Abstract
Heterocyst development was analyzed in mutants of the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120 bearing inactivated cox2 and/or cox3 genes, encoding heterocyst-specific terminal respiratory oxidases. At the morphological level, the cox2 cox3 double mutant (strain CSAV141) was impaired in membrane reorganization involving the so-called honeycomb system that in the wild-type strain is largely or exclusively devoted to respiration, accumulated glycogen granules at conspicuously higher levels than the wild type (in both vegetative cells and heterocysts), and showed a delay in carboxysome degradation upon combined nitrogen deprivation. Consistently, chemical analysis confirmed higher accumulation of glycogen in strain CSAV141 than in the wild type. No impairment was observed in the formation of the glycolipid or polysaccharide layers of the heterocyst envelope, consistent with the chemical detection of heterocyst-specific glycolipids, or in the expression of the heterocyst-specific genes nifHDK and fdxH. However, nitrogenase activity under oxic conditions was impaired in strain CSAV135 (cox3) and undetectable in strain CSAV141 (cox2 cox3). These results show that these dedicated oxidases are required for normal development and performance of the heterocysts and indicate a central role of Cox2 and, especially, of Cox3 in the respiratory activity of the heterocysts, decisively contributing to protection of the N2 fixation machinery against oxygen. However, in contrast to the case for other diazotrophic bacteria, expression of nif genes in Anabaena seems not to be affected by oxygen.
Biological nitrogen fixation represents a main input of N into the biosphere and is a limiting factor for primary productivity in vast oceanic areas, thus greatly influencing C and N cycling at a global scale (6, 30). The nitrogen-fixing reaction (i.e., N2 reduction to ammonium) catalyzed by most of the known nitrogenase complexes is extremely sensitive to oxygen, and bacteria able to carry out this process in oxic environments have developed different strategies to protect their N2 fixation machinery against oxygen. This problem is especially relevant in the case of diazotrophic cyanobacteria, because, their main life style being oxygenic photoautotrophy, they have to cope not only with external oxygen but also with that generated intracellularly by the operation of photosystem II (PSII). A remarkable way of protection of the N2 fixation machinery against oxygen is the differentiation of specialized cells called heterocysts that, in response to combined nitrogen deprivation, takes place in some filamentous cyanobacteria. In oxic environments, the N2 fixation machinery is confined to heterocysts, the only cells in which the nif genes are expressed (12, 34). Heterocysts exhibit distinct features aimed at increasing the efficiency of the N2 fixation reaction and at keeping free O2 in the cytoplasm at a low concentration. Thus, in the course of the differentiation process, heterocysts acquire supplemental envelope layers, lose activity of PSII and of photosynthetic CO2 fixation, and acquire specific hydrogenases and oxidases that contribute to the generation of ATP for the demanding diazotrophic metabolism. These oxidases consume traces of oxygen that, in spite of the barrier imposed by the enlarged cell wall, penetrate into the heterocyst (34).
Anabaena sp. strain PCC 7120 is a heterocyst-forming cyanobacterium whose entire genome has been sequenced (15). In this strain, three gene clusters encoding heme-copper-type terminal respiratory oxidases have been described (31). Each of the cox1 and cox2 clusters encodes the three subunits of an aa3-type cytochrome c oxidase similar to those present in other unicellular or heterocyst-forming cyanobacteria (23, 27, 28). In contrast, the polypeptides encoded in the cox3 cluster do not show the CuA and Mg2+ binding motifs characteristic of cytochrome oxidases, being more related to those of the so-called ARTO (or CtaII) terminal oxidases that are similar to heme-copper quinol oxidases (13, 22). The cox1 gene cluster is expressed in vegetative cells irrespective of the nitrogen regimen (14, 31). In contrast, the cox2 and cox3 gene clusters are expressed in response to combined nitrogen deprivation specifically in developing and mature heterocysts, and the operation of at least one of them is required for the diazotrophic growth of the cyanobacterium (31).
The present work involves a morphological, genetic, and biochemical analysis of mutant strains of Anabaena bearing inactivated versions of some cox genes, which was aimed at learning the function of the dedicated diazotrophic oxidases in heterocyst metabolism.
MATERIALS AND METHODS
Organisms and growth conditions.
