Abstract
Sphingomonas wittichii RW1 degrades chlorinated dibenzofurans and dibenzo-p-dioxins via meta cleavage. We used inverse PCR to amplify dxnB2, a gene encoding one of three meta-cleavage product (MCP) hydrolases identified in the organism that are homologues of BphD involved in biphenyl catabolism. Purified DxnB2 catalyzed the hydrolysis of 8-OH 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate (HOPDA) approximately six times faster than for HOPDA at saturating substrate concentrations. Moreover, the specificity of DxnB2 for HOPDA (kcat/Km = 1.2 × 107 M−1 s−1) was about half that of the BphDs of Burkholderia xenovorans LB400 and Rhodococcus globerulus P6, two potent polychlorinated biphenyl (PCB)-degrading strains. Interestingly, DxnB2 transformed 3-Cl and 4-OH HOPDAs, compounds that inhibit the BphDs and limit PCB degradation. DxnB2 had a higher specificity for 9-Cl HOPDA than for HOPDA but a lower specificity for 8-Cl HOPDA (kcat/Km = 1.7 × 106 M−1 s−1), the chlorinated analog of 8-OH HOPDA produced during dibenzofuran catabolism. Phylogenetic analyses based on structure-guided sequence alignment revealed that DxnB2 belongs to a previously unrecognized class of MCP hydrolases, evolutionarily divergent from the BphDs although the physiological substrates of both enzyme types are HOPDAs. However, both classes of enzymes have mainly small hydrophobic residues lining the subsite that binds the C-6 phenyl of HOPDA, in contrast to the bulky hydrophobic residues (Phe106, Phe135, Trp150, and Phe197) found in the class II enzymes that prefer substrates possessing a C-6 alkyl. Thr196 and/or Asn203 appears to be an important determinant of specificity for DxnB2, potentially forming hydrogen bonds with the 8-OH substituent. This study demonstrates that the substrate specificities of evolutionarily divergent hydrolases may be useful for degrading mixtures of pollutants, such as PCBs.
Chlorinated aromatic compounds, such as polychlorinated biphenyls (PCBs) and dibenzofurans, are among the most widespread, toxic, and/or persistent environmental pollutants. The Bph and Dxn/Dbf pathways responsible for the aerobic bacterial catabolism of biphenyl and dibenzofuran, respectively, have been extensively studied, in part due to their potential for remediating environments contaminated with the polychlorinated compounds (2, 10). The pathways share three homologous enzymes (Fig. 1): a multicomponent dioxygenase that catalyzes the initial step of ring hydroxylation, an extradiol dioxygenase that catalyzes oxygenolytic cleavage of the catecholic intermediate at the meta position to yield 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate (HOPDA) or ortho-substituted HOPDA, and a meta-cleavage product (MCP) hydrolase that catalyzes an unusual C—C bond cleavage of the HOPDA.
FIG. 1.
Microbial catabolism of dibenzofuran and biphenyl by the Dbf and Bph pathways, respectively. Carbon atoms 1, 6, and 8 of 8-OH HOPDA are numbered. The Dbf pathway enzymes are as follows: DxnA1 and DxnA2, large and small subunits of dioxin dioxygenase, respectively; RedA2, reductase of dioxin dioxygenase; Fdx3, ferredoxin of dioxin dioxygenase; DbfB, 2,2′,3-trihydroxybiphenyl dioxygenase; and DxnB, 8-OH HOPDA hydrolase. The Bph pathway enzymes are as follows: BphA and BphE, large and small subunits of biphenyl dioxygenase, respectively; BphG, reductase of biphenyl dioxygenase; BphF, ferredoxin of biphenyl dioxygenase; BphB, dihydrodiol dehydrogenase; BphC, 2,3-dihydroxybiphenyl dioxygenase; and BphD, HOPDA hydrolase.
A general limitation of bacterial catabolic pathways for the degradation of complex industrial mixtures is that one or more pathway enzymes lack the requisite broad substrate specificity to degrade all man-made (xenobiotic) congeners of the naturally occurring compounds, and some enzymes are susceptible to inhibition by the chlorinated metabolites. Studies have identified MCP hydrolases as a bottleneck in the Bph pathway, as summarized below, as well as in the toluene catabolic pathway (11). Substrate specificity studies of MCP hydrolases from potent PCB degraders, BphDLB400 from Burkholderia xenovorans LB400 and BphDP6 from Rhodococcus globerulus P6, revealed that these enzymes are inefficient in transforming HOPDA chlorinated at the 3 or 4 position (30, 31). In addition, these poorly transformed chlorinated metabolites are potential competitive inhibitors of the enzymes, thereby reducing the efficiency of transformation of all congeners, including unsubstituted HOPDA. Consistent with these studies, HOPDAs accumulate during the bacterial degradation of some PCBs (12). Interest in the inhibition of MCP hydrolases is further heightened with the recent discovery of HsaDH37Rv of Mycobacterium tuberculosis H37Rv (35). Formerly annotated as BphD, HsaDH37Rv appears to be involved in steroid metabolism during the survival of M. tuberculosis in the macrophage, hydrolyzing 4,5-9,10-diseco-3-hydroxy-5,9,17-trioxoandrosta-1(10),2-diene-4-oic acid (4,9-DSHA).
