Abstract
The hyperthermophilic archaeon Pyrococcus furiosus uses carbohydrates as a carbon source and produces acetate, CO2, and H2 as end products. When S0 is added to a growing culture, within 10 min the rate of H2 production rapidly decreases and H2S is detected. After 1 hour cells contain high NADPH- and coenzyme A-dependent S0 reduction activity (0.7 units/mg, 85°C) located in the cytoplasm. The enzyme responsible for this activity was purified to electrophoretic homogeneity (specific activity, 100 units/mg) and is termed NAD(P)H elemental sulfur oxidoreductase (NSR). NSR is a homodimeric flavoprotein (Mr, 100,000) and is encoded by PF1186. This designation was previously assigned to the gene encoding an enzyme that reduces coenzyme A disulfide, which is a side reaction of NSR. Whole-genome DNA microarray and quantitative PCR analyses showed that the expression of NSR is up-regulated up to sevenfold within 10 min of S0 addition. This primary response to S0 also involves the up-regulation (>16-fold) of a 13-gene cluster encoding a membrane-bound oxidoreductase (MBX). The cluster encoding MBX is proposed to replace the homologous 14-gene cluster that encodes the ferredoxin-oxidizing, H2-evolving membrane-bound hydrogenase (MBH), which is down-regulated >12-fold within 10 min of S0 addition. Although an activity for MBX could not be demonstrated, it is proposed to conserve energy by oxidizing ferredoxin and reducing NADP, which is used by NSR to reduce S0. A secondary response to S0 is observed 30 min after S0 addition and includes the up-regulation of genes encoding proteins involved in amino acid biosynthesis and iron metabolism, as well as two so-called sulfur-induced proteins termed SipA and SipB. This novel S0-reducing system involving NSR and MBX has been found so far only in the heterotrophic Thermococcales and is in contrast to the cytochrome- and quinone-based S0-reducing system in autotrophic archaea and bacteria.
The hyperthermophilic archaea are a group of microorganisms that grow optimally at temperatures of 80°C and above (48). Most of these microorganisms utilize elemental sulfur (S0) as a terminal electron acceptor and reduce it to H2S (17, 19). Those that use molecular H2 as the electron donor, such as Thermoproteus tenax and Acidianus ambivalens, are thought to have a respiratory system analogous to that found in mesophilic S0-reducing bacteria such as Wolinella succinogenes, where S0 reduction is accomplished by a membrane-bound respiratory system (17). On the other hand, the mechanism of S0 reduction by the heterotrophic hyperthermophilic archaea, such as Pyrococcus and Thermococcus species, is completely unknown. These organisms grow by fermentation with peptides as the carbon source, and most of them appear to be obligately dependent on S0 for optimal growth (48). The exceptions are those that are able to grow by fermentation of carbohydrates; one such exception is Pyrococcus furiosus, which grows equally well with or without S0 (9). Herein we have exploited this property to investigate the mechanism by which this prototypical heterotrophic hyperthermophile reduces S0 to H2S.
P. furiosus utilizes a range of both simple and complex carbohydrates and converts them to acetate, to CO2, to H2, and, if S0 is present, to H2S. Its glycolytic pathway has been extensively studied and served as one of the model systems for elucidating the modified Embden-Meyerhof pathway in archaea (39, 40, 51). The key feature of this pathway is that the classical enzymes glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and phosphoglycerate kinase are replaced by a single ferredoxin-linked enzyme, glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR, EC 1.2.1.-). This converts glyceraldehyde-3-phosphate to glycerate-3-phosphate without the generation of ATP (51). Consequently, only ferredoxin serves as the electron acceptor in glycolysis and no NAD(P)H is formed. The reason for this became apparent upon the discovery in P. furiosus of a membrane-bound hydrogenase (MBH) which evolved H2 from reduced ferredoxin in an energy-conserving manner via a proton motive force (41). The other oxidation step in the conversion of glucose to acetate is also coupled to the reduction of ferredoxin, and this is catalyzed by pyruvate ferredoxin oxidoreductase (POR) (4). Hence, in the production of acetate from glucose, all of the reductant is generated as reduced ferredoxin. Its oxidation via the MBH is thought to result in the conservation of energy equivalent to 1.2 ATP per glucose (41). Two cytoplasmic hydrogenases from P. furiosus have also been characterized (29), but these use NADPH, rather than ferredoxin, as the electron carrier, and their functions in fermentative metabolism are unclear. It is possible that one or both serve to recycle the H2 produced by the membrane-bound enzyme to generate NADPH for biosynthesis (29, 47).
For P. furiosus and related heterotrophic Thermococcales, S0 reduction, like H2 production, was proposed to be a mechanism for disposing of excess reductant (9, 43). H2 production is now regarded as an energy-conserving process (41), but it is not known if this is also true of S0 reduction. The S0 reduction system of the mesophilic bacterium Wolinella succinogenes is generally accepted as a model system for anaerobic S0 respiration in which H2S production is coupled to energy conservation (17). W. succinogenes uses H2 or formate as the electron donor, and their oxidation is coupled through cytochrome b and quinones to a membrane-bound, molybdopterin-containing sulfur reductase (8). A similar S0-reducing respiratory system has been characterized for other autotrophs, including the hyperthermophilic bacterium Aquifex aeolicus (13) and the hyperthermoacidophilic archaeon A. ambivalens (27), and it appears to be present in the H2-oxidizing hyperthermophilic archaea Pyrodictium brockii (36) and Pyrodictium abyssi (24).
P. furiosus and the other heterotrophic hyperthermophilic archaea seem to have a mechanism for S0 reduction that is different from that found for the autotrophic species. The available genome sequences of three Pyrococcus and one Thermococcus species (7, 11, 23, 38) do not contain obvious homologs of the molybdenum-containing sulfur reductase of W. succinogenes or A. ambivalens (2). There are also no reports of the presence of quinones or cytochromes in these organisms. Three enzymes from P. furiosus, the two cytosolic hydrogenases (29) and a sulfide dehydrogenase (30), have been previously reported to possess S0 reductase activity in vitro. However, both the activity and the expression of the two hydrogenases dramatically decreased in cells grown in the presence of S0 (1, 46). Similarly, the sulfide dehydrogenase is now thought to function in vivo as a ferredoxin:NADPH oxidoreductase (28), and the expression of its genes is related to the carbon source rather than to S0 (44). Consequently, none of these three enzymes is likely to play a role in S0 reduction in vivo.
In a previous study with P. furiosus, the effect of S0 on the expression of a selected group of genes (271) was investigated using a targeted DNA microarray by comparing cells grown for many generations (as batch cultures) in the presence or absence of S0 (46). While a significant number of genes were affected, including those encoding the two cytoplasmic hydrogenases (which were down-regulated in S0-grown cells), this study was limited by (i) the small number of genes analyzed and (ii) the use of batch-grown cells, where regulated genes involved in the primary metabolism of S0 could not be distinguished from those causing secondary or other effects. In the present work, the primary response of P. furiosus to S0 has been investigated using a kinetic approach, where S0 is added to a log-phase culture and changes in gene expression are analyzed using a complete genome DNA microarray. In addition, the enzyme responsible for NAD(P)H-linked S0 reduction was characterized independently by biochemical approaches, and the up-regulation of the gene encoding it (PF1186) was shown to be a component of the primary response of P. furiosus to S0 addition.
MATERIALS AND METHODS
Growth conditions.
P. furiosus (DSM 3638) was grown in the presence and absence of S0 with maltose as the primary carbon source. The growth medium was the same as previously reported (1) except that the yeast extract was 1.0 g/liter and cysteine (3 mM) was the reducing agent (Na2S was not added). Growth experiments to determine the effects of S0 addition were carried out in 100-ml serum bottles with 50-ml stirred (300 rpm) cultures or in a 20-liter custom fermentor (1). Cultures were grown until they reached mid-log phase (0.8 × 108 cells/ml), and S0 (J. T. Baker, Phillipsburg, NJ) was added to final concentrations of 5 and 2 g/liter, respectively. To prepare cell extracts, cells were rapidly cooled by pumping the culture from the 20-liter fermentor through a glass cooling coil, and the cells were collected by centrifugation (10,000 × g, 10 min) and fractionated as previously described (1). Approximately 7 liters of the culture was harvested before (time zero) and 1 h after S0 addition. To obtain RNA for microarray and quantitative PCR (QPCR) analyses, samples (2 liters each) were removed from the fermentor before and at various time points after the addition of sulfur and were cooled in ice and fractionated as described previously (46).
Gas analyses.
By use of the 100-ml cultures, samples (500 μl each) were taken from the liquid and headspace, and these were injected into 10-ml anaerobic vials containing an inner reaction vial (500-μl Eppendorf tube) surrounded by 0.1 M NaOH (1 ml) to capture H2S. Sulfuric acid (100 μl, 2.0 M) was added to the inner vial to release acid-labile sulfide from the liquid culture. H2S production was measured in the NaOH phase of the double-vial system with the methylene blue assay (6). H2 gas was detected in the headspace of the vials with a gas chromatograph (Shimadzu GC-8A). The Bradford method was used to estimate protein concentration for harvested cells with bovine serum albumin as a standard (5).