This study was carried out with the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120 (also called Nostoc sp. strain PCC 7120) and cox mutant derivatives CSAV135 (coxA3::C.S3), CSAV140 (coxB2::C.S3), and CSAV141 (coxB2::C.K3 coxA3::C.S3) (31). Cells were grown in BG110 (medium BG11 [25] without NaNO3) containing 8 mM NH4Cl and 16 mM TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid]-NaOH buffer (pH 7.5). All cultures were supplemented with 10 mM of NaHCO3 and bubbled with a mixture of CO2 and air (1%, vol/vol). Strains CSAV135 and CSAV140 were grown in the presence of 2 μg·ml−1 of streptomycin and 2 μg·ml−1 of spectinomycin. Strain CSAV141 was grown in the presence of 5 μg·ml−1 of neomycin, 2 μg·ml−1 of streptomycin, and 2 μg·ml−1 of spectinomycin. The amount of cyanobacteria in cell suspensions was estimated by their chlorophyll a (Chl) content. Chl was determined in methanolic extracts of the cells (16). For nitrogen step-down, filaments growing exponentially in NH4Cl-containing medium (3 to 5 μg of Chl·ml−1) were harvested at room temperature and either used directly or washed with and resuspended in BG110 medium and further incubated under culture conditions for the number of hours indicated for each experiment.
DNA isolation and analysis.
DNA fragments were purified from agarose gels with the GFX kit (Amersham Biosciences). Plasmid isolation from Escherichia coli, transformation of E. coli, digestion of DNA with restriction endonucleases, ligation with T4 ligase, and PCR were performed by standard procedures (2, 26).
RNA isolation and analysis.
RNA from whole filaments was isolated in the presence of ribonucleoside-vanadyl complex as previously described (18). For Northern analysis, 25 to 30 μg of RNA was loaded per lane and electrophoresed in 1% agarose denaturing formaldehyde gels. Transfer and fixation to Hybond-N+ membranes (Amersham Biosciences) were carried out using 0.1 M NaOH. Hybridization was performed at 65°C according to the recommendations of the manufacturers of the membranes. The nifH and fdxH probes were fragments of these genes amplified by PCR. The nifH probe was amplified using plasmid pCSAV60 (containing the nifH gene cloned in pGEM-T vector) as a template and oligonucleotides NH-1 (corresponding to positions −334 to −314 with respect to the translation start of nifH) and NH-4 (complementary to nucleotides +884 to +863 with respect to the translation start of nifH). The fdxH probe was amplified using plasmid pCSAV164 (containing the fdxH gene cloned in pGEM-T vector) as a template and oligonucleotides FH-1 (corresponding to nucleotides +3 to +20 with respect to the translation start of fdxH) and FH-2 (complementary to nucleotides +297 to +269 with respect to the translation start of fdxH). All probes were 32P labeled with a Ready-to-Go DNA labeling kit (Amersham Biosciences) using [α-32P]dCTP. Images of radioactive filters and gels were obtained and quantified with a Cyclone storage phosphor system and OptiQuant image analysis software (Packard).
Heterocyst isolation.
Heterocysts from wild-type or CSAV141 mutant strains were purified from induced filaments essentially as described previously (9), with minor modifications. Cultures were grown in ammonium-containing medium until they reached the exponential phase (3 to 5 μg Chl·ml−1). Cells were then washed with and resuspended in nitrogen-free medium, and incubated for approximately 48 h under growth conditions before the heterocyst-containing filaments were collected by centrifugation at 4°C. The cells were then suspended in buffer containing 50 mM imidazol, 0.5 mM EDTA, and 1 mM dithiothreitol; broken by two passages through a French press at 3,000 lb/in2; and centrifuged at 200× g for 10 min. The pellet, consisting mainly of heterocysts, was washed three to five times with the same buffer. The supernatant was centrifuged to collect the vegetative cells.
Glycogen determination.
The glycogen content of whole filaments, isolated heterocysts, or vegetative cells from filaments incubated for 48 h without combined nitrogen was determined as previously described (8). Filaments corresponding to about 5 to 8 μg of Chl, isolated heterocysts, or vegetative cells were suspended in 200 μl of a 2.5% (vol/vol) sulfuric acid solution and incubated in boiling water for 40 min. Glucose in the hydrolysate was then quantified by a colorimetric assay using the o-toluidine reagent. The protein concentration was determined with the DC protein assay (Bio-Rad).
Thin-layer chromatographic determination of lipids.
Lipids were extracted from whole filaments or isolated heterocysts with a 2:1 (vol/vol) mixture of chloroform and methanol and chromatographed on thin layers of Silica Gel as described by Nichols and Wood (21), except that extracts were concentrated under N2, and heterocyst glycolipids were identified as described previously (33).
