
K.P.L. was an NICHD Fellow of the Pediatric Scientist Development Program (NICHD Grant Award K12-HD00850). The work was also supported by a Mentored Clinical Scientist Development Award to K.P.L. (K08 AI070561), grant AI53669 to D.E.H., and grant GM58213 to R.K.
Footnotes
Published ahead of print on 6 April 2007.
REFERENCES
- 1.Ayebah, B., Y. C. Hung, and J. F. Frank. 2005. Enhancing the bactericidal effect of electrolyzed water on Listeria monocytogenes biofilms formed on stainless steel. J. Food Prot. 68:1375-1380. [DOI] [PubMed] [Google Scholar]
- 2.Bigot, A., H. Pagniez, E. Botton, C. Frehel, I. Dubail, C. Jacquet, A. Charbit, and C. Raynaud. 2005. Role of FliF and FliI of Listeria monocytogenes in flagellar assembly and pathogenicity. Infect. Immun. 73:5530-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borucki, M. K., J. D. Peppin, D. White, F. Loge, and D. R. Call. 2003. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 69:7336-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branda, S. S., J. E. Gonzalez-Pastor, S. Ben-Yehuda, R. Losick, and R. Kolter. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 98:11621-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Djordjevic, D., M. Wiedmann, and L. A. McLandsborough. 2002. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 68:2950-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dons, L., E. Eriksson, Y. Jin, M. E. Rottenberg, K. Kristensson, C. N. Larsen, J. Bresciani, and J. E. Olsen. 2004. Role of flagellin and the two-component CheA/CheY system of Listeria monocytogenes in host cell invasion and virulence. Infect. Immun. 72:3237-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fux, C. A., J. W. Costerton, P. S. Stewart, and P. Stoodley. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34-40. [DOI] [PubMed] [Google Scholar]
- 10.Giron, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 11.Grundling, A., L. S. Burrack, H. G. Bouwer, and D. E. Higgins. 2004. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proc. Natl. Acad. Sci. USA 101:12318-12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan, D., and R. Kolter. 2002. Why are bacteria refractory to antimicrobials? Curr. Opin. Microbiol. 5:472-477. [DOI] [PubMed] [Google Scholar]
- 13.Kearns, D. B., and R. Losick. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49:581-590. [DOI] [PubMed] [Google Scholar]
- 14.Kolter, R., and E. P. Greenberg. 2006. Microbial sciences: the superficial life of microbes. Nature 441:300-302. [DOI] [PubMed] [Google Scholar]
- 15.Lorber, B. 2005. Listeria monocytogenes, p. 2478-2484. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 6th ed., vol. 2. Elsevier Churchill Livingstone, Philadelphia, PA. [Google Scholar]
- 16.Moretro, T., and S. Langsrud. 2004. Listeria monocytogenes: biofilm formation and persistence in food-processing environments. Biofilms 1:107-121. [Google Scholar]
- 17.O'Neil, H. S., and H. Marquis. 2006. Listeria monocytogenes flagella are used for motility, not as adhesins, to increase host cell invasion. Infect. Immun. 74:6675-6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 19.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 20.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 21.Peel, M., W. Donachie, and A. Shaw. 1988. Temperature-dependent expression of flagella of Listeria monocytogenes studied by electron microscopy, SDS-PAGE and western blotting. J. Gen. Microbiol. 134:2171-2178. [DOI] [PubMed] [Google Scholar]
- 22.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 23.Schirm, M., M. Kalmokoff, A. Aubry, P. Thibault, M. Sandoz, and S. M. Logan. 2004. Flagellin from Listeria monocytogenes is glycosylated with β-O-linked N-acetylglucosamine. J. Bacteriol. 186:6721-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen, A., and D. E. Higgins. 2006. The MogR transcriptional repressor regulates nonhierarchal expression of flagellar motility genes and virulence in Listeria monocytogenes. PLoS Pathog. 2:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen, A., H. D. Kamp, A. Grundling, and D. E. Higgins. 2006. A bifunctional O-GlcNAc transferase governs flagellar motility through anti-repression. Genes Dev. 20:3283-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shetron-Rama, L. M., K. Mueller, J. M. Bravo, H. G. Bouwer, S. S. Way, and N. E. Freitag. 2003. Isolation of Listeria monocytogenes mutants with high-level in vitro expression of host cytosol-induced gene products. Mol. Microbiol. 48:1537-1551. [DOI] [PubMed] [Google Scholar]
- 27.Tsai, H. N., and D. A. Hodgson. 2003. Development of a synthetic minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 69:6943-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valle, J., A. Toledo-Arana, C. Berasain, J. M. Ghigo, B. Amorena, J. R. Penades, and I. Lasa. 2003. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48:1075-1087. [DOI] [PubMed] [Google Scholar]
- 29.Vatanyoopaisarn, S., A. Nazli, C. E. Dodd, C. E. Rees, and W. M. Waites. 2000. Effect of flagella on initial attachment of Listeria monocytogenes to stainless steel. Appl. Environ. Microbiol. 66:860-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou, J., L. L. Sharp, H. L. Tang, S. A. Lloyd, S. Billings, T. F. Braun, and D. F. Blair. 1998. Function of protonatable residues in the flagellar motor of Escherichia coli: a critical role for Asp32 of MotB. J. Bacteriol. 180:2729-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]