Enzyme assays.
Intact P. furiosus cells were harvested from fermentor mid-log-phase cultures by centrifugation (10,000 × g, 10 min) and were gently resuspended in fresh growth medium without S0 or maltose to a final protein concentration of ∼15 mg/ml. The production of H2 and H2S by intact cells using maltose (50 mM) as the source of reductant was measured with the 10-ml double-vial system described above containing 0.1 M NaOH (1 ml). The inner reaction vial (500 μl) contained the reaction mixture (100 μl), including 12.8 g/liter colloidal sulfur (Fluka, Milwaukee, WI) where indicated. NAD(P)H-dependent elemental sulfur oxidoreductase (NSR) activity was measured using the same double-vial system. The standard NSR assay mixture (50 μl) contained 50 mM phosphate buffer (pH 7.0), 10 mM NAD(P)H, 6.4 g/liter (wt/vol) colloidal sulfur, and 200 μM coenzyme A (CoA). The mixture was incubated for 5 min at 85°C, the reaction was stopped, and H2S was released by the addition to the reaction mixture of 100 μl 2 M H2SO4 and quantitated as described above. One unit of NSR catalyzed the production of 1 μmol of H2S per min under these conditions. Ferredoxin-linked H2S production was measured by the same method except that NADPH was omitted and the electron donor was ferredoxin reduced by the POR of P. furiosus. The 50-μl assay mixture contained 50 mM phosphate buffer (pH 7.0), 10 mM pyruvate, 1 mM CoA, 5 mM ADP, 10 μg ml−1 POR, 10 μM ferredoxin, and 6.4 g/liter colloidal sulfur. The addition of ADP allows the acetyl-CoA that is generated by the POR reaction to be utilized by acetyl-CoA synthetase (present in the cell extract), thereby preventing accumulation of acetyl-CoA. The reaction mixture was incubated at 85°C for 10 min, and H2S formation was determined as described above. The kinetic analyses were carried out under the same conditions except that NADPH, NADH, CoA, and CoA disulfide (CoA-S-S-CoA) concentrations were varied as indicated. POR and ferredoxin were purified from P. furiosus as described previously (45). Polysulfide was prepared as described previously (30), and the final concentration in the assay mixture was 11 mM. NAD(P)H, CoA, CoA-S-S-CoA, coenzyme M, glutathione, and dephospho- and desulfo-CoA were purchased from Sigma (St. Louis, MO).
RNA extraction and DNA microarray analyses.
Total RNA was extracted from cell extracts of P. furious by use of acid-phenol (46) and stored at −80°C until needed. The design and construction of DNA microarrays containing all of the predicted 2,192 open reading frames (ORFs) in the annotated genome of P. furiosus (37), preparation of cDNA from the RNA samples, and hybridization experiments were all performed as previously described (46). Fluorescently labeled cDNA was prepared using an ARES DNA labeling kit (Molecular Probes, Eugene, OR). The resulting amine-modified cDNA was purified using a QIAquick PCR purification kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions except that the wash buffer was replaced with 75% (vol/vol) ethanol and the cDNA was eluted with 45 μl of distilled water and dried under vacuum. The amine-modified cDNA was labeled with Alexa dye 488, 546, 594, or 647 (Molecular Probes) according to the manufacturer's instructions. The labeled cDNA was purified using a QIAquick PCR purification kit (QIAGEN) and dried under vacuum. Differentially labeled cDNAs derived from P. furiosus cells grown in the absence of S0 or from cells harvested at various times after S0 addition (up to 60 min) were pooled and hybridized to the DNA microarray, and the fluorescence intensities for the Alexa dyes were measured as described previously (46). For the microarray experiments, each log2 value represents an average of two hybridization experiments performed in triplicate using cDNA derived from two different cultures of P. furiosus. The log2 ratios were subjected to an unpaired t test function with two-tailed distribution to get the raw P values. The raw P values were subjected to a family-wise error rate correction by use of the Holm's step-down (18) procedure to give final P values. All microarray data are deposited in the NCBI GEO database (reference number GPL4688).
QPCR.
RNA was isolated as described above and further purified twice using the Absolutely RNA cleanup kit (Stratagene, La Jolla, CA) with an intermediate DNase (Ambion, Austin, TX) treatment (30 min, 37°C). cDNA was then prepared as described previously (53). The genes PF1186, PF2051, PF2052, PF1441-PF1453, PF1423-PF1436, and PF2025 were selected for study, and the constitutively expressed gene encoding the POR gamma subunit (PF0971) was selected as a control. Primers for the genes were designed using the program Array Designer v.1.16 (Premier Biosoft International, Palo Alto, CA). All QPCR experiments were carried out using an Mx3000P instrument (Stratagene) using the Brilliant SYBR green QPCR master mix (Stratagene). The comparative cycle threshold method was used to analyze the resulting data (2a), which are expressed as log2 changes (n-fold).
Purification of NSR from P. furiosus.
Frozen P. furiosus cell paste (100 g) grown on peptides in the presence of S0 (50) was lysed anaerobically by osmotic shock in 200 ml of 50 mM Tris-HCl (pH 8.0) under argon followed by sonication (Branson sonifier, 10 min, power setting 4). The cell extract was centrifuged at 120,000 × g for 1 h to fractionate the soluble cytoplasmic fraction from the insoluble membrane fraction. NSR was purified from the cytoplasmic fraction by anaerobic multistep chromatography using an Akta Basic (GE Healthcare, Piscataway, NJ). Unless otherwise stated, 50 mM Tris-HCl (pH 8.0) buffer was used, and all column chromatography materials were obtained from GE Healthcare. The cytoplasmic fraction (340 ml, 5,243 mg, 5,774 units) was loaded onto a DEAE-Sepharose column (150 ml) at a flow rate of 15 ml min−1. The column was washed with 2 column volumes (CV) of buffer and eluted with a NaCl gradient (0 to 1.0 M) over 20 CV. NSR eluted as 200 to 290 mM NaCl was applied to the column. Fractions with the highest specific activity were pooled (330 ml, 1,046 mg, 3,815 units) and, after being diluted with an equal volume of buffer, were loaded onto a Blue Sepharose column (40 ml) at a flow rate of 15 ml min−1, washed with 2 CV, and eluted with a linear gradient of NaCl (0 to 2.0 M over 20 CV). NSR eluted as a broad peak between 0.5 and 1.9 M NaCl. Active fractions were pooled (275 ml, 198 mg, 2,163 units), diluted threefold with buffer, loaded onto a hydroxyapatite (Bio-Rad) column (40 ml) at a flow rate of 10 ml min−1, and washed with 10 CV of buffer containing 5 mM phosphate (pH 7.4). Some of the activity (254 ml, 33 mg, 491 units) eluted during the wash and was purified separately, while the remainder (75 ml, 39 mg, 577 units) eluted when 129 to 162 mM phosphate was applied as part of a linear gradient of phosphate (5 to 500 mM in 18 CV). The active fractions from the wash and gradient elution steps were pooled separately, and each was diluted with an equal volume of 2.0 M (NH4)2SO4 in 50 mM Tris (pH 8.0) and loaded onto a Phenyl Sepharose HP column (20 ml) at a flow rate of 10 ml min−1. The column was washed with 2 CV of 1.0 M (NH4)2SO4 in 50 mM Tris (pH 8.0) and eluted with decreasing concentrations of (NH4)2SO4 (1.0 to 0 M over 20 CV). NSR activity was eluted as 575 to 465 mM (NH4)2SO4 was applied. The two NSR samples (11 ml, 3 mg, 317 units, and 12 ml, 2.3 mg, 224 units) from the Phenyl Sepharose columns were concentrated separately using a Q-Sepharose FF column (5 ml), and each was loaded onto a Bioscale Q5 column (Bio-Rad, Hercules, CA). NSR activity eluted as 130 to 150 mM NaCl was applied. The two NSR samples were indistinguishable by sodium dodecyl sulfate (SDS) gel analysis and specific activity and were combined to yield 2.0 mg with a total activity of 221 units.
Purification of recombinant NSR.