Nitrogenase activity.
After nitrogen step-down as described above, filaments from wild-type strain PCC 7120 and its derivatives CSAV135, CSAV140, and CSAV141 were incubated in BG110 medium under culture conditions for 18 h. After this period of time, the nitrogenase activity was determined (with samples of 2 ml containing 10 μg of Chl·ml−1) by the acetylene reduction assay under illumination in an atmosphere of 14% acetylene in air or under micro-oxic conditions. For achieving micro-oxic conditions, the 2-ml cell suspension in the vacuum-sealed flask was supplemented with 10 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) to inhibit oxygenic photosynthesis and bubbled with argon for 3 min. All cell suspensions (under air or under Ar-DCMU) were incubated for 1 h at 30°C under illumination before the addition of acetylene. Activity was calculated from linear rates of ethylene production.
Electron microscopy.
Filaments of the wild type and the three cox mutants were cultivated in fourfold-diluted A&A (A&A/4) medium (1) containing 5 mM KNO3 and bubbled with air enriched with 2% CO2. Nitrogen step-down was performed by washing exponentially growing filaments (3 to 5 μg of Chl·ml−1) with A&A/4 medium three times at room temperature. Cells were resuspended in A&A/4 medium and further incubated under culture conditions for 24 and 40 h. Cells were then harvested and fixed with 2.5% glutaraldehyde and 2% KMnO4 as described previously (3). After dehydration with increasing ethanol concentrations, the samples were incubated in a 1:1 mixture of Epon and propylene oxide at 37°C, followed by embedding in Epon for 24 h at 37°C and for 48 h at 60°C. Thin sections of 70 to 90 nm were transferred to copper grids and stained with uranyl acetate for 15 min and with lead citrate for 5 min. Samples were examined with a Zeiss EM109 electron microscope at 80 kV (7).
RESULTS
Heterocyst morphology in Anabaena cox mutants.
Electron microscopy of filaments of Anabaena strains with inactivated cox genes was conducted at various times after nitrogen step-down, with the aim of a comparison of morphological features of heterocyst differentiation in these mutants to those in the wild-type strain. Whereas no significant differences could be detected with any of the single mutants, namely, strains CSAV140 (coxB2::C.S3) and CSAV135 (coxA3::C.S3), the double mutant strain CSAV141 (coxB2::C.K3 coxA3::C.S3) was impaired to bring out some of the changes that in the wild type characterize the morphological differentiation of the heterocysts.
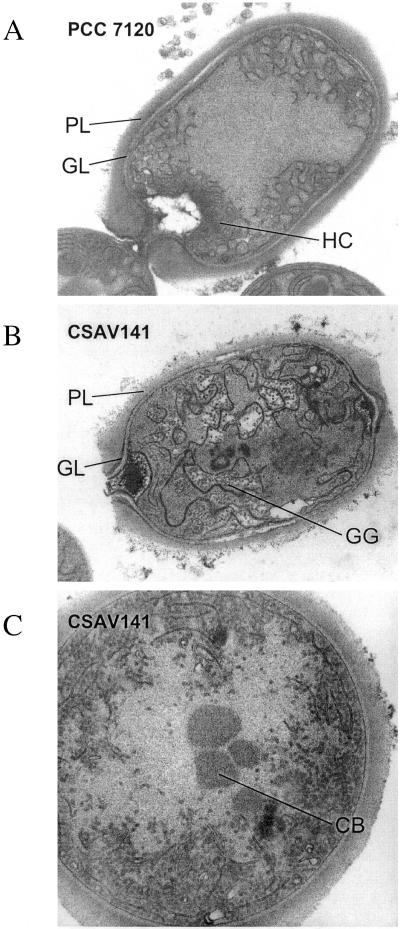
Besides the two membrane systems present in vegetative cells (the thylakoid system, containing photosynthetic and respiratory complexes, and the cytoplasmic membrane, exhibiting respiratory activity), heterocysts have a third membrane system devoted mainly to respiration. This consists of the spirally structured membranes situated towards the cell poles that have been called “honeycomb” membranes and that, being the sites where oxidation of diaminobenzidine takes place, are thought to include hemoprotein oxidases (19, 34). Whereas “honeycomb” membranes were clearly seen in heterocysts of strain PCC 7120 at 40 h after the onset of combined nitrogen deprivation, their formation could not be consistently detected in heterocysts of strain CSAV141 (Fig. 1), although some membrane reorganization and accumulation in the cell poles could occasionally be observed. Additionally, electron-dense deposits, likely corresponding to glycogen granules, accumulated in both heterocysts (Fig. 1) and vegetative cells (not shown) of strain CSAV141 at conspicuously higher levels than in the wild-type strain. No significant accumulation of these granules could be observed when strain CSAV141 was grown with ammonium as a nitrogen source (not shown). Occasionally carboxysomes (Fig. 1C) or material from degraded carboxysomes (dense nonpolar material) (Fig. 1B) could be observed in heterocysts of strain CSAV141. In the wild-type heterocysts, carboxysomes were completely degraded after 24 h of combined nitrogen deprivation (not shown). On the other hand, no significant impairment was observed in strain CSAV141 with regard to the formation of the glycolipid and polysaccharide layers of the heterocyst envelope (Fig. 1).
