Abstract
Surface proteins of Staphylococcus aureus fulfill many important roles during the pathogenesis of human infections and are anchored to the cell wall envelope by sortases. Although the chemical linkage of proteins to cell wall cross bridges is known, the mechanisms whereby polypeptides are distributed on the staphylococcal surface have not been revealed. We show here that protein A, the ligand of immunoglobulin, is unevenly distributed over the staphylococcal surface. Upon removal with trypsin, newly synthesized polypeptide is deposited at two to four discrete foci. During subsequent growth, protein A appears to be slowly distributed from these sites. When viewed through multiple focal planes by laser scanning microscopy, protein A foci are arranged in a circle surrounding the bacterial cell. This pattern of distribution requires the LPXTG sorting signal of protein A as well as sortase A, the transpeptidase that anchors polypeptides to cell wall cross bridges. A model is presented whereby protein A deposition at discrete sites coupled with cell wall synthesis enables distribution of protein A on the staphylococcal surface.
The envelope of gram-positive bacteria is comprised of murein sacculi (44). These rigid exoskeletal organelles with a diameter of 50 to 100 nm prevent osmotic lysis of bacteria in host tissues and function as a scaffold for the immobilization of protein, carbohydrate, and teichoic acid (12, 34). Murein is synthesized from precursor molecules in the bacterial cytoplasm, tethered to lipid carrier, and translocated across the plasma membrane (53). Penicillin binding proteins polymerize peptidoglycan precursors into linear glycan strands that are cross-linked with neighboring strands, thereby generating a single large murein macromolecule (23, 46, 53). Staphylococcus aureus peptidoglycan is composed of N-acetylmuramoyl-(l-Ala-d-iGln-l-Lys(Gly5)-d-Ala)-(β1-4)-N-acetylglucosamine (7, 13, 14, 32). Polymerization of repeating disaccharide units, N-acetylmuramoyl-(β1-4)-N-acetylglucosamine (MurNAc-GlcNAc), generates the glycan strands (3, 8). Amide bond formation between pentaglycine cross bridges (Gly5) and d-Ala of neighboring wall peptides provides for the formation of the three-dimensional murein network (54, 55).
Staphylococcal cell wall carbohydrate and teichoic acid are tethered to glycan strands (15, 31, 45), whereas covalently bound surface proteins such as protein A are linked to pentaglycine cross bridges (48, 56). The N-terminal portion of protein A with its five immunoglobulin (Ig) binding domains is located on the staphylococcal surface (17, 20, 51), while its C-terminal threonine residue is amide-linked to the murein sacculus (35). Protein A is synthesized in the cytoplasm as a precursor carrying an N-terminal signal peptide for initiation into the secretory pathway and a C-terminal sorting signal for incorporation into the envelope (49, 50). Sortase A cleaves the sorting signal between threonine and glycine of its LPXTG motif (29, 33, 58). Cleaved polypeptide is captured as a thioester-linked intermediate at the active site thiol of sortase (58). Nucleophilic attack of lipid II [C55-MurNAc-(l-Ala-d-iGln-l-Lys(Gly5)-d-Ala-d-Ala)-(β1-4)-GlcNAc] at this bond resolves the acyl enzyme (38, 43). Surface protein linked to peptidoglycan precursor is then incorporated into the cell wall envelope via transpeptidation and transglycosylation reactions (57, 60).
Fluorescence microscopy has been used to study the deposition of proteins on the surface of Streptococcus pyogenes (6, 18). Following removal of surface proteins with trypsin, the use of fluorophore-labeled antibodies and microscopy revealed the deposition of newly synthesized M proteins near the cell division sites of this chain-forming microbe (18). Upon further bacterial growth, the continuous deposition of M proteins over 2 h led to their distribution over the streptococcal surface (18). Recent studies that employed similar technologies corroborated that M protein deposition occurs near the cell division sites of S. pyogenes (4). However, protein F, another polypeptide that is also thought to be anchored to the cell wall envelope, is deposited at the old poles, opposite sites of new cell growth and division (4). Other investigators employed transmission electron microscopy of thin-sectioned streptococci and immunogold-labeling techniques, thereby revealing discrete accumulation sites of Sec machinery components, folding factors, and secretion substrates in transit (41, 42). Together, these studies suggest that, at least in S. pyogenes, the secretion and deposition of surface proteins may occur at discrete, perhaps even distinct, locations in the envelope. Thus, studies on the molecular nature of protein secretion or deposition into murein sacculi represent an important frontier for this research field.
Microscopic analysis of protein deposition in the envelope of gram-positive bacteria can take advantage of the rigid exoskeletal nature of murein sacculi (6). Once embedded within the three-dimensional network of murein, surface proteins are thought to be immobilized permanently (34). Thus, microscopic detection of newly synthesized and deposited surface protein should reveal the subcellular sites of protein secretion. Here, we sought to develop microscopy techniques for the study of protein deposition in the envelope of S. aureus. Previous work on staphylococci revealed enzymatic reactions for surface protein anchoring and molecular architectures of anchor structures; however, the secretion or distribution of proteins on cell surfaces has thus far not been considered. Unlike streptococci, where cell division planes lie parallel, S. aureus divides perpendicular to previous division planes (61, 63). Due to incomplete separation of cell walls under many growth conditions, staphylococci appear as grape-like clusters of cells (16), and this has hindered microscopic analysis of cell division and protein deposition on bacterial surfaces.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
S. aureus strains RN4220, Newman, and N315 were grown in tryptic soy broth (TSB) at 37°C. Erythromycin and chloramphenicol were used at 10 μg·ml−1 when necessary. The spa mutant strain SEJ2 and the srtA mutant strain SKM1 have been described previously (1, 52). Plasmids pSPA and pSPAΔLPETGE were transformed into strain SEJ2 by electroporation.
Trypsinization, fixation, and immunofluorescence microscopy.
S. aureus RN4220 was grown in TSB to mid-log phase (optical density at 660 nm [OD660] of 0.6), and 10 ml of culture was centrifuged for 5 min at 8,000 × g. The bacterial cell sediment was washed twice with 10 ml of phosphate-buffered saline (PBS; 10 mM sodium phosphate) and suspended in 5 ml of PBS containing 0.2 mg·ml−1 trypsin. Cells were incubated at 37°C for 1 h, washed twice with 10 ml of PBS, and suspended in 5 ml of TSB containing 1 mM phenylmethylsulfonyl fluoride. Cells were sonicated for 15 s, and 500-μl aliquots were removed and immediately fixed with 2.5% paraformaldehyde and 0.006% glutaraldehyde in 30 mM PBS (pH 7.4) for 20 min at room temperature. Cells were washed three times with 1 ml of PBS and suspended in 300 μl of PBS with 0.025% sodium azide. Remaining cells were incubated at 37°C, and 500-μl aliquots were removed and fixed after 5, 10, 15, 30, or 60 min. Cell suspensions (30 μl) were applied to l-polylysine-coated coverslips for 5 min, washed three times with 60 μl of PBS to remove nonadherent cells, and allowed to dry. Cells were rehydrated with 60 μl of PBS for 5 min and blocked with 3% bovine serum albumin in PBS for 45 min, followed by a 1-h incubation in the dark with Cy3-conjugated goat anti-rabbit IgG (1:1,000). Cells were then washed 15 times with 60 μl of PBS, and slides were prepared. Slides were viewed with an Olympus AX-70 fluorescence microscope, and images were captured with a charge-coupled-device (CCD) camera. Images were analyzed with ImageJ software, and data were assembled in Adobe Photoshop.