To clone the gene encoding NSR (PF1186), attB PCR primers were designed based on the Gateway cloning technology (Invitrogen) with a tobacco etch virus protease cleavage site two residues upstream of the start site on the N terminus. The forward and reverse primers were CTTACAAGTTTGTACAAAAAAGCAGGCTTAGAAAACCTGTATTTTCAGGGAGGAGAAAAGAAAAAGGTAGTCATAAT and CTTACCACTTTGTACAAGAAAGCTGGGTGTCACAAAACCCTGGCGAGGAC, respectively. Pfu polymerase (Stratagene) was used to amplify the gene of interest from P. furiosus genomic DNA, and the resulting PCR product was purified using a QIAquick PCR purification kit (QIAGEN). This was cloned into the destination vector pDEST C1 containing a six-His tag according to the manufacturer's protocols (Invitrogen). This destination vector was then transformed into the expression strain of Escherichia coli BL21(DE3)pRIL (Stratagene). These cells were grown aerobically on 2XYT medium in 2.8-liter Fernbach flasks (1 liter medium) at 37°C for 6 h with shaking at 200 rpm before induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After 16 h at 16°C, cells were harvested (10,000 × g, 20 min), resuspended in 100 ml 50 mM Tris-HCl (pH 7.4), degassed under argon, and frozen at −20°C. All purification procedures were carried out under anaerobic conditions, and all chemicals were acquired from Sigma (St. Louis, MO). Frozen cells (28 g [wet weight]) were thawed in the presence of lysozyme (200 μg ml−1), DNase I (5 μg ml−1), and phenylmethylsulfonyl fluoride (1 mM) and incubated with shaking at 37°C for 1 h. The cell extract was sonicated (Branson sonifier, 10 min, power setting 4), incubated at 80°C for 30 min, and then centrifuged (40,000 × g for 45 min) to remove denatured proteins. The heat-treated cytoplasmic extract (108 ml, 206 mg) containing 1,650 units of NSR activity was loaded onto a 12-ml Ni-nitrilotriacetic acid drip column (HIS-Select nickel affinity gel; Sigma) equilibrated with 50 mM Tris-HCl (pH 8.0) containing 0.5 M NaCl. NSR was eluted with 300 mM imidazole in the same buffer. The eluted His-tagged NSR protein (20 ml, 31 mg, 1,352 units) was incubated at 23°C for 3 h with AcTEV protease according to the manufacturer's protocols (Invitrogen), diluted 30-fold in 50 mM Tris-HCl (pH 8.0), and loaded onto the second Ni-nitrilotriacetic acid column. Protease-cleaved NSR without the N-terminal His tag (97 ml, 14.6 mg, 1,443 units) did not bind to the column, while residual His-tagged protein (18.6 ml, 14.9 mg, 1,044 units) was eluted with imidazole as described above. Both proteins were concentrated separately using a Q-Sepharose HP column (5 ml), where purified recombinant nontagged NSR (10 mg, 1,672 units) and His-tagged NSR (12.5 mg, 1,400 units) were obtained.
Other methods.
SDS-polyacrylamide gel electrophoresis analysis of purified NSR was performed using 4 to 20% Long Life gels (Life Therapeutics, Australia) with a Tris-HEPES buffer system. Samples were heated at 100°C for 10 min prior to loading. Gel filtration chromatography was performed using a Superdex S-200 column (320 ml; GE Healthcare) equilibrated with 50 mM Tris-HCl buffer (pH 8.0) containing 300 mM NaCl. Matrix-assisted laser desorption ionization was performed on a Bruker Autoflex (time of flight) mass spectrometer. SDS-polyacrylamide gel electrophoresis gel bands of purified NSR were excised, destained, and dehydrated with 50% acetonitrile in 50 mM NH4HCO3 and then digested with 15 μl of 10 μg/ml trypsin for 16 h. Peptides were then extracted from the gel slice by three 15-min washes (once with 50 mM NH4HCO3 and twice with 75% acetonitrile, 0.5% trifluoroacetic acid). Peptides were purified using NuTip C18 tips (Glygen Corp., Columbia, MD) and spotted (1 μl, containing α-cyano-4-hydroxycinnamic acid) directly on a matrix-assisted laser desorption ionization plate. Data analysis was performed in Protein Prospector v 3.2.1 using MS-Fit (http://prospector.ucsf.edu/).
RESULTS AND DISCUSSION
Effects of S0 on H2 and H2S production by intact cells.
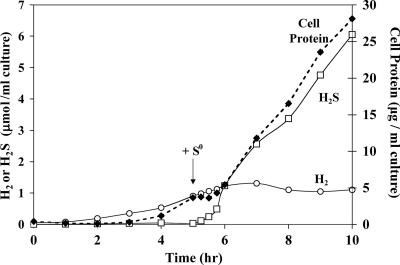
P. furiosus has been known to reduce S0 to H2S since its discovery (9). Under the growth conditions used here, the amount of H2S produced by cells grown for many generations in the presence S0 is comparable to the amount of H2 produced (per amount of cell protein) by cells grown without S0 (see Fig. S1 and S2 in the supplemental material), and cells produce insignificant amounts of the other gas (H2 in the presence of S0 and H2S in the absence of S0). Both sulfide and hydrogen production rates were closely correlated with cell density, although some abiotic production of sulfide was apparent at the early stage of growth (see Fig. S1 in the supplemental material). This is consistent with the results from a control experiment using the same S0-containing medium but lacking cells, which showed that sulfide was produced abiotically, reaching a concentration of approximately 0.5 mM after 1 h of incubation (data not shown). The effect of adding S0 to a growing P. furiosus culture is shown in Fig. 1. Within 10 min, H2S can be detected, and the rate of production rapidly increases in parallel with cell growth. Conversely, the rate of H2 production decreases to almost zero within 1 h after S0 addition. Clearly, P. furiosus prefers to use S0 as an electron acceptor rather than protons, and it rapidly adapts when S0 becomes available. In addition, when S0 is added, cell growth (as measured by total cellular protein) appears to stall for approximately 20 min before growth is coupled to H2S production. This suggests that a dramatic physiological response occurs within minutes of S0 addition.
FIG. 1.
Effect of S0 availability on the growth and production of H2 and H2S by P. furiosus. S0 (5 g/liter) was added (as indicated by the arrow) to a stirred maltose-grown culture (100 ml). Samples for total cell protein (closed diamonds), H2 production (open circles), and H2S production (open squares) were taken at the indicated times.
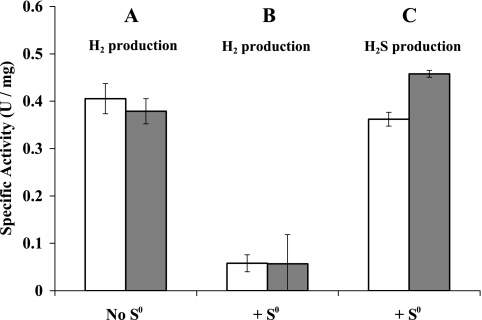
To determine if the decrease in H2 production by whole cells upon S0 addition was related to an effect of S0 on hydrogenase activity in vivo, cells were harvested before and 1 h after S0 addition and resuspended in fresh medium, and intact cell H2 production was measured using maltose as the source of reductant. As shown in Fig. 2A, both cell types exhibited high H2 production activities, showing that even after 1 h of exposure to S0, cells contained comparable amounts of the H2-producing MBH. Surprisingly, however, when the same assays were conducted with S0 added to the assay mixture (Fig. 2B), H2 production was clearly inhibited in both cell types. Under these conditions, both cell types have high S0 reduction activities (Fig. 2C). Consequently, growing cells exposed to S0 for 1 h, as illustrated in Fig. 1, have high hydrogenase activity but do not produce H2, since reductant is channeled preferentially to S0. Such cells therefore produce H2S rather than H2 due to changes in the pathway of electron flow rather than to the absence or presence of key enzymes. The enzyme primarily responsible for S0 reduction is discussed below.
FIG. 2.
Effect of elemental sulfur on H2 and H2S production using intact P. furiosus cells. Cells were harvested from a maltose-grown culture before (white bar) and 1 h after (gray bar) the addition of S0. Cells were resuspended in fresh medium (lacking S0 or maltose), and their ability to produce H2 was measured using maltose as the electron donor in the absence (A) and presence (B) of S0 (colloidal sulfur, 12.8 g/liter) in the assay medium. In the case where S0 was present, the ability of the intact cells to produce H2S (C) was also measured in the same assay vial.
Elemental sulfur reductase activities in cell extracts.
Cell extracts were prepared from intact cells harvested before and 1 h after the addition of S0 (see Fig. 1). Attempts were made to measure S0 reductase activity using either P. furiosus ferredoxin (reduced by P. furiosus POR) or NAD(P)H as the electron donor with cell extracts and with the cytoplasmic and membrane fractions after a 100,000 × g centrifugation step. Sodium dithionite and reduced dyes such as benzyl viologen and methyl viologen could not be used in these assays, as they readily reduce S0 abiotically. Significant ferredoxin-linked S0 reductase activity could not be measured (above the background) in any fraction, even though cell extracts and the membrane fraction exhibited ferredoxin-linked H2 production (data not shown; see reference 42). These results are in contrast to those obtained using the assays described above with intact cells, where S0 reductase activity, as well as hydrogenase activity, was measured using maltose as the electron donor.