FIG. 1.
Electron micrographs of heterocysts of strains PCC 7120 and CSAV141 (cox2 cox3). Nitrate-grown filaments incubated for 40 h under culture conditions in the absence of combined nitrogen were used for microscopy. HC, “honeycomb” membranes; GG, glycogen granules; GL, glycolipid layer; PL, polysaccharide layer; CB, carboxysomes. Original magnifications, ×12,000 (A and B) and ×30,000 (C).
Biochemical determination of glycolipids and glycogen in strains PCC 7120 and CSAV141.
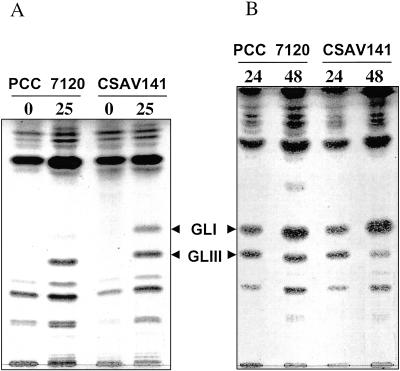
Glycolipids specific to the heterocysts, which constitute the laminated layer of the heterocyst envelope, can be readily identified by thin-layer chromatographic separation of lipids extracted from whole filaments or isolated heterocysts (33). In an analysis of lipids from both whole filaments grown with ammonium and incubated for 25 h in combined nitrogen-free medium and heterocysts isolated from them, heterocyst-specific glycolipids were observed in extracts from the wild-type and CSV141 strains (Fig. 2). This result is consistent with the normal appearance of the laminated layer of the heterocyst envelope in strain CSAV141 (see above).
FIG. 2.
Thin-layer chromatographic separation of lipids extracted from whole filaments (A) or isolated heterocysts (B) of strains PCC 7120 and CSAV141. Cells grown with ammonium (0) or grown with ammonium and incubated in the absence of combined nitrogen for the indicated number of hours, or heterocysts isolated from filaments incubated in the absence of combined nitrogen for the indicated number of hours, were used as described under Materials and Methods. GLI and GLIII, heterocyst envelope glycolipids I and III (10), respectively.
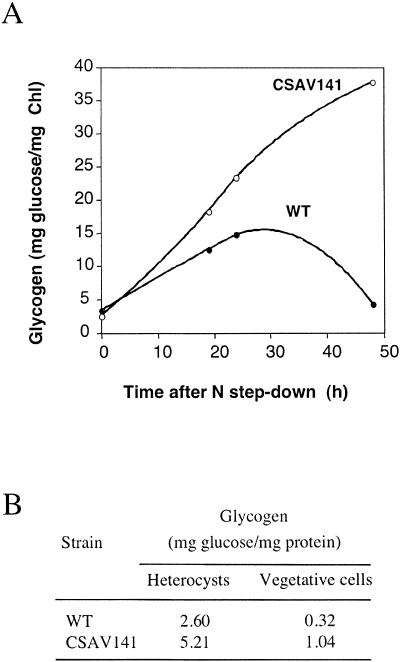
Glycogen content was determined during a time course of heterocyst development and in mature heterocysts. In the wild-type strain, the glycogen content in whole filaments increased during the first 24 h of combined nitrogen deprivation and then decreased to levels similar to those found in the ammonium-grown filaments (Fig. 3A). In contrast, the glycogen content in the CSAV141 mutant increased continuously, so that by 48 h after the onset of combined nitrogen deprivation, levels in the mutant were more than twice the maximum levels reached in the wild type (Fig. 3A). When the glycogen content was analyzed separately in vegetative cells and heterocysts from filaments incubated for 48 h in the absence of combined nitrogen, higher values were found in both types of cells from the CSAV141 mutant than in those from the wild type (Fig. 3B). These results are consistent with the conspicuous accumulation of glycogen-like granules detected by electron microscopy in the CSAV141 mutant (see above).