S. aureus strains RN4220, Newman, N315, RN4220, SEJ2, and SKM1 were grown in TSB to mid-log phase (OD660 of 0.6). Cells were prepared for immunofluorescence microscopy as described above. Protein A was stained with Cy3-IgG (Invitrogen) (1:1,000), fluorescein isothiocyanate (FITC)-IgG (Invitrogen) (1:25), or Alexa Fluor 647-IgG (Invitrogen) (1:50 or 1:250 dilution). Fluorophore conjugates to Ig preparations were goat anti-rabbit IgG.
Immunoblotting.
S. aureus strains RN4220, Newman, N315, SEJ2, and SKM1 were grown in TSB (with appropriate antibiotics as necessary) to mid-log phase (OD660 of 0.6). Two 1-ml aliquots of each strain were collected, centrifuged for 3 min at 8,000 × g, and washed with 1 ml of PBS or 1 ml of TSM (50 mM Tris-HCl [pH 7.5], 0.5 M sucrose, 10 mM MgCl2) and suspended in 1 ml of the same buffer containing 0.1 mg·ml−1 of lysostaphin. Following incubation at 37°C for 20 min, samples were centrifuged for 3 min at 8,000 ×g, and supernatants were removed (cell wall fractions). Both the lysate (total cell fraction) and supernatants (cell wall) were precipitated with 7.5% trichloroacetic acid. Protein sediment was suspended in 50 μl of loading buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblot analysis with monoclonal antibody SPA-27 (Sigma) or polyclonal antisera raised against either SrtA or L6 (cytoplasmic protein).
Confocal fluorescence microscopy.
S. aureus strain RN4220, SEJ2 (spa), SKM1 (srtA), SEJ2 (pSPA), or SEJ2 (pSPAΔLPETGE) was grown in TSB (with appropriate antibiotics as necessary) to mid-log phase (OD660 of 0.6), whereas S. aureus Newman cells were grown to late log phase (OD660 of 1.0). Cells were fixed and prepared for confocal microscopy as described above. Protein A was labeled with Alexa Fluor 647-IgG (1:250), and the cell wall was stained with 1 μg·ml−1 vancomycin-BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) conjugate (Invitrogen). Cells were viewed with a Leica SP5 AOBS spectral 2-photon confocal microscope. More specifically, cells were viewed with a Leica DMI6000 inverted microscope with conventional fluorescence (100-W Hg lamp) and differential interference contrast (DIC) optics under a 63× oil objective (numerical aperture, 1.4) with automatically optimized confocal pinhole apertures and images captured by three chilled photomultiplier tube fluorescence detectors (digital spectral definition in 1-nm increments) plus one transmitted light detector with 12-bit output and 15× digital zoom (effective pixel size, 30 nm). Fluorescence Z-scans were captured sequentially with a 488-nm line argon laser followed by a 633-nm line (10 mW) HeNe laser. Resonant scanning galvanometer mirrors (scan rate, 8,000 Hz) were used to collect frame-averaged (n = 64) z-series scans sampled in 50- to 60-nm steps. Captured images were analyzed with ImageJ software, and three-dimensional distribution was reconstructed.
RESULTS
Deposition of newly synthesized protein A on the staphylococcal surface.
Initial experiments established conditions to generate suspensions of single, nonclustered staphylococcal cells. Prior to microscopy, staphylococci were sedimented by centrifugation, washed in PBS, and dispersed by sonication. This protocol did not affect bacterial growth and viability. Following incubation with Cy3-IgG, staphylococcal display of surface protein was detected via binding of protein A to the Fc termini of Cy3-IgG in a fluorescence microscopy experiment. Fluorescence and merged fluorescence-DIC images for wild-type S. aureus detected the display of protein A on the bacterial surface (Fig. 1A).
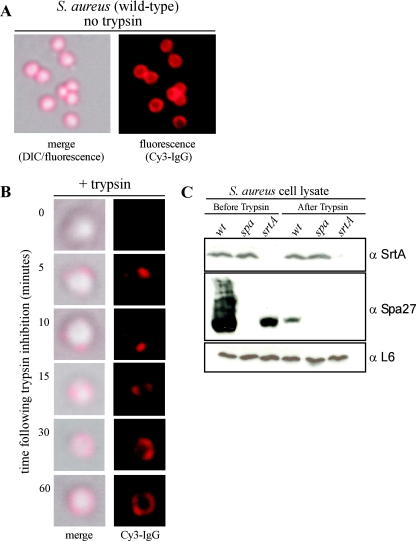
FIG. 1.
Deposition of newly synthesized protein A on the staphylococcal surface. (A) Protein A on the surface of S. aureus RN4220 cells was stained with Cy3-IgG, and DIC or fluorescence images were captured with an Olympus AX-70 fluorescence microscope and Himamatsu CCD camera. (B) Staphylococci were treated with trypsin to remove surface proteins, and the deposition of newly synthesized protein A on the cell surface at indicated times was visualized by staining with Cy3-IgG. DIC and fluorescence images were captured with a CCD camera. (C) Staphylococcal lysates before and after trypsin treatment were precipitated with trichloroacetic acid, washed in acetone, and separated on SDS-PAGE. Samples were subjected to immunoblotting with antibodies against sortase A (α-SrtA), protein A (α-Spa27), or ribosomal protein L6 (α-L6).
To reveal surface deposition of newly synthesized protein A, staphylococci were incubated with 200 μg·ml−1 trypsin for 60 min. Cells were washed in PBS and suspended in tryptic soy broth with protease inhibitor to quench all further proteolysis. At timed intervals, staphylococci were fixed with glutaraldehyde, and deposition of protein A was revealed by fluorescence microscopy (Fig. 1B). Cells that were fixed immediately following treatment with trypsin (0 min) did not reveal Cy3-IgG staining, indicating that the protease had removed all protein A from the staphylococcal surface (Fig. 1B). Within 5 min of incubation, as revealed by microscopy images, cells contained two to four discrete surface spots of Cy3-IgG staining. Upon incubation for 10 to 20 min, these areas of Cy3-IgG surface staining increased in size, and after 40 to 60 min, fluorescent signals covered most of the staphylococcal surface (Fig. 1B).