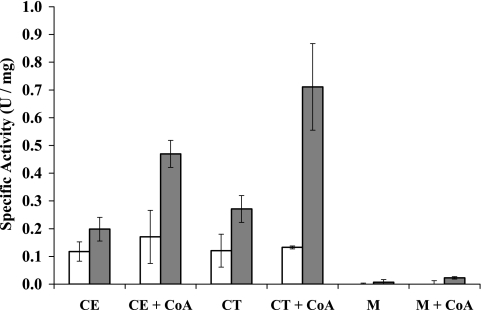
In contrast to ferredoxin-linked activity, NADPH-dependent S0 reductase activity was readily measured in cell extracts and in the cytosolic fraction but not in the membranes. As shown in Fig. 3, the activity was greatly stimulated by the addition of CoA to give a specific activity in the cytoplasm of 0.7 units/mg. The enzyme responsible for the activity was purified from the cytoplasm by use of anaerobic column chromatography. Only one peak of this NADPH- and CoA-dependent activity eluted from the first chromatography column, together with a minor CoA-independent peak of NADPH-linked S0-reducing activity, which was due to ferredoxin:NADPH oxidoreductase (28). The primary S0-reducing enzyme was purified by three subsequent chromatography steps to a specific activity in S0 reduction of approximately 100 units/mg, which represented a greater-than-140-fold purification compared to the cell extract. This NADPH NSR has the highest activity reported for any sulfur reductase-type enzyme system (13, 30, 33). The purified NSR preparation gave rise to a single protein band when analyzed by SDS-polyacrylamide electrophoresis (see Fig. S3 in the supplemental material), and this corresponded to a molecular weight of approximately 50,000. The apparent mass of the holoenzyme from analytical gel filtration was approximately 100 kDa, suggesting that NSR is a homodimer (data not shown). Analysis of the SDS gel band by trypsin digestion/mass spectrometry revealed that NSR corresponds to PF1186 (43% coverage), which is predicted to encode a protein of 48,720 Da, in agreement with the biochemical analyses of NSR.
FIG. 3.
NADPH- and CoA-dependent S0 reductase activities in cellular fractions of P. furiosus. The cells were obtained from a maltose-grown culture prior to (white bars) and 1 h after (gray bars) the addition of S0. The fractions are cell extract (CE), cytoplasm (CT [supernatant after 1 h at 120,000 × g]), and membrane (M [pellet after 1 h 120,000 × g]). Elemental sulfur reductase activity is expressed as units/mg.
The recombinant form of NSR with an N-terminal His tag was obtained by expression of PF1186 in E. coli. The corresponding enzyme was purified anaerobically by following its NADPH- and CoA-dependent S0 reductase activity. The purified preparation gave rise to a single band after electrophoretic analysis (see Fig. S3 in the supplemental material), and it eluted as an apparent homodimer after analytical gel filtration (data not shown). Purified recombinant NSR had a specific activity of 112 units/mg in the standard NSR assay. Although the sulfur reductase activity of NSR requires anaerobic conditions (the product sulfide is oxidized by oxygen), neither the native nor recombinant enzymes were oxygen sensitive (no loss of activity after exposure to air for 16 h at 23°C), and the two forms had very similar kinetic properties with respect to NADPH and CoA. The calculated apparent Km (and maximum rate of metabolism) values (for the native enzyme) were 8.5 mM (225 units/mg, using 200 μM CoA) and 18 μM (271 units/mg, using 10 mM NADPH) for NADPH and CoA, respectively. It should be noted, however, that the enzyme did not exhibit linear kinetics with respect to NADPH as the substrate (see Fig. S4 in the supplemental material), and this issue is discussed further below. The native and recombinant enzymes also utilized NADH and CoA-S-S-CoA as substrates, although the activities were about 50% lower (data not shown). The calculated apparent Km (and maximum rate of metabolism) values (for the native enzyme) were 3.3 mM (143 units/mg, using 200 μM CoA) and 10 μM (147 units/mg, using 10 mM NAPDH) for NADH and CoA-S-S-CoA, respectively. Colloidal sulfur at a concentration of 6.4 g/liter was used in these assays, and this appeared to be saturating. Approximately half-maximal activity was measured using 0.64 g/liter of colloidal sulfur.
In the NSR assays, the rate of sulfide production from colloidal sulfur was linear (up to 10 min) suggesting that this is the true substrate for the enzyme. A lag phase in sulfide production would be expected if polysulfide, which is generated by the reaction of sulfide with elemental sulfur, was the substrate for NSR. Accordingly, a less-than-twofold increase in activity was observed, both at pH 7.0 and at pH 9.0, when polysulfide (11 mM) was used as the substrate compared to when elemental sulfur (6.4 g/liter) was used. Polysulfide is stable only at pH values of ≥8 and readily dissociates to colloidal sulfur and sulfide at neutral pH (14). A much greater stimulation of activity would be observed if polysulfide were the preferred substrate, particularly at the higher pH. Presumably, the colloidal sulfur generated from polysulfide is a better substrate for NSR than the elemental sulfur typically added to the assay mixture (leading to an ∼2-fold increase in activity). The pH optimum for sulfide production (pH 6.5 [data not shown]) is also consistent with elemental sulfur rather than polysulfide being the substrate of NSR. Given the CoA dependence of the reaction, it is possible that polysulfide derivatives of CoA are generated during catalysis, and this is currently under investigation.
PF1186 is a member of a large family of flavin adenine dinucleotide (FAD)-dependent pyridine nucleotide-disulfide oxidoreductase genes (InterPro IPR013027). Accordingly, both native and recombinant forms of NSR were yellow in color and exhibited a UV/visible spectrum characteristic of flavoprotein (A460/A280 = 0.13 for the native enzyme). PF1186 and its homolog (PH0572) from P. horikoshii were previously proposed to function as NAD(P)H-dependent CoA-S-S-CoA reductase (CoADR) genes, and the recombinant form of the P. horikoshii enzyme was characterized (15). The aerobically purified P. horikoshii CoADR apoprotein was reconstituted with FAD and had a specific activity for CoA-S-S-CoA reduction of approximately 8.3 μmol/min/mg (at 75°C) using NADPH as the electron donor. The reported Km value for CoA-S-S-CoA (30 μM) is comparable to what was found (10 μM) in the present study using P. furiosus NSR in the S0 reduction assay, although the Km value for NADPH and P. horikoshii CoADR (<9 μM [15]) is 3 orders of magnitude lower than that which was determined with P. furiosus NSR (8.5 mM). The reason for this discrepancy is discussed below. It should be noted that for the P. furiosus enzyme, the specific activity for CoA-S-S-CoA reduction (6.0 μmol CoA-S-S-CoA reduced/min/mg) is about 20-fold lower than the activity that this enzyme exhibits in the S0 reduction assay. A second discrepancy is that P. horikoshii CoADR was reported to be a homotetramer (198 kDa [15]). This is in contrast to results presented here for P. furiosus NSR (92% sequence identity), which indicate that it is a homodimer. It was also reported that CoADR activity could not be purified from P. furiosus (15), but this attempt utilized cells grown in the absence of S0. Such cells would be expected to have a much lower content of the product of PF1186, according to the data presented in Fig. 2, a conclusion supported by the molecular analyses described below.
These results therefore suggest that the previously reported CoADR activity of the PF1186 homolog (in P. horikoshii) represents only a partial reaction of its true physiological function, which is now proposed to be CoA-dependent S0 reduction. Purified P. furiosus NSR did not reduce S0 to H2S in detectable amounts in the absence of CoA (or CoA-S-S-CoA), and this cofactor could not be replaced by dephospho- or desulfo-CoA, nor could it be replaced by glutathione or the methanogenic cofactor coenzyme M. The Km value for CoA (10 μM) is much lower than the intracellular concentration in P. furiosus (860 μM [20]), indicating that NSR would normally be saturated. The affinity of P. furiosus NSR for NADPH (Km, 8.5 mM) is surprisingly low, however, given the intracellular nicotinamide nucleotide concentration in P. furiosus (0.5 mM [34]). This high apparent Km value for NADPH may be related to the proposed mechanism of NADPH oxidation by NSR. P. horikoshii CoADR (15) is thought to react with two NADPH molecules, one to reduce the active site Cys (from the sulfenic acid derivative) to give the reduced enzyme (“EH2,” which could react with CoA-S-S-CoA, generating CoA) and one to produce the EH2NADPH active state (in which the FAD remains oxidized). Consequently, the prior kinetic analyses (15) might have measured only the first reaction, while the S0 reduction assay reported herein measures the second reaction. This would explain the difference in the kinetic constants for NADPH between this study and the earlier one (15), the nonlinear kinetics observed for S0 reduction (see Fig. S4 in the supplemental material), and the apparent high Km value for NADPH. A detailed study of the mechanism of S0 reduction by NSR is currently under way. Suffice it to say that since NSR contains only one cysteinyl residue, it is feasible that CoA provides the active site with a second thiol group to enable the two-electron reduction of S0, where the resulting disulfide is reduced by NADPH. Consequently, the ability of NSR to reduce CoA-S-S-CoA appears to be an artifactual side reaction of the catalytic mechanism and is not thought to have any physiological relevance.
Transcriptional analyses.