FIG. 3.
Glycogen content of whole filaments (A) or isolated vegetative cells and heterocysts (B) of strains PCC 7120 (wild type [WT]) and CSAV141. Filaments were grown with ammonium and incubated in the absence of combined nitrogen. For panel A, aliquots from each culture were withdrawn at the indicated times for glycogen determination. For panel B, at 48 h vegetative cells and heterocysts were separated as described in Materials and Methods and used for glycogen determination. For each panel, results for representative experiments are presented, from three each that were performed with similar results.
Expression of heterocyst-specific genes and nitrogenase activity in the cox mutants.
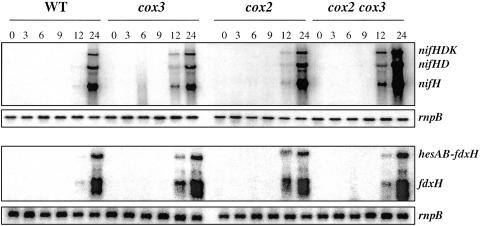
Expression of the nifHDK operon (encoding the nitrogenase enzyme complex) and of fdxH (encoding the heterocyst-specific ferredoxin, which likely represents a physiological electron donor to nitrogenase [24]) was analyzed upon nitrogen step-down in the cox mutants in comparison to the wild-type strain. Normal expression was observed with the nifH and fdxH probes in the three mutants analyzed. The nifH probe marked three transcripts, corresponding to nifHDK, nifHD, and nifH, respectively. The fdxH probe marked the hesAB-fdxH and fdxH transcripts (4, 5). Both the transcript amount and time course of transcript accumulation in the mutants were similar to those found in the wild type (Fig. 4).
FIG. 4.
Northern blot analysis of the expression of the nifHDK and hesAB-fdxH genes in cox mutant strains. RNA was isolated from cultures of the indicated strains grown with ammonium (0) or grown with ammonium, washed, and incubated in the absence of combined nitrogen for the indicated number of hours. Samples contained 25 to 30 μg of RNA, and hybridizations were carried out with a probe for the nifH or fdxH gene or for the rnpB gene (32), which was used as a loading and transfer control. WT, wild type.
Nitrogenase activity was determined in cox mutants in comparison to the wild-type strain in filaments that had been incubated for 18 h in the absence of combined nitrogen after growth with ammonium. Assays were carried out in the light under both oxic and micro-oxic conditions, by incubating the cells under air or under argon in the presence of DCMU to inhibit PSII-dependent O2 production (see Materials and Methods for details), respectively. The wild-type strain and strain CSAV140 exhibited higher activity levels under micro-oxic than under oxic conditions. Strain CSAV140 showed normal activity under air and activity similar to that in the wild type when assayed with DCMU under argon. Strains CSAV135 and CSAV141 exhibited substantial nitrogenase levels, although lower than those in the wild type, under micro-oxic conditions but exhibited considerably lower (in CSAV135) or undetectable (in CSAV141) levels under air (Table 1).
TABLE 1.
Nitrogenase activities of strain PCC 7120 and cox mutant strainsa
| Strain | Nitrogenase activity (%)b under:
|
|
|---|---|---|
| Air | Argon-DCMU | |
| PCC 7120 (wild type) | 100 | 160 |
| CSAV135 (coxA3::C.S3) | 37 | 108 |
| CSAV140 (coxB2::C.S3) | 103 | 145 |
| CSAV141 (coxB2::C.K3 coxA3::C.S3) | 0 | 75 |
Ammonium-grown filaments were incubated in the absence of combined nitrogen under culture conditions for 18 h and then used for nitrogenase activity determination under oxic and micro-oxic conditions as indicated in Materials and Methods.
Values are means of the data from three independent experiments with similar results and are percentages of the value for the wild-type strain under air, which corresponds to 11.9 nmol ethylene·μg Chl−1·h−1.
DISCUSSION
The heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120 contains three heme-copper terminal respiratory oxidases, two of which, Cox2 and Cox3, are dedicated diazotrophic oxidases (31). Thus, a cox2 cox3 double mutant does not grow diazotrophically and does not develop nitrogenase activity under oxic conditions. Here we wanted to further study the role of these oxidases in heterocyst development and protection of the N2 fixation machinery against oxygen in these differentiated cells.