MCP hydrolases are members of the α/β-hydrolase superfamily (28). The hydrolyzed C—C bond lies between a dienoate (C-1 to C-5) (Fig. 1) and a carbonyl (C-6). Hydrolysis is mediated by conserved Ser-His-Asp residues reminiscent of the catalytic triad of proteases. The proposed catalytic mechanism involves His-mediated ketonization of the dienoate (18, 23), followed by Ser-mediated hydrolysis at C-6. It is unclear whether the catalytic Ser attacks C-6 directly to form an acyl-enzyme intermediate or whether it activates a water molecule which then attacks C-6 (9, 18, 23, 24). Crystal structures have been reported for five MCP hydrolases: BphDLB400 (18), BphDRHA1 from Rhodococcus sp. strain RHA1 (26), CumDIP01 from Pseudomonas fluorescens IP01, involved in cumene degradation (13), CarCJ3 from Janthinobacterium sp. strain J3, involved in carbazole degradation (14), and MhpC (7). All comprise a “core domain,” consisting of an eight-stranded β-sheet flanked by α-helices, and a “lid domain,” which occurs as an insertion into the core domain. The active site, located between the core and lid domains, includes the conserved Ser, His, and Asp residues and is divided into two subsites: P (polar) and NP (nonpolar), located on each side of the catalytic serine. The P subsite binds the dienoate moiety, and the NP subsite accommodates the C-6 substituent, a phenyl ring in the case of HOPDA.
Sphingomonas wittichii RW1, one of the best-characterized dibenzofuran-degrading bacteria (37), is able to grow on either dibenzofuran or dibenzo-p-dioxin as a sole organic substrate (36). In addition, the strain partially transforms 2-Cl, 3-Cl, 4-Cl, and 2,3-diCl dibenzofurans to the corresponding chlorinated hydroxysalicylates (36). It is not entirely clear which enzymes are responsible for these transformations, as the bacterium possesses multiple homologues of key catabolic enzymes. For example, Armengaud and coworkers isolated four genes that encode α subunits of ring-hydroxylating dioxygenases and two genes that encode β subunits (2). The bacterium also contains at least two extradiol dioxygenases and three MCP hydrolases (2, 4). Two of the MCP hydrolases, DxnB and DxnB2 (previously H1 and H2), were purified and shown to hydrolyze HOPDA and 8-hydroxy HOPDA (4). In addition, the gene encoding a third potential MCP hydrolase was cloned (2). DxnB2 has been reported to be more related to MCP hydrolases involved in the catabolism of monocyclic aromatic compounds than to enzymes such as BphD, which are involved in the degradation of bicyclic aromatics (15). This result, derived from amino acid sequence comparisons, is somewhat surprising as the substrates of DxnB and BphD differ by a single hydroxyl group on the phenyl ring. Better characterization of the DxnB enzymes may clarify the phylogenetic relationships of MCP hydrolases and provide insight into why S. wittichii RW1 contains multiple isozymes.
Herein, we report the cloning, sequencing, and heterologous expression of dxnB2. Heterologously produced DxnB2 was purified, and steady-state kinetic studies revealed that the enzyme possesses useful activities towards chlorinated HOPDAs. We generated a structure-based sequence alignment of MCP hydrolases and used this to perform phylogenetic analyses. The results identified a previously unrecognized class of MCP hydrolases and revealed possible structural determinants of substrate specificity in the various classes.
MATERIALS AND METHODS
Chemicals.
Chlorinated dihydroxybiphenyls were synthesized as previously reported (27, 30). 2,2′,3-Trihydroxybiphenyl (THB) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). HOPDAs were prepared as described previously (30). Other chemicals were of analytical grade and were obtained from ICN Chemicals, Sigma-Aldrich, and BDH.
Bacterial strains and plasmids.
S. wittichii RW1 was purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). This bacterium was grown at 30°C in mineral medium (DSMZ medium 457) with crystalline dibenzofuran or 5 mM sodium salicylate as a carbon source. The vectors and bacterial strains used to test the expression of dxnB2 were the same as those described for bphDP6 and bphDLB400 (30, 32).
Recombinant DNA techniques.