Trypsin removal of protein A from the staphylococcal surface was also examined by immunoblotting with monoclonal antibody anti-Spa27 (Fig. 1C). Prior to trypsin treatment, a spectrum of protein A molecules linked to different sizes of peptidoglycan fragments was detected by immunoblotting. A sortase A mutant strain (srtA) accumulated only small amounts of P2 precursor species, i.e., protein A without signal peptide but with an uncleaved C-terminal sorting signal (28). As expected, a protein A (spa) mutant strain did not generate monoclonal antibody Spa-27-specific immune-reactive signals (Fig. 1C). Following trypsin treatment, almost all protein A immune-reactive species disappeared, consistent with the view that protease treatment had removed surface proteins. Immunoblotting with sortase-specific antibodies (anti-SrtA) revealed that the transpeptidase, which resides in staphylococcal membranes, had not been removed by treatment of staphylococci with trypsin (Fig. 1C). As a control, trypsin treatment also did not affect the abundance of ribosomal subunit L6, a polypeptide that resides in the bacterial cytoplasm. Together, these results suggest that, upon protease-mediated removal of surface proteins but not of sortase, the deposition of newly synthesized protein A is at least initially restricted to two to four discrete sites. Continuous deposition of protein A near these sites may eventually provide for the distribution of anchored polypeptide over the bacterial surface.
Distribution of protein A on staphylococcal surfaces.
We entertained a model whereby surface protein deposition into murein sacculi occurs in the immediate vicinity of a few discrete sites whose subcellular location may be altered upon expansion of the staphylococcal envelope. If so, immobilized protein A on bacterial surfaces should be highly abundant in the vicinity of its deposition sites and low or even absent where these sites are not or have not been. Simply put, it is unlikely that the molecular mechanisms sustaining such a model could achieve a uniform distribution of protein A on the bacterial surface. Thus, if uniform distribution of protein A on staphylococcal surfaces were observed, such an argument would allow us to reject the hypothesis of localized deposition.
In previous work, we used IgG labeled with FITC and Cy3 to detect protein A staining (28, 50). In captured images of FITC-IgG-labeled cells, the distribution of protein A on the bacterial surface appears generally uniform (Fig. 2A). For Cy3-IgG-labeled bacteria, we observed both uniform as well as uneven distributions of protein A (Fig. 2A). Prompted by these observations as well as by wavelength-dependent differences in optical parameters such as signal-to-noise ratio and resolution, we used a third fluorophore for protein A labeling experiments. FITC (λ518) emits green light, whereas Cy3 (λ570) emits light with an orange spectrum. Compared to these fluorophores, Alexa Fluor 647 (λ668) displays a much higher signal-to-noise ratio and fluorescence emission with far-red spectral properties (25). Alexa Fluor 647-IgG labeling and fluorescence microscopy, indeed, revealed a remarkably irregular surface distribution of protein A (Fig. 2A). Thus, considering that staphylococci are small, round cells with a diameter of 0.7 to 0.9 μM, FITC-IgG or Cy3-IgG labeling, with a low signal-to-noise ratio, may mask the uneven distribution of protein A, which was observed with Alexa Fluor 647-IgG labeling.
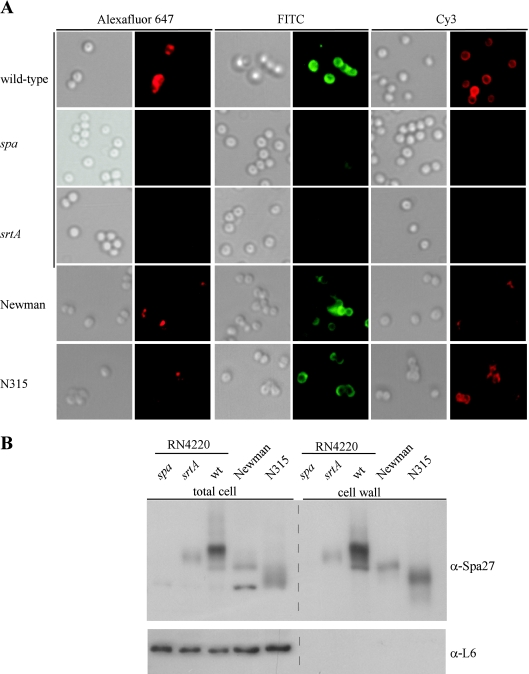
FIG. 2.
Distribution of protein A on the surface of staphylococcal strains. (A) S. aureus RN4220 (wild type) and isogenic spa and srtA mutants were incubated with Alexa Fluor 647-IgG, FITC-IgG, or Cy3-IgG, and DIC or fluorescence images were captured with a Olympus AX-70 fluorescence microscope and Himamatsu CCD camera. As a control for protein A distribution on human clinical isolates, S. aureus Newman and N315 strains were analyzed with the same technology. (B) Total cell extracts or cell wall fractions generated by degradation of murein sacculi with lysostaphin obtained from S. aureus RN4220 (wild-type [wt]) and isogenic spa and srtA mutants or from strains Newman and N315. Samples were separated by SDS-PAGE and then analyzed by immunoblotting with monoclonal antibody specific for protein A (α-Spa27) or with polyclonal antiserum raised against purified L6 ribosomal protein.
Both Alexa Fluor 647-IgG and Cy3-IgG labeling specifically detected the surface display of protein A, as staphylococcal mutants lacking the structural gene for protein A (spa) did not generate fluorescence under these experimental conditions (Fig. 2A). FITC-IgG labeling generated background fluorescence in spa mutants, indicating that most, but certainly not all, of the signal intensity in images captured with this fluorophore were generated by Ig binding to protein A (Fig. 2A).
To determine whether the distribution of protein A over the staphylococcal surface requires its anchoring to the cell wall envelope, S. aureus sortase mutants (srtA) were examined by fluorescence microscopy. Captured images revealed either no staining for protein A or small, punctuate fluorescence signals with FITC-IgG, Cy3-IgG, or Alexa Fluor 647-IgG. These data indicate that sortase-mediated anchoring of protein A to the cell wall envelope facilitates its distribution over the staphylococcal surface.
S. aureus RN4220, a laboratory strain for molecular genetic experiments, harbors mutations in the virulence gene regulators agr and sigB, which increase expression of surface proteins while reducing the abundance of exotoxins (21, 36). As the preceding experiments were conducted with strain RN4220, we sought to subject clinical strains to fluorescence microscopy and selected two human isolates, S. aureus strains Newman and N315 (9, 22), for our study. As expected, fluorescence signals generated via binding of IgG conjugates to protein A were significantly reduced for S. aureus Newman and N315 compared to S. aureus RN4220 (Fig. 2A). FITC-IgG or Cy3-IgG generated diffuse fluorescent signals emanating from the surface of S. aureus Newman or N315, whereas Alexa Fluor 647-IgG binding produced a much more irregular and punctuate staining. Thus, it appears that the uneven surface distribution of protein A is similar in both laboratory and clinical strains.