Growth studies showed that P. furiosus has a rapid response to the addition of S0, with H2S detected within 10 min and a pause in growth for approximately 20 min (Fig. 1). Transcriptional analyses of the complete genome (2,192 ORFs [37]) were therefore conducted on RNA extracted from cells harvested 10, 20, 30, and 60 min after S0 addition. In spite of the apparent dramatic physiological response (Fig. 1), only 19 ORFs were significantly up-regulated more than threefold (P < 0.05) within 10 min of adding S0, and a total of 34 were down-regulated. These ORFs are shown in Tables 1 and 2, respectively, together with those that are part of corresponding and potentially regulated operons. The regulation of these 34 ORFs is proposed to represent the primary response to S0. QPCR analyses were carried out with selected key ORFs to assess the validity of the DNA microarray data, and the results (Fig. 4) are discussed below. In analyzing the data, advantage was taken of the availability of the genome sequences of three other members of the Thermococcales order, Pyrococcus abyssi, P. horikoshii, and Thermococcus kodakaraensis (7, 11, 23). All three utilize S0, and it is assumed that they use the same mechanism as that of P. furiosus. Therefore, if an S0-regulated ORF in P. furiosus is not conserved in the other three species, this calls into question a direct role in primary S0 metabolism.
TABLE 1.
ORFs whose expression is up-regulated within 10 min after the addition of elemental sulfur to growing P. furiosus cells
| Function and ORFa | Description | Fold changeb |
|---|---|---|
| PF0094 | Protein disulfide oxidoreductase (12) | 3.7 |
| [Unknown transporter] | ||
| PF0261c | [Nucleic acid binding, OB-fold] | 3.5 |
| PF0262c | [Acriflavin resistance protein] | 2.9 |
| PF1186 | NAD(P)H sulfur reductase (this work) | 3.7 |
| [Membrane-bound oxidoreductase (42)] | ||
| PF1441 | MbxN | 4.3 |
| PF1442 | MbxL | 5.0 |
| PF1443 | MbxK | ND |
| PF1444 | MbxJ | 8.7 |
| PF1445 | MbxM | 4.6 |
| PF1446 | MbxH | 5.5 |
| PF1447 | MbxH | 6.0 |
| PF1448 | MbxG | 6.9 |
| PF1449 | MbxF | 4.7 |
| PF1450 | MbxD | 8.0 |
| PF1451 | MbxC | 8.6 |
| PF1452 | MbxB | 9.2 |
| PF1453 | MbxA | 12 |
| [Transcriptional regulation] | ||
| PF2051 | [Bacterial regulatory protein ArsR] | 6.5 |
| PF2052 | [Conserved hypothetical protein] | 5.6 |
Potential operons and their potential functions are indicated in bold. Descriptions for the ORFs are derived from the best hit in the Interpro database (http://www.ebi.ac.uk/interpro/), given within square brackets, and/or are from the indicated references/source in cases where there are experimental data to support the ORF assignment (given without brackets).
All changes (n-fold) are statistically significant (P < 0.05) unless indicated. ND, not determined.
No homolog present in the genome sequence of T. kodakaraensis.
TABLE 2.
ORFs whose expression is down-regulated within 10 min after the addition of elemental sulfur to growing P. furiosus cells
| Function and ORFa | Description | Fold changeb |
|---|---|---|
| PF0450 | [Glutamine synthetase, catalytic region] | 4.2 |
| [Cobalt transport] | ||
| PF0529d | [Cobalt transport protein (CbiQ)] | 5.1 |
| PF0530d | [Conserved hypothetical protein] | 2.6 |
| PF0531d | [Cobalamin (vitamin B12) biosynthesis CbiM] | 5.6 |
| PF0559 | [Hydrogenase maturation protein HypF] | 6.3 |
| PF0736e | [Conserved hypothetical protein] | 5.0 |
| PF0736.1e | [Conserved hypothetical protein] | 4.1 |
| Hydrogenase I (31) | ||
| PF0891 | Hydrogenase I beta | 4.4 |
| PF0892 | Hydrogenase I gamma | 4.9 |
| PF0893 | Hydrogenase I delta | 4.0 |
| PF0894 | Hydrogenase I alpha | 2.9 |
| PF0913c | [Formylmethanofuran dehydrogenase, subunit E] | 6.1 |
| PF0915 | [Cytochrome c biogenesis protein] | 3.0 |
| [Unknown] | ||
| PF0925f | [Radical SAM] | 9.4 |
| PF0926f | [Conserved hypothetical protein] | 10.2 |
| Hydrogenase IIg (32) | ||
| PF1329 | Hydrogenase II beta | 11.3 |
| PF1330 | Hydrogenase II gamma | 7.8 |
| PF1331 | Hydrogenase II delta | 10.0 |
| PF1332 | Hydrogenase II alpha | 4.4 |
| Membrane-bound hydrogenase (42) | ||
| PF1423 | MbhA | 4.4 |
| PF1424 | MbhB | 7.2 |
| PF1425 | MbhC | 6.5 |
| PF1426 | MbhD | 8.0 |
| PF1427 | MbhE | 6.8 |
| PF1428 | MbhF | 6.7 |
| PF1429 | MbhG | ND |
| PF1430 | MbhH | 5.2 |
| PF1431 | MbhI | 5.7 |
| PF1432 | MbhJ | ND |
| PF1433 | MbhK | 4.0 |
| PF1434 | MbhL | 3.7 |
| PF1435 | MbhM | 2.5 |
| PF1436 | MbhN | 2.4 |
| PF1621d | [Fibronectin, type III-like fold] | 4.2 |
For explanation, see Table 1, footnote a.
All changes (n-fold) are statistically significant (P < 0.05) unless indicated. ND, not determined.
No homolog present in the genome sequence of T. kodakaraensis.
No homologs in the genome sequences of P. horikoshii and P. abyssi.
Unique to P. furiosus.
No homologs in the genome sequences of T. kodakaraensis, P. horikoshii, and P. abyssi.
No homologs in the genome sequences of T. kodakaraensis and P. horikoshii.
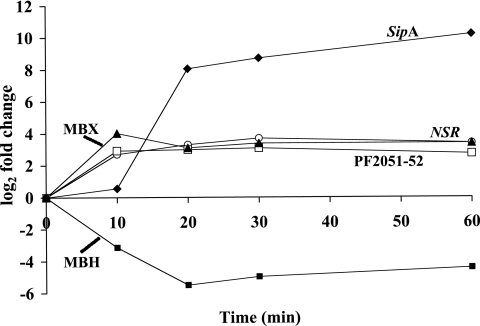
FIG. 4.
Real-time PCR analysis of the effect of S0 addition on the transcription of key genes. A 15-liter P. furiosus culture was grown with maltose as the carbon source and S0 was added in mid-log phase. RNA samples were prepared at 0, 10, 20, 30, and 60 min after S0 addition. The genes are those for NSR (PF1186 [open circles]), MBH (PF1423-PF1436 [closed squares]), MBX (PF1441-PF1453 [closed triangles]), SipA (PF2025 [closed diamonds]), and putative regulators (PF2051 and PF2052 [open squares]). In the case of potentially coregulated operons, the average response of all ORFs within the operon was plotted.
Primary response to S0 (up-regulated ORFs).
Within 10 min of the addition of S0 to a growing culture of P. furiosus, the expression of the gene encoding NSR (PF1186) was up-regulated by 3.7-fold according to the DNA microarray (Table 1) and by 7.0-fold using QPCR analysis (Fig. 4). These data are consistent with NSR playing a key and primary role in the response of P. furiosus to S0. As shown in Table 1, of the remaining 18 ORFs whose expression is immediately up-regulated upon S0 addition, 13 of them are arranged as a gene cluster, PF1441-PF1453. QPCR shows that they are up-regulated by an average of 16-fold (Fig. 4). This 13-ORF cluster was previously proposed to encode a second MBH, a membrane-bound oxidoreductase (MBX), as it shows high sequence identity and conservation of gene order with the 14 ORFs that encode the MBH (42). However, MBX lacks two key residues that coordinate the NiFe catalytic site of the hydrogenase (47). Moreover, the up-regulation of MBX is a primary response to S0, and it has been previously shown that cells grown for multiple generations with S0 lack significant hydrogenase activity (1), in further support of the contention that MBX is not a hydrogenase. Accordingly, the 14 ORFs that encode MBH, in addition to the 8 ORFs that encode the two cytoplasmic hydrogenases, hydrogenases I and II, are dramatically down-regulated within 10 min of S0 addition (Table 2 and Fig. 4). These data therefore indicate that, as a primary response to S0, the MBX cluster replaces the homologous MBH cluster (Fig. 4). The Mbh and Mbx operons are both highly conserved, both in sequence and in gene order, in P. horikoshii, P. abyssi, and T. kodakaraensis (49), in agreement with the proposed metabolic importance of these complexes in S0 and H2 metabolism.
MBH and MBX are part of the NADH dehydrogenase complex I/hydrogenase family, which includes NADH:quinone oxidoreductases (NUO or complex I), F420 quinone oxidoreductases, and energy-converting hydrogenases (Ech) (10, 16). These complexes consist of a core of six homologous subunits and through evolution and recruitment of additional subunits diverged into complexes with different physiological functions (10). For example, the core enzymes of MBH and MBX (MbxH, J-N and MbhH, J-N, respectively) are supplemented with six subunits of a ubiquitous family of cation/proton antiporters (49). Previous studies have shown that MBH is an energy-conserving complex in which the oxidation of ferredoxin and the reduction of protons are coupled to the generation of a proton motive force (41). MBH lacks homologs of the NADH- and flavin-binding subunits (NuoEFG) of the typical complex I (10, 16) and couples the oxidation of ferredoxin, rather than NADH, to proton pumping and in this case H2 production. The remarkable gene conservation and sequence similarity between MBH and MBX suggests that they have very similar functions.