DNA was manipulated, and genomic DNA of S. wittichii RW1 was isolated using established protocols (29). DNA amplification reaction mixtures typically contained 20 to 100 ng of DNA template, 0.3 μM of each primer, 0.25 mM deoxynucleoside triphosphates, 1.5 mM MgCl2, and 1.75 U Pfx high-fidelity DNA polymerase (Boehringer Mannheim) in a total volume of 25 μl. PCR was performed using 10 to 30 of the following cycles: denaturation at 94°C for 1 min, annealing at 48°C to 56°C (depending on primers) for 1 min, and extension at 72°C for 2 min. Amplicons were purified using a QIAquick PCR purification kit (QIAGEN).
The dxnB2 gene was amplified from S. wittichii RW1 genomic DNA using a three-step PCR procedure. First, a fragment of dxnB2 was amplified using degenerate primers. The sense primer was GCGGAATTCATATGTTYGARCARTTYGAR, in which the underlined sequence encodes the first six amino acids, MFEQFE, derived from the N-terminal sequence of purified H2 (4). The antisense primer was CCGCCCGGGATCCTGIRCCCARTGICCRCA, in which the underlined sequence corresponds to a highly conserved amino acid sequence, CGHW, found near the C terminus of MCP hydrolases and the bold sequence corresponds to partially conserved codons encoding alanine and glutamine. Restriction sites (EcoRI and NdeI on the sense primer and XmaI and BamHI on the antisense primer) were introduced to facilitate insertion of the resultant amplicon into pT7-7 (33). Amplification yielded a single fragment of the expected 0.8-kbp size. The inserts of two independently obtained clones were sequenced. In the second step, the flanking dxnB2 DNA was amplified using inverse PCR. In this reaction, the template consisted of circularized fragments of genomic DNA that had been generated through PstI digestion and religation using T4 DNA ligase. The primers for inverse PCR were based on the terminal sequences of the 0.8-kbp amplicon: GGGGGAATTCAATGTAATGGGTACG and GCTAGGATCCCGCAGGCGATC. The resultant 1.6-kbp amplicon was inserted into pT7-7 by using EcoRI and BamHI. In the third and final step, dxnB2 was amplified in its entirety together with the upstream Shine-Dalgarno sequence from genomic DNA, using primers based on the nucleotide sequences of two clones containing the amplicon obtained by inverse PCR: GGGGTCTAGAATTGCCGGTCGGTGAATATTTG and GGGGCTGCAGCGATCAATCCAGCTTTCGCGAAG. This third PCR yielded a 0.84-kbp amplicon that was cloned into pT7-7 by using XbaI and PstI, yielding pT7DXN1. The nucleotide sequences of cloned inserts were determined, and the insert was subcloned into pEMBL18 (6) and pVLT31 to yield pEMDXN1 and pVLTDXN1, respectively. To prevent the production of DxnB2 as a fusion protein with the LacZ α-peptide by using pEMDXN1, the pEMBL18 construct was digested with BamHI (upstream of the XbaI insertion site of dxnB2), the 5′ overhangs were blunted with Klenow DNA polymerase, and the construct was recircularized by ligation. The production of DxnB2 was tested in three systems: Escherichia coli BL21(DE3) containing pT7DXN1, E. coli DH5α containing pEMDXN1, and Pseudomonas putida KT2442 containing pVLTDXN1.
Protein purification.
DxnB2 was purified from E. coli DH5α containing pEMDXN1. Freshly transformed cells were grown in LB broth at 37°C. When the optical density at 600 nm of the culture reached 0.6, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a concentration of 0.5 mM. Incubation was continued overnight, after which the cells were harvested by centrifugation. The cell pellet obtained from 4 liters of culture (16 g [wet weight]) was resuspended in 15 ml of buffer A (20 mM sodium HEPES, pH 7.5) and disrupted by double passage through a French press operated at 20,000 lb/in2. The cell debris was removed by ultracentrifugation (150,000 × g, 60 min), and the supernatant was filtered through a 0.45-μm filter. The filtered supernatant (∼20 ml, 0.085 U/mg, 570 mg) was divided into two portions, each of which was loaded onto a Source 15Q (GE Healthcare) anion-exchange column (2 by 9 cm) equilibrated with buffer A. Proteins were eluted using a gradient of 70 to 220 mM NaCl over 10 column volumes. Activity-containing fractions eluting at ∼140 mM NaCl were pooled, concentrated to about 10 ml by ultrafiltration (Amicon YM 10 membrane), and added to an equal volume of buffer A containing 1 M ammonium sulfate. This mixture (0.29 U/mg, 160 mg) was divided into two portions, each of which was loaded onto a 1- by 9-cm column of phenyl-Sepharose (GE Healthcare) that had been equilibrated with buffer A containing 20% saturation of ammonium sulfate. Proteins were eluted with a gradient of 20% to 0% saturation of ammonium sulfate over 4 column volumes. Activity-containing fractions which eluted at ∼10% saturation of ammonium sulfate were pooled, concentrated, and exchanged into buffer A by ultrafiltration. The protein preparation (∼11 ml, 0.36 U/mg, 61 mg), was then loaded onto a MonoQ HR10/10 (GE Healthcare) column equilibrated with buffer B (20 mM Tris-sulfate, pH 7.5). The proteins were eluted using a gradient of buffer B containing 30 to 150 mM sodium sulfate over 15 column volumes. Activity-containing fractions eluting at ∼60 mM sodium sulfate were concentrated to about 1 ml (0.44 U/mg, 39 mg), flash frozen as beads in liquid nitrogen, and stored at −80°C.