As a measure for protein A abundance and anchoring, murein sacculi of staphylococci were digested with lysostaphin, a glycyl-glycine endopeptidase that liberates cell wall-anchored protein A (47, 48). Following centrifugation and separation of sedimented protoplasts from cell wall lysate, samples of both fractions were subjected to immunoblotting with monoclonal antibody (anti-Spa27) (Fig. 2B). Lysostaphin cleavage solubilized protein A from the murein sacculi of S. aureus RN4220, Newman, and N315 strains. As expected, protein A (which differs in molecular size and SDS-PAGE mobility between staphylococcal strains) was more abundant in the cell wall fraction of S. aureus RN4220 than in the Newman or N315 strains. Immunoblotting revealed the P2 precursor in total cell extracts and envelope preparations of the srtA mutant. As expected, samples derived from the spa mutant strain did not generate immune-reactive species. As a control for proper fractionation, ribosomal L6 protein was detected in the total cell fractions but not in cell wall lysates (Fig. 2B).
Localization of protein A with laser scanning confocal microscopy.
To map deposition sites of protein A on the surface of the spherical S. aureus cell, we sought to reveal the division site within this orb and wondered whether this might be achieved by analyzing clusters of incompletely separated cells. For example, in streptococci, where spherical cells divide in parallel to previous division planes, the incomplete separation of cells and their chain-like appearance allow accurate predictions of future division planes and mapping of surface protein deposition sites (10). However, S. aureus divides perpendicular to previous division sites, and future division sites may be positioned in two different geometric planes of cell spheres, both of which lie perpendicular to the previous division plane (61, 63). However, the position of cell division sites cannot be predicted by simply viewing contours of cells in clusters of incompletely separated staphylococci.
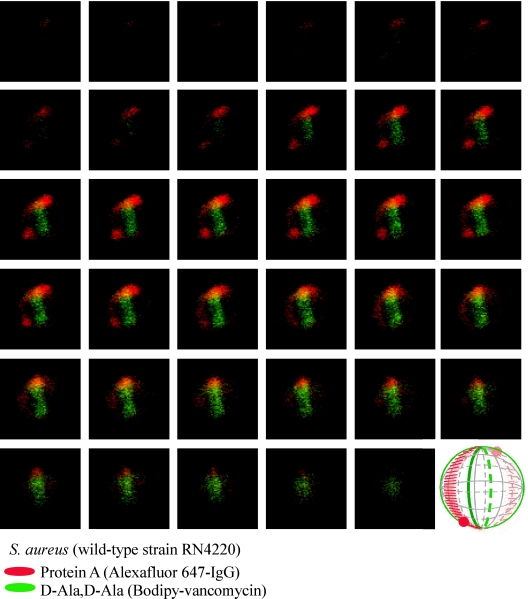
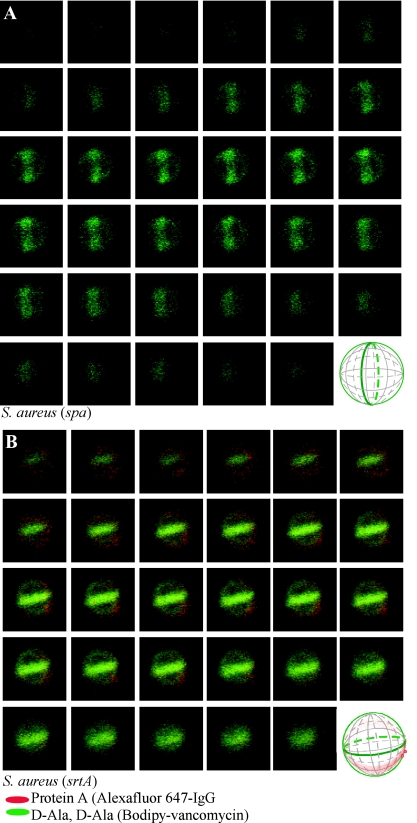
Fluorophore conjugates of vancomycin, a glycopeptide antibiotic that binds with high affinity to d-Ala-d-Ala (62), have been used to reveal the location of peptidoglycan pentapeptide precursors (lipid II) in the murein sacculus and have revealed the “cross wall,” a layer of newly synthesized cell wall that separates dividing staphylococcal daughter cells (16, 39, 40). Labeling of staphylococci with vancomycin-BODIPY conjugate and sequential laser scanning confocal microscopy in optical planes separated by 50 to 60 nm along the z axis generated series of images that were used to reconstruct a three-dimensional fluorescence pattern of the murein sacculus. In dividing cells, both the murein sacculus and cross wall can be visualized, the latter of which separates two daughter cells. We used only images of dividing cells for topological mapping of the surface distribution of protein A in S. aureus RN4220. Figure 3 shows a series of images for a representative staphylococcal cell that was labeled with vancomycin-BODIPY conjugate and Alexa Fluor 647-IgG. Reconstruction of fluorescence signals into a three-dimensional spherical orb revealed the murein sacculus and cross wall. Abundant protein A staining occurred at two discrete sites, herein named foci, that were positioned in close proximity but juxtaposed on opposite sites of the cross wall (Fig. 3). More diffuse and weaker protein A staining was observed in a ring-like structure around the murein sacculus that traversed the cross wall in the vicinity of protein A foci. Outside of the ring-like staining pattern for protein A, very little or no fluorescence was observed, suggesting that large surface areas of the murein sacculus had not been decorated with this surface protein.
FIG. 3.
Protein A localization in a dividing S. aureus RN4220 cell. Laser scanning confocal microscopy images (z series with 50-nm increments) of S. aureus RN4220. Protein A was labeled with Alexa Fluor 647-IgG (red fluorescence), and cell wall pentapeptide was stained with BODIPY-vancomycin (green fluorescence). Central regions of intense green fluorescence reveal the cross wall, the cell wall layer separating two daughter cells. Each display item is derived from merged images of confocal scans of separate laser line channels. Aggregate data were used to build a three-dimensional model of protein A deposition in the cell wall, which is shown as a diagram in the lower right corner.