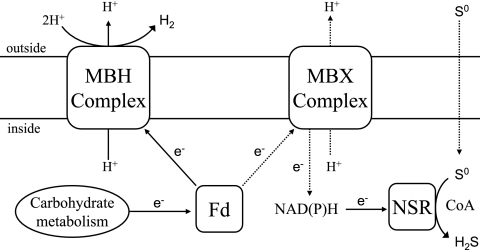
As illustrated in Fig. 5, MBX is proposed, like MBH, to oxidize ferredoxin and to conserve energy by pumping protons. The question then becomes, what entity does MBX reduce, thereby ultimately leading to S0 reduction? The most logical explanation is that MBX reduces NADP and NADPH is reoxidized by NSR as S0 is reduced to H2S (Fig. 5). However, we were unable to measure ferredoxin-dependent reduction of NADP or of S0 using a membrane preparation obtained from P. furiosus cells harvested from 10 to 60 min after S0 addition or using cells grown for many generations with S0. Since the amount of S0 reduced by growing P. furiosus cells approximates the amount of H2 produced on a cellular protein basis (see Fig. S2 in the supplemental material), it would seem likely that MBX and MBH conserve comparable amounts of energy during growth with and without S0, respectively, and that most if not all of the reductant used to reduce S0 flows through MBX. We speculate that our inability to measure an activity associated with MBX is due to instability of the complex. In this regard, it should be noted that MBH readily loses its ability to use ferredoxin as an electron donor during membrane fractionation (42, 47). Similarly, bacterial complex I has yet to be isolated as an intact complex (54). Attempts to stabilize P. furiosus MBX are in progress.
FIG. 5.
Proposed pathways of electron flow in Pyrococcus furiosus in the presence and absence of S0. Fd, ferredoxin.
In addition to NSR and MBX, the DNA microarray data (Table 1) show that the expression of the gene encoding a glutaredoxin-like protein termed protein disulfide oxidoreductase (PDO) (12, 25), PF0094, is up-regulated almost fourfold as part of the primary response to S0 (Table 1). A specific role for PF0094 as a PDO in P. furiosus has yet to be established. It has been proposed that in Pyrococcus species PDO is reduced by a thioredoxin reductase (PF1442 [22]) and that it could be an electron carrier for ribonucleotide reductase (RNR, PF0440 [3]). However, neither PF1442 nor PF0440 were part of the primary response to S0, although their expressions were up-regulated 60 min after S0 addition (by 2.3- and 3.8-fold, respectively; data not shown). Since homologs of PDO and thioredoxin reductase (and RNR) are widespread throughout the archaea, including those that do not utilize S0 (25, 35), it would seem unlikely that PDO has a specific role in reducing S0. To investigate this, the recombinant form of the PF0094 protein was obtained using published procedures (12). However, the purified protein had no effect on the S0 reduction activity of purified NSR activity or on the ability of P. furiosus membranes to couple ferredoxin oxidation to the reduction of NADP or S0 (data not shown).
In addition to those encoding NSR, MBX, and PDO, there are only four other ORFs that are significantly up-regulated within 10 min of S0 addition (Table 1), and these appear to be present as two operons. PF2051 and PF2052 are both annotated as transcriptional regulators, but only PF2051 contains regulatory domains (IPR001845), while PF2052 contains a nucleotide triphosphate pyrophosphohydrolase domain (IPR004518). Both ORFs most likely form an operon, as their sequences overlap by 22 nucleotides, and this synteny is conserved in the genome sequences of the other three Thermococcales. It is possible that these regulators play a key role in the primary response to S0, although the nature of the effector is unknown. The other two ORFs that are regulated, PF0261 and PF0262, overlap by 12 nucleotides. PF0262 shows homology to multidrug efflux systems (IPR001036), while PF0261 has a nucleic acid binding fold (IPR008994). Homologs of both ORFs are found in the genomes of the other two Pyrococcus species but not in the genome sequence of T. kodakaraensis, so their role in S0 metabolism is unclear.
Primary response to S0 (down-regulated ORFs).
The most striking feature of the list of 34 ORFs whose expression is down-regulated within 10 min of S0 addition (Table 2) is that 22 of them contain the structural genes of the three hydrogenases and another involves hydrogenase maturation. It was already known that cells grown for many generations with S0 contain only very low hydrogenase activity (1), and it is now apparent that the biosynthesis of all three hydrogenases is rapidly curtailed within minutes of S0 addition, indicating a complete shutdown at the genetic level of H2 metabolism in the presence of S0 (Table 2 and Fig. 4). Cells continue to produce H2 for 2 h or so after S0 addition (Fig. 1) due to the existing hydrogenase protein in the cell, but by 1 h the rate is <10% of the rate of H2S production, and eventually no H2 is produced. The small amount of H2 subsequently consumed (Fig. 1) may reflect a differential stability between the MBH (less stable) and the cytoplasmic enzymes, which are proposed to consume H2 and reduce NADP (29). The hydrogenase genes are well conserved within the Thermococcales order, although hydrogenase II, whose exact function is unknown, is absent from P. horikoshii and T. kodakaraensis (29).
It is difficult to rationalize the roles of the remaining 11 ORFs that are part of the primary response to S0 (Table 2), particularly when not all of them are conserved in the other three Thermococcales. PF0450 encodes a putative glutamine synthetase but it was not regulated in cells grown for many generations with S0 (46), and the fact that it is affected so quickly after S0 addition is puzzling. While PF0450 has homologs in the other sequenced Thermococcales, this is not the case for the other S0-responsive ORFs, suggesting that they may not be playing essential roles in S0 metabolism. PF0528-PF0531 appears to form an operon and is annotated as a cobalt transporter. This operon is conserved in T. kodakaraensis but not in P. horikoshii or P. abyssi. Similarly, PF1621 contains a fibronectin-like fold (IPR008957) but has a homolog only in T. kodakaraensis. Since P. horikoshii and P. abyssi do not utilize sugars like P. furiosus and T. kodakaraensis, perhaps the latter two ORF systems are involved in sugar metabolism. PF0925-PF0926 is predicted to contain a radical SAM domain, but close homologs are absent from P. horikoshii, P. abyssi, and T. kodakaraensis. PF0736 and PF0736.1 are hypothetical ORFs on opposing strands and show little sequence similarity to any protein in the NCBI database (2). PF0913 is a homolog of subunit E of formylmethanofuran dehydrogenase, an enzyme found in methanogens, but this is not the catalytic subunit and its function is unknown (52).
Secondary response to S0.
The ORFs that are up-regulated only 10 min after S0 addition are assumed to represent the primary response, and all remain up-regulated at 30 min. At this time, an additional 27 ORFs are up-regulated more than threefold (or are part of a potentially regulated operon), and these appear to represent a secondary response to S0 (Table 3). This is supported by the fact that most of them (15 of 27) are involved in metabolism of glutamate or branched-chain amino acids. PF0204-PF206 and PF1852 are potentially involved in glutamate biosynthesis (21, 44) and these might compensate for down-regulation (by 4.1-fold) of the ORF that encodes glutamate dehydrogenase (data not shown), although why is not clear. Three ORFs involved in iron metabolism are also up-regulated by S0 (Table 3). Presumably, the production of intracellular sulfide (by cytoplasmic NSR) might lead to insoluble iron sulfides, and ORFs involved in ferrous iron transport (PF0857) and iron-sulfur cluster biosynthesis (PF1285, PF1286) are up-regulated in response to the products of S0 reduction.
TABLE 3.