The fact that in Anabaena sp. strain PCC 7120 nitrogenase levels are even higher under argon in the presence of DCMU than under air suggests that this organism can generate substantial amounts of reducing equivalents and ATP for the nitrogenase-catalyzed reduction of N2 under micro-oxic conditions, at least within the experimental setting here used. Under these conditions, nitrogenase activity can be supported by photosystem I (PSI)-dependent generation of ATP and PSI-reduced ferredoxin (34). Accordingly, the three cox mutants, bearing inactive cox2 and/or cox3 gene clusters, exhibit substantial nitrogenase activity in the presence of DCMU under argon. The increased nitrogenase activity under micro-oxic conditions in the wild type could reflect some negative effect of the low levels of oxygen that still may be present in heterocysts under oxic conditions and/or could reflect more efficient operation of PSI under micro-oxic conditions. The latter might result from competition for electrons between the photosynthetic and respiratory chains under oxic conditions. Under these conditions, the price of diverting electrons from the more efficient system of ATP (and reducing equivalent) generation would be paid to maintain adequate low oxygen levels inside the heterocyst.
The fact that nitrogenase levels in strain CSAV135 (but not CSAV140) are much lower than those in the wild type under air, with the differences between the two strains being smaller under argon-DCMU, indicates a principal role of Cox3 in nitrogenase protection from oxygen in the heterocyst. Some contribution also of Cox2 to this protection can be inferred from the observation that under air, nitrogenase levels are still appreciable in the single cox3 mutant but are undetectable in the double cox2 cox3 mutant (Table 1; see also reference 31). It seems that although in the presence of Cox3 the activity of Cox2 would be dispensable, Cox2 can provide some protection in the absence of Cox3. This different contribution to protection against oxygen may be related to the different natures of these two oxidases: Cox2 is a cytochrome c oxidase, whereas Cox3 seems to be a quinol oxidase, which might have higher affinity for oxygen than the cytochrome oxidase. Also, it might reflect different amounts of the two oxidases in the heterocyst. Whether the large difference in nitrogenase activity between strains CSAV135 and PCC 7120 under air in the light also reflects a negative effect of the lack of Cox3 on ATP production in the heterocysts is unknown.
We asked whether inactivation of the cox2 and/or cox3 genes has an effect on heterocyst development and nif gene expression in Anabaena. Strain CSAV141 is impaired in the formation of the heterocyst “honeycomb” membranes (see Fig. 1), indicating that the presence of the Cox2 and Cox3 oxidases has a role in the structural differentiation of these membranes. This could also contribute to explain the lower nitrogenase activity of cox mutants in comparison to the wild type under micro-oxic conditions.
The results presented in this work indicate that the nifHDK and hesAB-fdxH operons are induced normally in the cox mutants under oxic conditions (Fig. 4). This suggests that there is not a requirement for Cox2 and Cox3 activities for further gene expression during the process of heterocyst differentiation. On one hand, these results indicate that the impairment in nitrogenase activity exhibited by the mutants does not result from secondary effects on expression of these genes provoked by the abnormally high levels of O2 likely present in their heterocysts. On the other hand, these results contrast with the situation in many well-studied diazotrophs in which expression of nif genes responds to the oxygen concentration (17, 29). The development of the cyanobacterial heterocyst to shelter the nitrogen fixation machinery under oxic conditions could have eliminated the need for direct control of nif gene expression by oxygen in these organisms.
Strain CSAV141, which does not express nitrogenase activity under oxic conditions, exhibits an abnormally high abundance of glycogen in both vegetative cells and heterocysts specifically when incubated in the absence of combined nitrogen (Fig. 1 and 3). This accumulation can be due to increased synthesis, decreased degradation, or both. Although a lack of heterocyst-specific Cox2 and Cox3 activities would not affect respiration in vegetative cells (which anyway is negligible in the light [20]) and hence respiration-driven catabolism of internal carbohydrates, a lack of N2 reduction to ammonium in the double mutant would reduce operation of the glutamine synthetase/glutamate synthetase pathway, which represents the principal manner of consumption of 2-oxoglutarate, a main product of C assimilation in cyanobacteria. Thus, carbon compounds accumulated in the cox double mutant under nitrogen deprivation in the light could favor glycogen synthesis in this strain. This rationale is consistent with the observed time course of glycogen accumulation in whole filaments of the wild-type strain (Fig. 3A), which increases at the onset of combined nitrogen deprivation until 24 to 30 h, when active nitrogenase is established in the heterocysts, and decreases thereafter. In addition, in the absence of combined nitrogen, mutant CSAV141 will have an anomalously high C-to-N balance, a parameter determinant for expression of N-regulated genes in cyanobacteria (11). The possibility that this high C-to-N balance has a regulatory influence on glycogen synthesis or degradation in vivo, thus linking carbon metabolism to N control, could be considered and represents a subject worthy of future research.