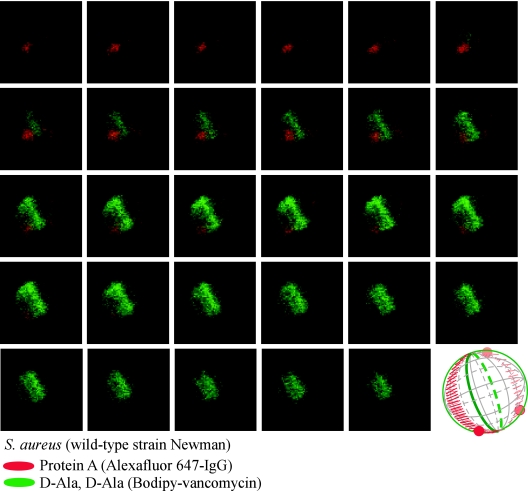
We used fluorescence microscopy to analyze 75 dividing cells that had been treated with trypsin and observed staphylococci without foci (57 cells) and with two (16 cells), three (1 cell), or four (1 cell) foci 5 min after protease quenching (data not shown). In staphylococci that had not been treated with trypsin, analysis of 77 cells labeled with Alexa Fluor 647-IgG revealed 2 cells with zero foci, 36 cells with two foci, 25 cells with three foci, and 14 cells with four foci (data not shown). Thus, most cells harbor two to four foci of protein A staining on their surfaces, and almost all cells displayed a ring-like structure of diffuse surface protein staining. These results suggest that the absolute number of foci per cell may not impact the distribution of protein A on the cell surface. To test whether a similar pattern of protein A distribution occurred in other staphylococcal strains, the human clinical isolate S. aureus Newman was subjected to vancomycin-BODIPY conjugate and Alexa Fluor 647-IgG labeling followed by sequential laser scanning confocal microscopy. We observed a similar pattern of ring-like distribution of protein A over cell surfaces marked by two to four foci of abundant surface protein staining (Fig. 4). These foci were again positioned in the immediate vicinity and on opposite sites of the cross wall separating the two daughter cells. Taken together, these data suggest that the observed ring-like distribution of protein A occurs in laboratory stains and in clinical isolates.
FIG. 4.
Protein A localization in a dividing S. aureus Newman cell. Laser scanning confocal microscopy images (z series with 50-nm increments) of S. aureus Newman. Protein A was labeled with Alexa Fluor 647-IgG (red fluorescence), and cell wall pentapeptide was stained with BODIPY-vancomycin (green fluorescence). Protein A and cell wall images of pentapeptide precursors were collected as described in the legend of Fig. 3. Aggregate data were used to build a three-dimensional model of protein A deposition in the cell wall, which is shown as a diagram in the lower right corner.
Protein A distribution in spa and srtA mutant staphylococci.
To establish an experimental system that may be perturbed by intra- and extragenic mutations, we first asked whether all Alexa Fluor 647-IgG-generated signals in laser scanning confocal microscopy experiments required the presence of the structural gene for protein A. Deletion of the structural protein A gene (24) (spa) in S. aureus RN4220 generated strain SEJ2, which failed to react with Alexa Fluor 647-IgG (Fig. 5A). BODIPY-vancomycin staining of murein sacculi was indistinguishable between wild-type parent and spa mutant strains, indicating that all Alexa Fluor 647-IgG-generated fluorescent signals must be derived from its binding to protein A (Fig. 5A).
FIG. 5.
Protein A distribution in dividing S. aureus cells requires sortase A. Laser scanning confocal microscopy images (z series with 50-nm increments) of S. aureus mutants lacking the protein A gene (spa) (A) or the sortase A gene (srtA) (B). Protein A was labeled with Alexa Fluor 647-IgG (red fluorescence), and cell wall pentapeptide was stained with BODIPY-vancomycin (green fluorescence). Protein A and cell wall images of pentapeptide precursors were collected as described in the legend of Fig. 3. Aggregate data were used to build three-dimensional models of protein A deposition in the cell wall, which are shown as diagrams in the lower right corners of the panels.
Covalent attachment of protein A to cell wall peptidoglycan occurs via a transpeptidation reaction that is catalyzed by sortase A (srtA) (28). When cells were analyzed by vancomycin-BODIPY conjugate and Alexa Fluor 647-IgG staining, fewer than 20% of the cells generated during growth of an srtA mutant strain revealed a very faint, ring-like distribution of protein A (Fig. 5B). Foci of abundant protein A staining in these cells were either absent or greatly reduced in size and signal intensity. More than 80% of staphylococci lacking the srtA gene failed to react with Alexa Fluor 647-IgG (Fig. 2).
Protein A distribution requires a functional sorting signal.
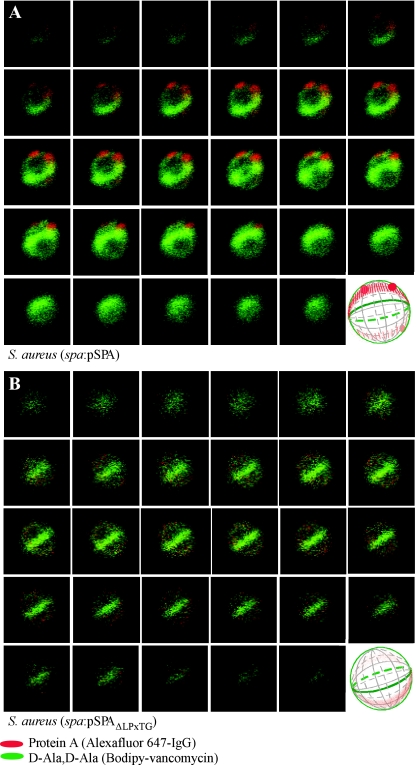
Transformation of spa mutant staphylococci with a plasmid encoding wild-type spa not only complemented the observed expression defect but also restored protein A deposition to its ring-like distribution pattern with two to four foci. Protein A anchoring to the cell wall envelope absolutely requires the presence of an LPXTG motif sorting signal at the C-terminal end of the polypeptide (49), which is cleaved by sortase and then incorporated into the amide bond that tethers surface proteins to pentaglycine cross bridges (29). We sought to determine whether the surface distribution of protein A required its anchoring to the cell wall envelope. S. aureus mutants lacking spa were transformed with a plasmid encoding the protein A variant (SPAΔLPXTG) without the LPXTG motif (50). Because SPAΔLPXTG harbors the remainder of the sorting signal including its hydrophobic domain and charged tail, this variant is retained in staphylococcal membranes but not linked to the cell wall envelope (50). Transformation of spa mutant staphylococci with pSPAΔLPXTG failed to restore protein A expression and surface deposition to wild-type levels. Instead, we observed cells with markedly reduced (0.06% of wild-type) protein A staining and without foci (Fig. 6B). When present, surface distribution of SPAΔLPXTG occurred in a ring-like manner that lacked the apparent foci for more abundant protein A staining that were observed in wild-type cells (Fig. 6B).
FIG. 6.
Protein A distribution in dividing S. aureus cells requires a functional LPXTG sorting signal. S. aureus mutants lacking the protein A gene (spa) were transformed with plasmid encoding wild-type protein A (Spa) (A) or a variant lacking the LPXTG motif (SpaΔLPXTG) (B). Laser scanning confocal microscopy images (z series with 50-nm increments) of S. aureus mutants lacking the protein A gene (spa) or the sortase A gene (srtA). Protein A and cell wall images of pentapeptide precursors were collected as described in the legend of Fig. 3. Aggregate data were used to build three-dimensional models of protein A deposition in the cell wall, which are shown as diagrams in the lower right corners of the panels.