Recombinant BphDLB400, BphDP6, and 2,3-dihydroxybiphenyl dioxygenase (DHBD) of B. xenovorans LB400 were purified as described previously (30, 32).
Determination of protein concentration, purity, and molecular mass.
Protein concentrations were determined using the Bradford method (3a), using bovine serum albumin as the standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using a Bio-Rad Mini-Protean II apparatus and stained with Coomassie blue according to established procedures (29). Molecular weight markers of the range 14,000 to 200,000 were used (Bio-Rad).
Steady-state kinetics.
Enzymatic activity was measured by following the consumption of the yellow substrate on a Varian Cary 3 spectrophotometer equipped with a thermojacketed cuvette holder. Standard activity assays were performed at 25°C using 0.1 M phosphate, pH 7.5, containing 10 μM HOPDA as previously described (32). One unit of enzymatic activity is defined as the quantity of enzyme required to consume 1 μmol of HOPDA or substituted HOPDA per minute. Extinction coefficients for HOPDA and monochlorinated and dichlorinated HOPDAs were reported previously (30). Extinction coefficients for trichlorinated HOPDAs were determined by measuring the absorbance of solutions containing weighed amounts of trichlorinated DHB (0.1 M phosphate, pH 7.5, 25°C) to which an excess amount of DHBD had been added. Steady-state kinetic parameters were evaluated using 0.1 M phosphate, pH 7.5, at 25°C essentially as described previously (32). Initial velocities were evaluated using substrate concentrations that typically ranged from 0.2 to 10 times the Km value for that substrate. Appropriate equations were fitted to the initial velocities determined at different substrate concentrations by using the least-squares and dynamic weighting options of LEONORA (5). Best-fit parameters were calculated using a minimum of 10 independent data points. The validity of the kinetic models was evaluated using residual plots.
The enzyme-catalyzed hydrolysis of 8-OH HOPDA was monitored at 438 nm using 1 mM potassium phosphate, pH 7.5, at 8°C and an extinction coefficient of 16.2 mM−1 cm−1, determined as described above. The extinction coefficient for HODPA under these conditions was unchanged from the previously reported value (30). Due to its low stability, 8-OH HOPDA was generated in situ by adding an excess of DHBD to assay mixtures containing 5 or 30 μM of THB. The rates of enzyme-catalyzed reactions were calculated from the first 30 s of the progress curves and were corrected for nonenzymatic transformation of 8-OH HODPA.
Sequence analyses.
All sequences were obtained from the Entrez server at the National Center for Biotechnology Information. Crystal structures were obtained from the Protein Data Bank (http://www.pdb.org/) (accession numbers in parentheses) for MhpCW3110 (1U2E), BphD1RHA1 (1C4X), CumDIP01 (1IUP), CarCJ3 (1J1I), and BphDLB400 (2OG1). A structure-guided alignment of eight MCP hydrolases was obtained in a two-step process. First, the sequences of eight MCP hydrolases of interest were aligned in an automated fashion using the program ClustalX version 1.83 (34) and its default parameters. Second, this alignment was modified using information obtained by superposing the Cα backbones of the structures of MhpCW3110, BphD1RHA1, CumDIP01, and CarCJ3 on that of BphDLB400, using the least-squares superposition commands of the program O with default parameters (19). The initial transformation relating each of the four pairs of structures was obtained by superposing the two structures, using the Lsq_exp command and explicitly defined pairs of Cα atoms (i.e., those of the conserved catalytic residues). The Lsq_imp command was then used to improve the superposition by locating the longest matching fragments between the two molecules, as determined by the superposition of Cα atoms (20). The ClustalX-generated alignment was visually checked against the well-superimposed segments in the structures to eliminate any program-generated errors, such as misaligned residues or segments. Well-superimposed segments included all α-helices and β-sheets as well as some of the loops. An alignment containing more sequences was obtained by using ClustalX and defining the structure-guided eight-sequence alignment as a profile. The final alignment was inspected to ensure that it was consistent with the structure-guided alignment. Phylogenetic analyses were performed using the maximum-likelihood program of PHYLIP (8). Bootstrap analyses were performed using 100 data sets. Trees were calculated using default parameters except for the jumbling parameter, J, which was set at 10.