ORFs whose expression is up-regulated within 30 min after the addition of elemental sulfur to growing P. furiosus cells
| Function and ORFa | Description | Fold changeb |
|---|---|---|
| [Glutamate biosynthesis] | ||
| PF0204f | [Glutamate synthase, large subunit] | 4.0 |
| PF0205f | [Ferredoxin-dependent glutamate synthase] | 2.8 |
| PF0206f | [Glutamate synthase, large subunit region 3] | 3.2 |
| PF0686e | [Hypothetical protein] | 3.1 |
| PF0704 | [Protein of unknown function DUF302] | 3.1 |
| PF0857 | [Ferrous iron transport protein B, N terminal] | 3.2 |
| [Branched-chain amino acid biosynthesis] | ||
| PF0935 | [Acetolactate synthase, large subunit] | 4.6 |
| PF0936 | [Acetohydroxy acid isomeroreductase] | 4.7 |
| PF0937 | [Pyruvate carboxyltransferase] | 5.0 |
| PF0938 | [3-Isopropylmalate dehydratase large subunit] | 3.5 |
| PF0939 | [3-Isopropylmalate dehydratase small subunit] | 4.3 |
| PF0940 | [Isocitrate/isopropylmalate dehydrogenase] | 3.7 |
| PF0941 | [2-Isopropylmalate/homocitrate synthase] | 3.1 |
| PF0942 | [6-Phosphogluconate dehydratase] | 3.2 |
| [Transcription factor] | ||
| PF0984 | [Hypothetical protein] | 3.3 |
| PF0986 | [Transcription factor TFIIS] | 3.4 |
| [Iron-sulfur cluster assembly] | ||
| PF1285f | [SufBD] | 2.3 |
| PF1286f | [SufBD] | 3.2 |
| [Branched-chain amino acid biosynthesis] | ||
| PF1678 | [Alpha-isopropylmalate/homocitrate synthase] | 5.2 |
| PF1679 | [3-Isopropylmalate dehydratase large subunit] | 6.9 |
| PF1680 | [3-Isopropylmalate dehydratase small subunit] | 4.9 |
| [Ribosome] | ||
| PF1823 | [Ribosomal L23 protein] | 2.6 |
| PF1824 | [Ribosomal protein L4/L1e] | 4.0 |
| PF1852 | [Glutamate synthase (21)] | 3.4 |
| [Sulfur-induced proteins(46)] | ||
| PF2025 | SipA | 9.3 |
| PF2026 | SipB | 6.5 |
| PF2029 | [Hypothetical protein] | 3.7 |
For explanation, see Table 1, footnote a.
All changes (n-fold) are statistically significant (P < 0.05) unless indicated. ND, not determined.
c No homolog present in the genome sequence of T. kodakaraensis.
d No homologs in the genome sequences of P. horikoshii and P. abyssi.
Unique to P. furiosus.
No homologs in the genome sequences of T. kodakaraensis, P. horikoshii, and P. abyssi.
In a previous study (46) involving cells grown for many generations with S0, two highly regulated S0-dependent ORFs were characterized as “sulfur-induced proteins” A and B (SipA and SipB). We were surprised to find that these are part of the secondary, rather than the primary, response to S0, although the response is quite dramatic and appears to represent an on/off switch. By QPCR analysis, SipA and SipB are up-regulated over 400-fold and 26-fold, respectively, 30 min after S0 addition (Fig. 4). They are conserved in the four Thermococcales members whose genomes have been sequenced, but their physiological function still remains a mystery. A potential transcription factor, PF0986, which is up-regulated 30 min after S0 addition might be involved in coordinating the secondary responses to S0. PF0986 is a homolog of the characterized TFIIS in Methanococcus thermolithotrophicus, which is involved in RNA proofreading (26). PF0986 is potentially in an operon with PF0984, encoding a small 59-residue protein, both of which are conserved in the sequenced Thermococcales (2). However, the relationship of these ORFs to transcriptional regulation and S0 metabolism is not clear at this point.
In conclusion, while the addition of S0 to a culture of P. furiosus cells causes growth to stall, indicating a large metabolic shift (Fig. 1), only two key enzymes that appear to be directly involved in S0 reduction, MBX and NSR, were identified. As shown in Fig. 5, MBX and NSR are proposed to be the key enzymes responsible for the reoxidation of ferredoxin and NAD(P)H, respectively. Another primary response to S0 availability is the concomitant shutdown of H2 metabolism, resulting in the preferential transfer of reducing equivalents to the reduction of S0 rather than protons (Fig. 5). This novel S0-reducing system involving NSR and MBX is so far unique to the heterotrophic Thermococcales and contrasts with the cytochrome- and quinone-based S0-reducing system in autotrophic archaea (and bacteria). Future research will focus on elucidating the precise role of MBX and the mechanism of S0 reduction by NSR.
Supplementary Material
Acknowledgments
This research was funded by grants (FG05-95ER20175 and FG02-05ER15710) from the Department of Energy.
We thank Peter S. Horanyi for providing the pDEST C1 Gateway expression vector, Cindy Lim for preliminary QPCR studies, Scott D. Hamilton-Brehm for assistance in constructing the microarrays, Frank E. Jenney, Jr. and Angeli L. Menon for many helpful discussions, and Farris L. Poole II for bioinformatics analyses.
Footnotes
Published ahead of print on 20 April 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Adams, M. W. W., J. F. Holden, A. L. Menon, G. J. Schut, A. M. Grunden, C. Hou, A. M. Hutchins, F. E. Jenney, Jr., C. Kim, K. Ma, G. Pan, R. Roy, R. Sapra, S. V. Story, and M. F. Verhagen. 2001. Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183:716-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Applied Biosystems. 2001. Bulletin 2. Applied Biosystems, Foster City, CA.
- 3.Arner, E. S., and A. Holmgren. 2000. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 267:6102-6109. [DOI] [PubMed] [Google Scholar]
- 4.Blamey, J. M., and M. W. Adams. 1993. Purification and characterization of pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. Biochim. Biophys. Acta 1161:19-27. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J. S., and L. E. Mortenson. 1977. Inhibition of methylene blue formation during determination of the acid-labile sulfide of iron-sulfur protein samples containing dithionite. Anal. Biochem. 79:157-165. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, G. N., V. Barbe, D. Flament, M. Galperin, R. Heilig, O. Lecompte, O. Poch, D. Prieur, J. Querellou, R. Ripp, J. C. Thierry, J. Van der Oost, J. Weissenbach, Y. Zivanovic, and P. Forterre. 2003. An integrated analysis of the genome of the hyperthermophilic archaeon Pyrococcus abyssi. Mol. Microbiol. 47:1495-1512. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich, W., and O. Klimmek. 2002. The function of methyl-menaquinone-6 and polysulfide reductase membrane anchor (PsrC) in polysulfide respiration of Wolinella succinogenes. Eur. J. Biochem. 269:1086-1095. [DOI] [PubMed] [Google Scholar]
- 9.Fiala, G., and K. O. Stetter. 1986. Pyrococcus furiosus sp-nov represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100-degrees C. Arch. Microbiol. 145:56-61. [Google Scholar]
- 10.Friedrich, T., and D. Scheide. 2000. The respiratory complex I of bacteria, archaea and eukarya and its module common with membrane-bound multisubunit hydrogenases. FEBS Lett. 479:1-5. [DOI] [PubMed] [Google Scholar]
- 11.Fukui, T., H. Atomi, T. Kanai, R. Matsumi, S. Fujiwara, and T. Imanaka. 2005. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res. 15:352-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guagliardi, A., D. de Pascale, R. Cannio, V. Nobile, S. Bartolucci, and M. Rossi. 1995. The purification, cloning, and high level expression of a glutaredoxin-like protein from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 270:5748-5755. [DOI] [PubMed] [Google Scholar]
- 13.Guiral, M., P. Tron, C. Aubert, A. Gloter, C. Iobbi-Nivol, and M. T. Giudici-Orticoni. 2005. A membrane-bound multienzyme, hydrogen-oxidizing, and sulfur-reducing complex from the hyperthermophilic bacterium Aquifex aeolicus. J. Biol. Chem. 280:42004-42015. [DOI] [PubMed] [Google Scholar]
- 14.Gun, J., A. D. Modestov, A. Kamyshny, D. Ryzkov, V. Gitis, A. Goifman, O. Lev, V. Hultsch, T. Grischek, and E. Worch. 2004. Electrospray ionization mass spectrometric analysis of aqueous polysulfide solutions. Microchim. Acta 146:229-237. [Google Scholar]
- 15.Harris, D. R., D. E. Ward, J. M. Feasel, K. M. Lancaster, R. D. Murphy, T. C. Mallet, and E. J. Crane III. 2005. Discovery and characterization of a coenzyme A disulfide reductase from Pyrococcus horikoshii. Implications for this disulfide metabolism of anaerobic hyperthermophiles. FEBS J. 272:1189-1200. [DOI] [PubMed] [Google Scholar]
- 16.Hedderich, R., and L. Forzi. 2005. Energy-converting [NiFe] hydrogenases: more than just H2 activation. J. Mol. Microbiol. Biotechnol. 10:92-104. [DOI] [PubMed] [Google Scholar]
- 17.Hedderich, R., O. Klimmek, A. Kroger, R. Dirmeier, M. Keller, and K. O. Stetter. 1999. Anaerobic respiration with elemental sulfur and disulfides. FEMS Microbiol. Rev. 22:353-381. [Google Scholar]