DISCUSSION
Protein A, a virulence factor of S. aureus strains that binds to the Fc portion of Igs and to von Willebrand factor during host infection (11, 19, 30), has been used as a model system to understand surface protein anchoring to the cell wall envelope of gram-positive bacteria (26). Following translocation across the cytoplasmic membrane and signal peptide cleavage of P1 precursors, the protein A P2 intermediate is retained in the secretory pathway and cleaved by sortase A between the threonine and the glycine of its LPXTG motif sorting signal (28, 33). Subsequent amide bond formation between the C-terminal threonine and the pentaglycine cross bridge of lipid II generates the P3 intermediate, which is finally incorporated into murein sacculi via the transpeptidation and transglycosylation reactions of cell wall biosynthesis (27, 59, 60).
S. aureus, which has spherical gram-positive cells that divide perpendicular to previous cell division planes, is thought to synthesize peptidoglycan de novo at the cross wall, a newly formed peptidoglycan layer that eventually separates daughter cells (16, 39, 40). Upon separation of the cross wall at its midline, the hemispherical portions of newly synthesized murein sacculi are thought to expand and assume their final shape. Here, we asked the question, Where is protein A incorporated into the murein sacculi of S. aureus cells? Using plain and confocal scanning fluorescence microscopy, foci of protein A secretion and deposition into the cell wall envelope were observed. These foci were arranged into a ring-like structure that encircled the bacterial surface with diffuse staining connecting all foci. Compared to the circular cross wall, the protein A ring is located in its vicinity but tilted at an angle, thereby positioning protein A deposition sites and areas of de novo cell wall synthesis in the vicinity of one another. This surprising result implies that protein A is not deposited into murein sacculi in a random fashion all over the bacterial surface. Rather, protein A secretion and incorporation into murein sacculi seem to occur in a well-organized manner that orchestrates cell wall synthesis and protein targeting. Preliminary work with two other surface proteins suggests that these molecules may also be distributed and anchored to the cell wall of S. aureus as is reported here for protein A (A. C. DeDent and O. Schneewind, unpublished data). Our results should not, however, be interpreted to mean that sortase A must also be specifically localized to the foci of surface protein assembly. Currently, we do not know the distribution of sortase A, as the transpeptidase is not accessible to either protease or antibody on the bacterial surface. Green fluorescent protein fusions to the N terminus of type I transmembrane proteins such as sortase A can give rise to proteolytic cleavage and aggregation, causing the derived location of membrane proteins to inaccurately reflect their physiological distribution. Depending on the genetic background of S. aureus strains, sortase A recognizes 17 to 21 different surface protein substrates, and future work will need to accomplish a comprehensive analysis of substrate and sortase localization.
Previous work used antibodies raised against streptococcal M protein to study the deposition of proteins on the surface of gram-positive bacteria (5). This experimental system has one considerable advantage: streptococci divide in parallel to previous cell division planes (6). Thus, simple inspection of streptococcal chains reveals the future cell division sites. By using trypsin to remove M proteins and FITC-labeled antibodies to reveal new protein on the bacterial surface, previous studies observed deposition of newly synthesized M protein at cell division sites (18). During further growth, surface areas of the cell wall envelope that had been decorated with M proteins continued to expand, suggesting that the continued incorporation of polypeptide into growing murein sacculi ensures that most, or perhaps all, bacterial surfaces are decorated with protein (18). Recent work demonstrated the genetic requirement of sortase A in group A streptococci for cell wall anchoring of M protein (2), even though the chemical nature of anchoring, the cell wall anchor structure, and peptidoglycan substrates for this reaction are still unknown. Further studies revealed that protein F, a surface protein lacking the YSIR(K/G)S motif found in the M protein signal peptide, is anchored not only at cell division sites but also at the old poles of streptococcal chains (4). Other experiments suggest that surface protein deposition at this unique site is governed by the presence of specific signal peptides (4). Genome sequences of S. aureus strains encode 18 to 23 different surface proteins (37). Some, but not all, of these harbor signal peptides with a YSIR(K/G)S motif (1). It is not yet known whether these signal peptides control the fate of protein deposition on staphylococcal surfaces.
Electron microscopy and fluorescence microscopy have not yet revealed a ring-like structure or foci of M protein deposition in streptococci. We think it is highly likely that the observed zones of M protein deposition near the streptococcal cross wall form a ring around these spherical cells (4, 18). However, we cannot predict whether these technologies may also reveal foci of M protein deposition. Microscopy work on some secreted streptococcal proteins, HtrA and SpeB, suggests that these molecules may be secreted at a single defined focus, named the ExPortal (41, 42). Further, foci of protein secretion were also defined as sites of secretion machinery (SecA) localization (41, 42). The latter aspect of this model has recently been challenged, as other investigators observed a more even distribution of secretion machinery components in the plasma membrane (4).
It is certainly tempting to speculate that the observed foci of protein A deposition on staphylococcal surfaces may surround designated protein secretion sites, perhaps the equivalent of the ExPortal in S. aureus. However, current technologies cannot yet define the molecular nature of these sites. In our model, the deposition sites for protein A are defined as foci in murein sacculi, a notable distinction from the ExPortal, which defines localized secretion as a site in the cytoplasmic membrane (41). Future work will need to reveal the molecular nature of protein A deposition sites or of membrane ExPortals, thereby providing the frontier in this field with mechanistic insight on protein secretion and surface distribution.
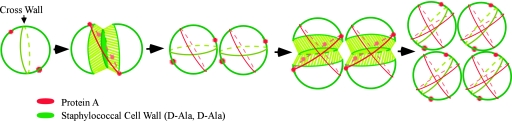
In summary, we propose that protein A first emerges on the cell surface at discrete locations, with at least two but not more than four foci that encircle spherical cells (Fig. 7). In dividing cells, protein A rings do not align with the cross wall (septum), which suggests that they are positioned in a different plane, possibly representing future division sites. As dividing daughter cells grow and expand, peptidoglycan as well as protein A incorporation at the cross wall may be increased, generating a more diffuse pattern of protein deposition. Finally, as cells set up new division planes, new protein A deposition foci may evolve in a ring positioned perpendicular to the previous plane, thereby generating broader areas of surface decoration with protein A (Fig. 7).
FIG. 7.
Diagram to illustrate a hypothesis on the deposition of protein A in the cell wall envelope of S. aureus. The cross wall, a thick layer of newly synthesized peptidoglycan, separates two newly formed daughter cells and is indicated as a green ring structure. Two to four foci of protein A staining (red dots) mark sites for the secretion and deposition of newly synthesized surface protein into murein sacculi. As cells grow and the cross wall expands, surface protein deposition forms a ring-like structure that traverses areas of cell wall synthesis. Once cell wall synthesis and separation as well as surface protein ring formation have been completed, new cross walls and foci of protein A deposition are formed perpendicular to previous planes of division and surface protein anchoring.