RESULTS
Cloning of dxnB2.
The dxnB2 gene was cloned by inverse PCR. Degenerate primers for the initial amplification were based on the published N-terminal amino acid sequence of purified DxnB2 (formerly H2) and a C-terminal motif, CGHW, that is conserved in MCP hydrolases. The histidine and tryptophan residues of this motif are thought to assist in the substrate binding and the ketonization that precedes C—C bond hydrolysis in the proposed catalytic cycle (7, 18). Inverse PCR yielded a 2,234-bp fragment containing the entire dxnB2 gene. The dxnB2 gene was 840 bp long. The 5′ end of the gene encodes the N-terminal amino acid sequence reported for DxnB2 purified from S. wittichii RW1 (4). The 5′ end of a second open reading frame was deduced downstream of the dxnB2 gene. This partial open reading frame encodes a fragment of 311 amino acid residues that shares sequence identity with salicylate hydroxylases (e.g., 65% amino acid sequence identity to the α subunit of salicylate hydroxylase from Pseudomonas aeruginosa JB2 [16]). This result is consistent with DxnB2 being involved in dibenzofuran catabolism, as salicylate would be one of the products of the hydrolase-catalyzed reaction in such a pathway. The nucleotide sequence of the 2.2-kbp fragment was submitted to NCBI (accession no. DQ975235).
Sequence and phylogenetic analyses.
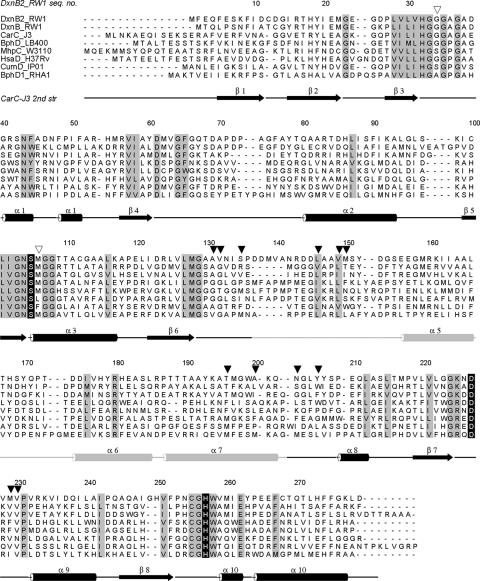
The amino acid sequences of MCP hydrolases were aligned with the help of information derived from the crystallographic structures of BphDLB400 (18), MhpCW3110 (7), CarCJ3 (14), CumDIP01 (13), and BphDRHA1 (26). The alignment of eight representative sequences, including DxnB2, is presented in Fig. 2. Conserved residues in these enzymes include the catalytic triad (Ser105, His257, and Asp227 of DxnB2) (Fig. 2) and the residues that comprise the P subsite, which binds the dienoate moiety shared by all substrates. These residues include those that interact with the C-1 carboxylate and C-2 hydroxyl of the dienoate moiety: Gly35, Asn43, Asn104, Arg180, His257, and Trp258. The residues comprising the NP subsite, which binds the C-6 substituent of the substrate, occur at positions 106, 131, 132, 135, 146, 149, 150, 196, 200, 203, 206, 229, and 230 in DxnB2 (Fig. 2) and are less well conserved.
FIG. 2.
Structure-based sequence alignment of MCP hydrolases. Sequences are grouped according to class (see Fig. 3), with GenBank accession numbers in parentheses. Class III: DxnB2_RW1, DxnB2RW1 from S. wittichii RW1 (DQ975235); DxnB_RW1, DxnBRW1 from S. wittichii RW1 (CAA51367); CarC_J3, CarCJ3 from Janthinobacterium sp. strain J3 (BAC56745). Class I: BphD_LB400, BphDLB400 from B. xenovorans LB400 (P47229); MhpC_W3110, MhpCW3110 from E. coli W3110 (BAA13054); HsaD_H37Rv, HsaDH37Rv from M. tuberculosis H37Rv (NP_218086). Class II: CumD_IP01, CumDIP01 from Pseudomonas fluorescens IP01 (BAA12150). Other: BphD1_RHA1, BphDRHA1 from Rhodococcus sp. strain RHA1 (BAC92715). The enzymes of known structure are CarCJ3, BphDLB400, MhpCW3110, CumDIP01, and BphDRHA1. Conserved residues are shaded in gray. The catalytic residues (Ser105, His257, and Asp227 in DxnB2) appear as white characters on a black background. Residues comprising the oxyanion hole are indicated with open arrowheads. Residues comprising the NP subsite and therefore interacting with the C-6 substituent of the substrate are indicated with filled arrowheads. The secondary structure (2nd str) for CarCJ3 (15) is noted underneath the alignment, with black and light gray representing the core domain and lid domain, respectively. seq., sequence.