- 18.Holm, S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6:65-70. [Google Scholar]
- 19.Huber, R., H. Huber, and K. O. Stetter. 2000. Towards the ecology of hyperthermophiles: biotopes, new isolation strategies and novel metabolic properties. FEMS Microbiol. Rev. 24:615-623. [DOI] [PubMed] [Google Scholar]
- 20.Hummel, C. S., K. M. Lancaster, and E. J. Crane III. 2005. Determination of coenzyme A levels in Pyrococcus furiosus and other Archaea: implications for a general role for coenzyme A in thermophiles. FEMS Microbiol. Lett. 252:229-234. [DOI] [PubMed] [Google Scholar]
- 21.Jongsareejit, B., R. N. Rahman, S. Fujiwara, and T. Imanaka. 1997. Gene cloning, sequencing and enzymatic properties of glutamate synthase from the hyperthermophilic archaeon Pyrococcus sp. KOD1. Mol. Gen. Genet. 254:635-642. [DOI] [PubMed] [Google Scholar]
- 22.Kashima, Y., and K. Ishikawa. 2003. A hyperthermostable novel protein-disulfide oxidoreductase is reduced by thioredoxin reductase from hyperthermophilic archaeon Pyrococcus horikoshii. Arch. Biochem. Biophys. 418:179-185. [DOI] [PubMed] [Google Scholar]
- 23.Kawarabayasi, Y., M. Sawada, H. Horikawa, Y. Haikawa, Y. Hino, S. Yamamoto, M. Sekine, S. Baba, H. Kosugi, A. Hosoyama, Y. Nagai, M. Sakai, K. Ogura, R. Otsuka, H. Nakazawa, M. Takamiya, Y. Ohfuku, T. Funahashi, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K. Aoki, and H. Kikuchi. 1998. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 5:55-76. [DOI] [PubMed] [Google Scholar]
- 24.Keller, M., and R. Dirmeier. 2001. Hydrogen-sulfur oxidoreductase complex from Pyrodictium abyssi. Methods Enzymol. 331:442-451. [DOI] [PubMed] [Google Scholar]
- 25.Ladenstein, R., and B. Ren. 2006. Protein disulfides and protein disulfide oxidoreductases in hyperthermophiles. FEBS J. 273:4170-4185. [DOI] [PubMed] [Google Scholar]
- 26.Lange, U., and W. Hausner. 2004. Transcriptional fidelity and proofreading in Archaea and implications for the mechanism of TFS-induced RNA cleavage. Mol. Microbiol. 52:1133-1143. [DOI] [PubMed] [Google Scholar]
- 27.Laska, S., F. Lottspeich, and A. Kletzin. 2003. Membrane-bound hydrogenase and sulfur reductase of the hyperthermophilic and acidophilic archaeon Acidianus ambivalens. Microbiology 149:2357-2371. [DOI] [PubMed] [Google Scholar]
- 28.Ma, K., and M. W. Adams. 2001. Ferredoxin:NADP oxidoreductase from Pyrococcus furiosus. Methods Enzymol. 334:40-45. [DOI] [PubMed] [Google Scholar]
- 29.Ma, K., and M. W. Adams. 2001. Hydrogenases I and II from Pyrococcus furiosus. Methods Enzymol. 331:208-216. [DOI] [PubMed] [Google Scholar]
- 30.Ma, K., and M. W. Adams. 1994. Sulfide dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus: a new multifunctional enzyme involved in the reduction of elemental sulfur. J. Bacteriol. 176:6509-6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, K., R. N. Schicho, R. M. Kelly, and M. W. Adams. 1993. Hydrogenase of the hyperthermophile Pyrococcus furiosus is an elemental sulfur reductase or sulfhydrogenase: evidence for a sulfur-reducing hydrogenase ancestor. Proc. Natl. Acad. Sci. USA 90:5341-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma, K., R. Weiss, and M. W. Adams. 2000. Characterization of hydrogenase II from the hyperthermophilic archaeon Pyrococcus furiosus and assessment of its role in sulfur reduction. J. Bacteriol. 182:1864-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng, K. Y., R. Sawada, S. Inoue, K. Kamimura, and T. Sugio. 2000. Purification and some properties of sulfur reductase from the iron-oxidizing bacterium Thiobacillus ferrooxidans NASF-1. J. Biosci. Bioeng. 90:199-203. [DOI] [PubMed] [Google Scholar]
- 34.Pan, G., M. F. Verhagen, and M. W. Adams. 2001. Characterization of pyridine nucleotide coenzymes in the hyperthermophilic archaeon Pyrococcus furiosus. Extremophiles 5:393-398. [DOI] [PubMed] [Google Scholar]
- 35.Pedone, E., D. Limauro, R. D'Alterio, M. Rossi, and S. Bartolucci. 2006. Characterization of a multifunctional protein disulfide oxidoreductase from Sulfolobus solfataricus. FEBS J. 273:5407-5420. [DOI] [PubMed] [Google Scholar]
- 36.Pihl, T. D., L. K. Black, B. A. Schulman, and R. J. Maier. 1992. Hydrogen-oxidizing electron transport components in the hyperthermophilic archaebacterium Pyrodictium brockii. J. Bacteriol. 174:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole, F. L., II, B. A. Gerwe, R. C. Hopkins, G. J. Schut, M. V. Weinberg, F. E. Jenney, Jr., and M. W. Adams. 2005. Defining genes in the genome of the hyperthermophilic archaeon Pyrococcus furiosus: implications for all microbial genomes. J. Bacteriol. 187:7325-7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robb, F. T., D. L. Maeder, J. R. Brown, J. DiRuggiero, M. D. Stump, R. K. Yeh, R. B. Weiss, and D. M. Dunn. 2001. Genomic sequence of hyperthermophile, Pyrococcus furiosus: implications for physiology and enzymology. Methods Enzymol. 330:134-157. [DOI] [PubMed] [Google Scholar]
- 39.Ronimus, R. S., and H. W. Morgan. 2003. Distribution and phylogenies of enzymes of the Embden-Meyerhof-Parnas pathway from archaea and hyperthermophilic bacteria support a gluconeogenic origin of metabolism. Archaea 1:199-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakuraba, H., and T. Ohshima. 2002. Novel energy metabolism in anaerobic hyperthermophilic archaea: a modified Embden-Meyerhof pathway. J. Biosci. Bioeng. 93:441-448. [DOI] [PubMed] [Google Scholar]
- 41.Sapra, R., K. Bagramyan, and M. W. Adams. 2003. A simple energy-conserving system: proton reduction coupled to proton translocation. Proc. Natl. Acad. Sci. USA 100:7545-7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sapra, R., M. F. Verhagen, and M. W. W. Adams. 2000. Purification and characterization of a membrane-bound hydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 182:3423-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schicho, R. N., K. Ma, M. W. Adams, and R. M. Kelly. 1993. Bioenergetics of sulfur reduction in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 175:1823-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schut, G. J., S. D. Brehm, S. Datta, and M. W. Adams. 2003. Whole-genome DNA microarray analysis of a hyperthermophile and an archaeon: Pyrococcus furiosus grown on carbohydrates or peptides. J. Bacteriol. 185:3935-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schut, G. J., A. L. Menon, and M. W. W. Adams. 2001. 2-Ketoacid oxidoreductases from Pyrococcus furiosus and Thermococcus litoralis. Methods Enzymol. 331:144-158. [DOI] [PubMed] [Google Scholar]
- 46.Schut, G. J., J. Zhou, and M. W. W. Adams. 2001. DNA microarray analysis of the hyperthermophilic archaeon Pyrococcus furiosus: evidence for a new type of sulfur-reducing enzyme complex. J. Bacteriol. 183:7027-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva, P. J., E. C. van den Ban, H. Wassink, H. Haaker, B. de Castro, F. T. Robb, and W. R. Hagen. 2000. Enzymes of hydrogen metabolism in Pyrococcus furiosus. Eur. J. Biochem. 267:6541-6551. [DOI] [PubMed] [Google Scholar]
- 48.Stetter, K. O. 1996. Hyperthermophilic procaryotes. FEMS Microbiol. Rev. 18:149-158. [Google Scholar]
- 49.Swartz, T. H., S. Ikewada, O. Ishikawa, M. Ito, and T. A. Krulwich. 2005. The Mrp system: a giant among monovalent cation/proton antiporters? Extremophiles 9:345-354. [DOI] [PubMed] [Google Scholar]
- 50.Verhagen, M. F., A. L. Menon, G. J. Schut, and M. W. Adams. 2001. Pyrococcus furiosus: large-scale cultivation and enzyme purification. Methods Enzymol. 330:25-30. [DOI] [PubMed] [Google Scholar]
- 51.Verhees, C. H., S. W. Kengen, J. E. Tuininga, G. J. Schut, M. W. Adams, W. M. De Vos, and J. Van Der Oost. 2003. The unique features of glycolytic pathways in Archaea. Biochem. J. 375:231-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vorholt, J. A., M. Vaupel, and R. K. Thauer. 1996. A polyferredoxin with eight [4Fe-4S] clusters as a subunit of molybdenum formylmethanofuran dehydrogenase from Methanosarcina barkeri. Eur. J. Biochem. 236:309-317. [DOI] [PubMed] [Google Scholar]
- 53.Weinberg, M. V., G. J. Schut, S. Brehm, S. Datta, and M. W. Adams. 2005. Cold shock of a hyperthermophilic archaeon: Pyrococcus furiosus exhibits multiple responses to a suboptimal growth temperature with a key role for membrane-bound glycoproteins. J. Bacteriol. 187:336-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yagi, T., and A. Matsuno-Yagi. 2003. The proton-translocating NADH-quinone oxidoreductase in the respiratory chain: the secret unlocked. Biochemistry 42:2266-2274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.