Acknowledgments
We thank Vytas Bindokas, Director of the University of Chicago BSD Light Microscopy Core Facility, for assistance with microscopy experiments and members of our laboratory for discussion.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases, Infectious Disease Branch (AI38897), to O.S. A.D. acknowledges support from the Molecular Cell Biology Training Grant T32GM007183 at the University of Chicago.
Footnotes
Published ahead of print on 6 April 2007.
REFERENCES
- 1.Bae, T., and O. Schneewind. 2003. The YSIRK-G/S motif of staphylococcal protein A and its role in efficiency of signal peptide processing. J. Bacteriol. 185:2910-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett, T. C., and J. R. Scott. 2002. Differential recognition of surface proteins in Streptococcus pyogenes by two sortase gene homologs. J. Bacteriol. 184:2181-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boneca, I. G., Z. H. Huang, D. A. Gage, and A. Tomasz. 2000. Characterization of Staphylococcus aureus cell wall glycan strands, evidence for a new β-N-acetylglucosaminidase activity. J. Biol. Chem. 275:9910-9918. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson, F., M. Stalhammar-Carlemalm, K. Flardh, C. Sandin, E. Carlemalm, and G. Lindahl. 2006. Signal sequence directs localized secretion of bacterial surface proteins. Nature 442:943-946. [DOI] [PubMed] [Google Scholar]
- 5.Cole, R. M. 1965. Bacterial cell wall replication followed by immunofluorescence. Bacteriol. Rev. 29:326-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, R. M., and J. J. Hahn. 1962. Cell wall replication in Streptococcus pyogenes. Science 135:722-724. [DOI] [PubMed] [Google Scholar]
- 7.de Jonge, B. L. M., Y. S. Chang, D. Gage, and A. Tomasz. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. J. Biol. Chem. 267:11248-11254. [PubMed] [Google Scholar]
- 8.Dmitriev, B. A., F. V. Toukach, O. Holst, E. T. Rietschel, and S. Ehlers. 2004. Tertiary structure of Staphylococcus aureus cell wall murein. J. Bacteriol. 186:7141-7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 10.Fischetti, V. A. 1989. Streptococcal M protein: molecular design and biological behavior. Clin. Microbiol. Rev. 2:285-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster, T. J., and M. Höök. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 12.Ghuysen, J.-M. 1968. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol. Rev. 32:425-464. [PMC free article] [PubMed] [Google Scholar]
- 13.Ghuysen, J.-M., and J. L. Strominger. 1963. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. II. Separation and structure of the disaccharides. Biochemistry 2:1119-1125. [DOI] [PubMed] [Google Scholar]
- 14.Ghuysen, J.-M., D. J. Tipper, C. H. Birge, and J. L. Strominger. 1965. Structure of the cell wall of Staphylococcus aureus strain Copenhagen. VI. The soluble glycopeptide and its sequential degradation by peptidases. Biochemistry 4:2245-2254. [DOI] [PubMed] [Google Scholar]
- 15.Ghuysen, J.-M., D. J. Tipper, and J. L. Strominger. 1965. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. IV. The teichoic acid-glycopeptide complex. Biochemistry 3:474-485. [DOI] [PubMed] [Google Scholar]
- 16.Giesbrecht, P., T. Kersten, H. Maidhof, and J. Wecke. 1998. Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin. Microbiol. Mol. Biol. Rev. 62:1371-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guss, B., M. Uhlén, B. Nilsson, M. Lindberg, J. Sjöquist, and J. Sjödahl. 1984. Region X, the-cell-wall-attachment part of staphylococcal protein A. Eur. J. Biochem. 138:413-420. [DOI] [PubMed] [Google Scholar]
- 18.Hahn, J. J., and R. M. Cole. 1963. Streptococcal M antigen location and synthesis, studied by immunofluorescence. J. Exp. Med. 118:659-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartleib, J., N. Kohler, R. Dickinson, G. Chhatwal, J. Sixma, O. Hartford, T. J. Foster, G. Peters, B. Kehrl, and M. Herrmann. 2000. Protein A is the von Willebrand factor binding protein of Staphylococcus aureus. Blood 96:2149-2156. [PubMed] [Google Scholar]
- 20.Jensen, K. 1958. A normally occurring staphylococcus antibody in human serum. Acta Pathol. Microbiol. Scand. 44:421-428. [Google Scholar]
- 21.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mitsutani-Ui, N. Kobayashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 23.Labischinski, H., and H. Maidhof. 1994. Bacterial peptidoglycan: an overview and evolving concepts, p. 23-38. In J.-M. Ghuysen and R. Hakenbeck (ed.), Bacterial cell wall, vol. 27. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 24.Löfdahl, S., B. Guss, M. Uhlén, L. Philipson, and M. Lindberg. 1983. Gene for staphylococcal protein A. Proc. Natl. Acad. Sci. USA 80:697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majlof, L., and P. Forsgren. 1993. Confocal microscopy: important considerations for accurate imaging, p. 79-94. In B. Matsumoto (ed.), Cell biological applications of confocal microscopy, vol. 38. Academic Press, San Diego, CA. [Google Scholar]
- 26.Marraffini, L. A., A. C. DeDent, and O. Schneewind. 2006. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol. Mol. Biol. Rev. 70:192-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marraffini, L. A., H. Ton-That, Y. Zong, S. V. L. Narayana, and O. Schneewind. 2004. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. IV. A conserved arginine residue is required for efficient catalysis of sortase A. J. Biol. Chem. 279:37763-37770. [DOI] [PubMed] [Google Scholar]
- 28.Mazmanian, S. K., G. Liu, E. R. Jensen, E. Lenoy, and O. Schneewind. 2000. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA 97:5510-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazmanian, S. K., G. Liu, H. Ton-That, and O. Schneewind. 1999. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285:760-763. [DOI] [PubMed] [Google Scholar]
- 30.Moks, T., L. Abrahmsen, B. Nilsson, U. Hellman, J. Sjöquist, and M. Uhlén. 1986. Staphylococcal protein A consists of five IgG-binding domains. Eur. J. Biochem. 156:3577-3588. [DOI] [PubMed] [Google Scholar]
- 31.Munoz, E., J.-M. Ghuysen, and H. Heymann. 1967. Cell walls of Streptococcus pyogenes type 14. C polysaccharide-peptidoglycan and G polysaccharide-peptidoglycan complexes. Biochemistry 6:3659-3670. [DOI] [PubMed] [Google Scholar]