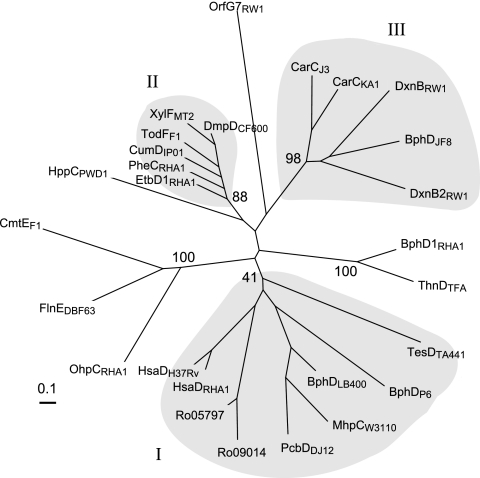
Phylogenetic analysis based on the alignment indicates that there are three classes of hydrolases (Fig. 3) that appear to be consistent with enzyme specificity. Classes I and II were identified as groups I and III, respectively, in a previous phylogenetic analysis (15). The former consists mostly of enzymes that are involved in the catabolism of biphenyl and steroids, such as BphDLB400 and HsaDH37Rv, respectively. This class also includes MhpCW3110, which transforms a 2-hydroxy-6-oxo-2,4-dienoate (HODA) with a propionate at the C-6 position (22). While the bootstrap values for the clustering of BphDP6 and TesDTA441 with class I enzymes are low, the respective physiological roles of these enzymes are consistent with their belonging to this group. Class II MCP hydrolases appear to be involved in alkylbenzene degradation and transform HODAs that have an uncharged alkyl substituent at C-6. Members of this family are tightly clustered with high amino acid sequence similarity between any two members.
FIG. 3.
Dendrogram of best tree obtained by alignment of 27 MCP hydrolases. The scale represents the distance of divergence. The sequences not in Fig. 2 are as follows (with GenBank accession numbers in parentheses): CarCKA1, CarC from Sphingomonas sp. strain KA1 (BAC56762); BphDJF8, BphD from Bacillus sp. strain JF8 (BAC79225); BphDP6, BphD from Rhodococcus globerulus P6 (AAB17100); TodFF1, TodF from Pseudomonas putida F1 (P23133); XylFMT2, XylF from Pseudomonas putida MT2 (CAC86804); DmpDCF600, DmpD from Pseudomonas sp. strain CF600 (P19076); PheCRHA1, EtbD1RHA1, HsaDRHA1, Ro05797RHA1, Ro09014RHA1, and OhpCRHA1, MCP hydrolases from Rhodococcus sp. strain RHA1 (ABG99128, BAA31163, BAA98136, ABG97574, ABH00058, and ABG92355, respectively); ThnDTFA, ThnD from Sphingopyxis macrogoltabida TFA (AAG18490); HppCPWD1, HppC from R. globerulus PWD1 (AAB81313); TesDTA441, TesD from Comamonas testosteroni TA441 (BAC67693); PcbDDJ12, PcbD from Pseudomonas sp. strain DJ12 (BAA07955); CmtEF1, CmtE from Pseudomonas putida F1 (BAB17778); FlnEDBF63, FlnE from Terrabacter sp. strain DBF63 (BAE45094); and OrfG7RW1, putative hydrolase from S. wittichii RW1 (CAA11193).
Two of the three MCP hydrolases of S. wittichii RW1, DxnB2 and DxnB, belong to the previously unrecognized class III. This class appears to comprise enzymes such as CarCJ3 that are involved in the catabolism of dibenzofuran, carbazole, and related compounds. The catabolism of such compounds is initiated by angular dioxygenation. Thus, the substrate of the class III MCP hydrolases is a HOPDA possessing an ortho-substituted phenyl ring (Fig. 1). Although the annotation of BphDJF8 from Bacillus sp. strain JF8 (25) suggests that this class III enzyme is involved in biphenyl catabolism, the ring-hydroxylating dioxygenase encoded by the operon containing bphDJF8 is most closely related to enzymes that catalyze angular dioxygenation, strongly suggesting that the natural substrate of BphDJF8 is also an ortho-substituted HOPDA. The third putative MCP hydrolase of S. wittichii RW1 (2), encoded by orfG7, is evolutionarily divergent from other MCP hydrolases. Although the catalytic triad residues (Ser105, Asp227, and His257; DxnB2 numbering) are conserved in OrfG7, most of OrfG7's P subsite residues differ from those found in the other MCP hydrolases. For example, Gly35, Asn43, Asn104, and Arg180 are replaced with Leu, Thr, Thr, and Ile, respectively.