Acknowledgments
We thank Enrique Martínez-Force for help with lipid analysis and Angelika Kühn for help with electron microscopy.
This work was supported by grant BFU2004-00872 from the Ministerio de Educación y Ciencia, Spain, and Programa de Acciones Integradas from the Ministerio de Educación y Ciencia (Spain) (HA2003-0159)/PPP-Spanien from DAAD, Germany.
Footnotes
Published ahead of print on 6 April 2007.
REFERENCES
- 1.Allen, M. B., and D. I. Arnon. 1955. Studies on nitrogen-fixing blue-green algae. I. Growth and nitrogen fixation by Anabaena cylindrica Lemm. Plant Physiol. 30:366-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2006. Current protocols in molecular biology. Greene Publishing/Wiley-Interscience, New York, NY.
- 3.Black, K., W. J. Buikema, and R. Haselkorn. 1995. The hglK gene is required for localization of heterocyst-specific glycolipids in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 177:6440-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böhme, H., and R. Haselkorn. 1988. Molecular cloning and nucleotide sequence analysis of the gene coding for heterocyst ferredoxin from the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Gen. Genet. 214:278-285. [DOI] [PubMed] [Google Scholar]
- 5.Borthakur, D., M. Basche, W. J. Buikema, P. B. Borthakur, and R. Haselkorn. 1990. Expression, nucleotide sequence and mutational analysis of two open reading frames in the nif gene region of Anabaena sp. strain PCC 7120. Mol. Gen. Genet. 221:227-234. [DOI] [PubMed] [Google Scholar]
- 6.Falkowski, P. G. 1997. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 387:272-274. [Google Scholar]
- 7.Fiedler, G., M. Arnold, S. Hannus, and I. Maldener. 1998. The DevBCA exporter is essential for envelope formation in heterocysts of the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 27:1193-1202. [DOI] [PubMed] [Google Scholar]
- 8.Forchhammer, K., and N. Tandeau de Marsac. 1995. Functional analysis of the phosphoprotein PII (glnB gene product) in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 177:2033-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Häger, K.-P., G. Danneberg, and H. Bothe. 1983. The glutamate synthase in heterocysts of Nostoc muscorum. FEMS Microbiol. Lett. 17:179-183. [Google Scholar]
- 10.Haury, J. F., and C. P. Wolk. 1978. Classes of Anabaena variabilis mutants with oxygen-sensitive nitrogenase activity. J. Bacteriol. 136:688-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero, A., A. M. Muro-Pastor, and E. Flores. 2001. Nitrogen control in cyanobacteria. J. Bacteriol. 183:411-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrero, A., A. M. Muro-Pastor, A. Valladares, and E. Flores. 2004. Cellular differentiation and the NtcA transcription factor in filamentous cyanobacteria. FEMS Microbiol. Rev. 28:469-487. [DOI] [PubMed] [Google Scholar]
- 13.Howitt, C. A., and W. F. Vermaas. 1998. Quinol and cytochrome oxidases in the cyanobacterium Synechocystis sp. PCC 6803. Biochemistry 37:17944-17951. [DOI] [PubMed] [Google Scholar]
- 14.Jones, K. M., and R. Haselkorn. 2002. Newly identified cytochrome c oxidase operon in the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120 specifically induced in heterocysts. J. Bacteriol. 184:2491-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko, T., Y. Nakamura, C. P. Wolk, T. Kuritz, S. Sasamoto, A. Watanabe, M. Iriguchi, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, M. Kohara, M. Matsumoto, A. Matsuno, A. Muraki, N. Nakazaki, S. Shimpo, M. Sugimoto, M. Takazawa, M. Yamada, M. Yasuda, and S. Tabata. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:205-213. [DOI] [PubMed] [Google Scholar]
- 16.Mackinney, G. 1941. Absorption of light by chlorophyll solutions. J. Biol. Chem. 140:315-322. [Google Scholar]
- 17.Martínez-Argudo, I., R. Little, N. Shearer, P. Johnson, and R. Dixon. 2005. Nitrogen fixation: key genetic regulatory mechanisms. Biochem. Soc. Trans. 33:152-156. [DOI] [PubMed] [Google Scholar]