- 32.Munoz, E., J.-M. Ghuysen, M. Lehy-Bouille, J.-F. Petit, H. Heymann, E. Bricas, and P. Lefrancier. 1966. The peptide subunit N-(l-alanyl-d-isoglutamyl)-l-lysyl-d-alanine in cell wall peptidoglycans of Staphylococcus aureus strain Copenhagen, Micrococcus roseus R27, and Streptococcus pyogenes group A, type 14. Biochemistry 5:3748-3764. [Google Scholar]
- 33.Navarre, W. W., and O. Schneewind. 1994. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol. Microbiol. 14:115-121. [DOI] [PubMed] [Google Scholar]
- 34.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and the mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarre, W. W., H. Ton-That, K. F. Faull, and O. Schneewind. 1998. Anchor structure of staphylococcal surface proteins. II. COOH-terminal structure of muramidase and amidase-solubilized surface protein. J. Biol. Chem. 273:29135-29142. [DOI] [PubMed] [Google Scholar]
- 36.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48:1429-1449. [DOI] [PubMed] [Google Scholar]
- 37.Pallen, M. J., A. C. Lam, M. Antonio, and K. Dunbar. 2001. An embarrassment of sortases—a richness of substrates. Trends Microbiol. 9:97-101. [DOI] [PubMed] [Google Scholar]
- 38.Perry, A. M., H. Ton-That, S. K. Mazmanian, and O. Schneewind. 2002. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. III. Lipid II is an in vivo peptidoglycan substrate for sortase-catalyzed surface protein anchoring. J. Biol. Chem. 277:16241-16248. [DOI] [PubMed] [Google Scholar]
- 39.Pinho, M. G., and J. Errington. 2003. Dispersed mode of Staphylococcus aureus cell wall synthesis in the absence of the division machinery. Mol. Microbiol. 50:871-881. [DOI] [PubMed] [Google Scholar]
- 40.Pinho, M. G., and J. Errington. 2005. Recruitment of penicillin-binding protein PBP2 to the division site of Staphylococcus aureus is dependent on its transpeptidation substrates. Mol. Microbiol. 55:799-807. [DOI] [PubMed] [Google Scholar]
- 41.Rosch, J., and M. Caparon. 2004. A microdomain for protein secretion in gram-positive bacteria. Science 304:1513-1515. [DOI] [PubMed] [Google Scholar]
- 42.Rosch, J. W., and M. G. Caparon. 2005. The ExPortal: an organelle dedicated to the biogenesis of secreted proteins in Streptococcus pyogenes. Mol. Microbiol. 58:959-968. [DOI] [PubMed] [Google Scholar]
- 43.Ruzin, A., A. Severin, F. Ritacco, K. Tabei, G. Singh, P. A. Bradford, M. M. Siegel, S. J. Projan, and D. M. Shlaes. 2002. Further evidence that a cell wall precursor [C55-MurNAc-(peptide)-GlcNAc] serves as an acceptor in a sorting reaction. J. Bacteriol. 184:2141-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salton, M. R. J. 1952. Cell wall of Micrococcus lysodeikticus as the substrate of lysozyme. Nature 170:746-747. [DOI] [PubMed] [Google Scholar]
- 45.Sanderson, A. R., J. L. Strominger, and S. G. Nathenson. 1962. Chemical structure of teichoic acid from Staphylococcus aureus, strain Copenhagen. J. Biol. Chem. 237:3603-3613. [PubMed] [Google Scholar]
- 46.Scheffers, D. J., and M. G. Pinho. 2005. Bacterial cell wall synthesis: new insights from localization studies. Microbiol. Mol. Biol. Rev. 69:585-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schindler, C. A., and V. T. Schuhardt. 1964. Lysostaphin: a new bacteriolytic agent for the Staphylococcus. Proc. Natl. Acad. Sci. USA 51:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneewind, O., A. Fowler, and K. F. Faull. 1995. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science 268:103-106. [DOI] [PubMed] [Google Scholar]
- 49.Schneewind, O., D. Mihaylova-Petkov, and P. Model. 1993. Cell wall sorting signals in surface protein of gram-positive bacteria. EMBO 12:4803-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneewind, O., P. Model, and V. A. Fischetti. 1992. Sorting of protein A to the staphylococcal cell wall. Cell 70:267-281. [DOI] [PubMed] [Google Scholar]
- 51.Sjödahl, J. 1977. Repetitive sequences in protein A from Staphylococcus aureus. Arrangement of five regions within the protein, four being highly homologous and Fc-binding. Eur. J. Biochem. 73:343-351. [DOI] [PubMed] [Google Scholar]
- 52.Stranger-Jones, Y. K., T. Bae, and O. Schneewind. 2006. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 103:16942-16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strominger, J. L. 1968. Penicillin-sensitive enzymatic reactions in bacterial cell wall synthesis. Harvey Lect. 64:179-213. [PubMed] [Google Scholar]
- 54.Tipper, D. J. 1968. Alkali-catalyzed elimination of D-lactic acid from muramic acid and its derivatives and the determination of muramic acid. Biochemistry 7:1441-1449. [Google Scholar]
- 55.Tipper, D. J., and J. L. Strominger. 1965. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-d-alanyl-alanine. Proc. Natl. Acad. Sci. USA 54:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ton-That, H., K. F. Faull, and O. Schneewind. 1997. Anchor structure of staphylococcal surface proteins. I. A branched peptide that links the carboxyl terminus of proteins to the cell wall. J. Biol. Chem. 272:22285-22292. [DOI] [PubMed] [Google Scholar]
- 57.Ton-That, H., H. Labischinski, B. Berger-Bachi, and O. Schneewind. 1998. Anchor structure of staphylococcal surface proteins. III. Role of the FemA, FemB, and FemX factors in anchoring surface proteins to the bacterial cell wall. J. Biol. Chem. 273:29143-29149. [DOI] [PubMed] [Google Scholar]
- 58.Ton-That, H., G. Liu, S. K. Mazmanian, K. F. Faull, and O. Schneewind. 1999. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. USA 96:12424-12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ton-That, H., S. K. Mazmanian, L. Alksne, and O. Schneewind. 2002. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. II. Cysteine 184 and histidine 120 of sortase A form a thiolate imidazolium ion pair for catalysis. J. Biol. Chem. 277:7447-7452. [DOI] [PubMed] [Google Scholar]
- 60.Ton-That, H., and O. Schneewind. 1999. Anchor structure of staphylococcal surface proteins. IV. Inhibitors of the cell wall sorting reaction. J. Biol. Chem. 274:24316-24320. [DOI] [PubMed] [Google Scholar]
- 61.Tzagoloff, H., and R. Novick. 1977. Geometry of cell division in Staphylococcus aureus. J. Bacteriol. 129:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walsh, C. T. 1993. Vancomycin resistance: decoding the molecular logic. Science 261:308-309. [DOI] [PubMed] [Google Scholar]
- 63.Yamada, S., M. Sugai, H. Komatsuzawa, S. Nakashima, T. Oshida, A. Matsumoto, and H. Suginaka. 1996. An autolysin ring associated with cell separation of Staphylococcus aureus. J. Bacteriol. 178:1565-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]