Production and purification of DxnB2.
Among three tested expression systems, DxnB2 was produced to the highest level using E. coli DH5α containing pEMDXN1 based on SDS-PAGE analyses. The heterologously produced DxnB2 was purified using anion-exchange and hydrophobic interaction chromatography. Thirty-nine milligrams of purified protein with a specific activity of 0.44 U/mg was obtained from 4 liters of cell culture. The protein was of >99% purity as judged from SDS-PAGE. The molecular weight of the purified protein (∼31,000) corresponds to that of DxnB2 partially purified from its native host (4) as well as to the molecular mass deduced from the nucleotide sequence of the gene (30.4 kDa).
Steady-state kinetic analysis.
The steady-state kinetic parameters of DxnB2 for 8-OH HOPDA, its likely natural substrate, could not be reliably determined under the standard assay conditions due to the compound's rapid nonenzymatic transformation (half-life [t1/2], ∼18 s), consistent with previous reports (21). Reducing the concentration of phosphate to 1 mM increased the t1/2 of 8-OH HOPDA an order of magnitude (t1/2, ∼3 min), and lowering the temperature to 8°C resulted in a further fivefold increase (t1/2, ∼15 min). Under these conditions, DxnB2 catalyzed the hydrolysis of 5 μM 8-OH HOPDA at a rate of 0.30 ± 0.09 U/mg (mean ± standard error). The rate at 30 μM 8-OH HOPDA was similar, indicating that the enzyme was saturated, and the kcat value was 0.15 ± 0.04 s−1 (1 mM potassium phosphate, pH 7.5, 8°C). The kcat value for HOPDA under these conditions was approximately sixfold lower (0.026 ± 0.001 s−1). By contrast, BphDLB400 catalyzed the hydrolysis of HOPDA (kcat, ∼1.4 ± 0.1 s−1) an order of magnitude more rapidly than for 8-OH HOPDA (kcat, ∼0.11 ± 0.01 s−1) under these conditions.
Under the standard assay conditions, DxnB2 catalyzed the efficient hydrolysis of HOPDA: the specificity constant (kcat/Km) was within a factor of two of those previously determined for BphDLB400 and BphDP6, whose physiological substrate is HOPDA (Table 1). Interestingly, the specificity of DxnB2 for 8-Cl HOPDA, a steric analog of the enzyme's physiological substrate 8-OH HOPDA, was hydrolyzed with a specificity constant that was over an order of magnitude lower than for HOPDA. As observed for the BphDs, other HOPDAs that were chlorinated on the phenyl ring tended to be better substrates of DxnB2 than HOPDA. However, the specificity of DxnB2 appears to be distinct in that it preferentially hydrolyzed 9-Cl HOPDA over 10-Cl HOPDA, in contrast to the BphDs in which this specificity is reversed.
TABLE 1.
Steady-state kinetic parameters of DxnB2, BphDP6, and BphDLB400 with different chlorinated HOPDAsa
| HOPDA substituent | BphDP6
|
BphDLB400
|
DxnB2
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Km (μM) | kcat (s−1) | kcat/Km (105) (M−1 s−1) | Km (μM) | kcat (s−1) | kcat/Km (105) (M−1 s−1) | Km (μM) | kcat (s−1) | kcat/Km (105) (M−1 s−1) | Ksi (mM) | |
| None | 0.47 (0.02) | 7.6 (0.01) | 162 (3) | 0.19 (0.01) | 4.18 (0.03) | 225 (6) | 0.039 (0.006) | 0.47 (0.02) | 118 | 36.5 (7.8)d |
| 3-Cl | 6.9 (0.3) | 0.0172 (0.004) | 0.0252 (0.007) | 0.54 (0.03) | 0.0089 (0.0001) | 0.16 (0.01) | 1.19 (0.09) | 0.25 (0.01) | 2.1 | 39.9 (4.9)d |
| 4-Cl | 0.40 (0.02)b | ND | ND | 3.6 (0.2)b | 0.00059 (6 × 10−5)b | 0.0016 (0.0002)b | ND | ND | ND | ND |
| 4-OH | 4.4 (0.2)b | ND | ND | 0.95 (0.04)b | 0.0055 (0.0008)b | 0.058 (0.008)b | 1.34 (0.23) | 0.087 (0.007) | 0.65 | 15.6 (2.4)d |
| 5-Cl | 18 (1) | 1.03 (0.04) | 0.57 (0.03) | 4.9 (0.1) | 1.53 (0.01) | 3.11 (0.05) | 0.48 (0.04) | 0.41 (0.01) | 8.6 | 27.6 (3.1)d |
| 8-Cl | 1.29 (0.04) | 1.74 (0.02) | 13.5 (0.3) | 0.33 (0.02) | 2.10 (0.03) | 64 (3) | 0.29 (0.03) | 0.48 (0.02) | 17 (1) | NA |