- 18.Muro-Pastor, A. M., A. Valladares, E. Flores, and A. Herrero. 2002. Mutual dependence of the expression of the cell differentiation regulatory protein HetR and the global nitrogen regulator NtcA during heterocyst development. Mol. Microbiol. 44:1377-1385. [DOI] [PubMed] [Google Scholar]
- 19.Murry, M. A., A. G Olafsen, and J. R. Benemann. 1981. Oxidation of diaminobenzidine in the heterocysts of Anabaena cylindrica. Curr. Microbiol. 6:201-206. [Google Scholar]
- 20.Murry, M. A., and C. P. Wolk. 1989. Evidence that the barrier to the penetration of oxygen into heterocysts depends upon two layers of the cell envelope. Arch. Microbiol. 151:469-474. [Google Scholar]
- 21.Nichols, B. W., and B. J. B. Wood. 1968. New glycolipid specific to nitrogen-fixing blue-green algae. Nature 217:767-768. [Google Scholar]
- 22.Pils, D., and G. Schmetterer. 2001. Characterization of three bioenergetically active respiratory terminal oxidases in the cyanobacterium Synechocystis sp. strain PCC 6803. FEMS Microbiol. Lett. 203:217-222. [DOI] [PubMed] [Google Scholar]
- 23.Pils, D., C. Wilken, A. Valladares, E. Flores, and G. Schmetterer. 2004. Respiratory terminal oxidases in the facultative chemoheterotrophic and dinitrogen fixing cyanobacterium Anabaena variabilis strain ATCC 29413: characterization of the cox2 locus. Biochim. Biophys. Acta 1659:32-45. [DOI] [PubMed] [Google Scholar]
- 24.Razquin, P., S. Schmitz, M. L. Peleato, M. F. Fillat, C. Gómez-Moreno, and H. Böhme. 1995. Differential activities of heterocyst ferredoxin, vegetative cell ferredoxin, and flavodoxin as electron carriers in nitrogen fixation and photosynthesis in Anabaena sp. Photosynthesis Res. 43:35-40. [DOI] [PubMed] [Google Scholar]
- 25.Rippka, R., J. Deruelles, J. B. Waterbury, M. Herdman, and R. Y. Stanier. 1979. Generic assignments, strain stories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1-61. [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 27.Schmetterer, G., D. Alge, and W. Gregor. 1994. Deletion of cytochrome c oxidase genes from the cyanobacterium Synechocystis sp. PCC 6803: evidence for alternative respiratory pathways. Photosynthesis Res. 42:43-50. [DOI] [PubMed] [Google Scholar]
- 28.Schmetterer, G., A. Valladares, D. Pils, S. Steinbach, M. Pacher, A. M. Muro-Pastor, E. Flores, and A. Herrero. 2001. The coxBAC operon encodes a cytochrome c oxidase required for heterotrophic growth in the cyanobacterium Anabaena variabilis strain ATCC 29413. J. Bacteriol. 183:6429-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sciotti, M. A., A. Chanfon, H. Hennecke, and H. M. Fischer. 2003. Disparate oxygen responsiveness of two regulatory cascades that control expression of symbiotic genes in Bradyrhizobium japonicum. J. Bacteriol. 185:5639-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ting, C., G. Rocap, J. King, and S. W. Chisholm. 2002. Cyanobacterial photosynthesis in the oceans: the origins and significance of divergent light-harvesting strategies. Trends Microbiol. 10:134-142. [DOI] [PubMed] [Google Scholar]
- 31.Valladares, A., A. Herrero, D. Pils, G. Schmetterer, and E. Flores. 2003. Cytochrome c oxidase genes required for nitrogenase activity and diazotrophic growth in Anabaena sp. PCC 7120. Mol. Microbiol. 47:1239-1249. [DOI] [PubMed] [Google Scholar]
- 32.Vioque, A. 1997. The RNase P RNA from cyanobacteria: short tandemly repeated repetitive (STRR) sequences are present within the RNase P RNA gene in heterocyst-forming cyanobacteria. Nucleic Acids Res. 25:3471-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkenbach, F., C. P. Wolk, and M. Jost. 1972. Lipids of membranes and of the cell envelope in heterocysts of a blue-green alga. Planta 107:69-80. [DOI] [PubMed] [Google Scholar]
- 34.Wolk, C. P., A. Ernst, and J. Elhai. 1994. Heterocyst metabolism and development. p. 769-823. In D. A. Bryant (ed.), The Molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.