| 9-Cl | 0.43 (0.01) | 15.3 (0.1) | 360 (4) | 0.46 (0.03) | 3.9 (0.1) | 85 (4) | 0.11 (0.04) | 5.1 (0.6) | 460 (140) | 5.9 (1.8)d |
| 10-Cl | 0.65 (0.01) | 33.7 (0.2) | 517 (8) | 0.13 (0.01) | 3.13 (0.04) | 246 (13) | 0.084 (0.009) | 0.88 (0.03) | 105 (8) | 3.7 (0.3)d |
| 9,10-diCl | 0.75 (0.03) | 29.0 (0.3) | 387 (13) | 0.154 (0.009) | 2.23 (0.03) | 143 (7) | 0.11 (0.05) | 2.5 (0.3) | 219 (6) | 10 (4)d |
| 9,11-diCl | 1.04 (0.01) | 11.2 (0.2) | 108 (3) | 0.031 (0.001) | 0.144 (0.001) | 47 (2) | 0.049 (0.007) | 2.59 (0.06) | 530 (60) | NA |
| 3,9,11-triCl | 60 (3)b | 0.0039 (0.0002)c | NA | 0.33 (0.03) | 0.0166 (0.0004) | 0.50 (0.04) | 0.7 (0.1) | 0.13 (0.01) | 1.7 (0.1) | 13 (4)d |
Experiments were performed using 0.1 M potassium phosphate, pH 7.5, at 25°C. The kinetic parameters of BphDLB400 and BphDP6 for all but triCl HOPDA have previously been published (30, 31). Values in parentheses indicate standard errors. NA, not applicable due to observed behavior; ND, not determined.
Kic, determined from inhibition experiments, was calculated from specific activities and/or by assuming that Kic is ∼Km.
Calculated using a specific activity of 8.04 × 10−2 U/mg.
Substrate inhibition model fitted to data.
The most remarkable aspect of DxnB2's substrate specificity is its ability to catalyze the hydrolysis of HOPDAs substituted on the dienoate moiety at reasonable rates. By contrast, the BphDs were essentially inhibited by 3-Cl and 4-OH HOPDAs. The latter is the nonenzymatic transformation product of 4-Cl HODPA that is rapidly formed in aqueous solutions (31). Thus, the specificity constant of DxnB2 for 3-Cl and 4-OH HOPDAs is 1 or 2 orders of magnitude greater than those of the BphDs. Consistent with these data, 3,9,11-triCl HODPA is also a better substrate for DxnB2 than for either of the BphDs.
Another distinguishing feature of the DxnB2 steady-state hydrolysis of HOPDAs was the occurrence of substrate inhibition. This was observed for all substrates except for 8-Cl HOPDA and 9,11-Cl HOPDA. By contrast, substrate inhibition was not observed for either BphD. In DxnB2, the Ksi values were in the millimolar range while the Km values were in the micromolar range.
DISCUSSION
The gene encoding DxnB2 of S. wittichii RW1 was cloned using inverse PCR, and the heterologously produced enzyme was purified and characterized. Several lines of evidence indicate that DxnB2 is involved in the catabolism of dibenzofurans. First, the enzyme possessed a significant substrate preference for 8-OH HOPDA over HOPDA, in contrast to what was observed for BphDLB400. Second, dxnB2 is located immediately upstream of a salicylate hydroxylase, an enzyme predicted to succeed DxnB2 in the catabolism of dibenzofuran. Finally, our phylogenetic analysis indicates that DxnB2 is most similar to hydrolases such as DxnB and CarC, which are involved in the degradation of dibenzofuran and the structurally related carbazole, respectively. The occurrence of multiple DxnB isozymes in S. wittichii RW1 is consistent with the proposal that this bacterium's ability to degrade a range of dioxins and dibenzofurans is due to the higher number of homologues of various aromatic catabolic genes present in its genome than in those of other bacterial strains (2-4).
In a previous phylogenetic analysis, DxnB-type MCP hydrolases were classified together with MCP hydrolases involved in the degradation of alkylbenzenes (15). The current analysis is based on a structure-guided sequence alignment. Moreover, the previous classification scheme included α/β hydrolases that are not MCP hydrolases, such as dehalogenases and epoxide hydrolases (15), which were omitted from our analyses. Nevertheless, it is interesting that the DxnB-type hydrolases appear to be more distinct from the BphD-type hydrolases than are the HsaD-type enzymes, considering that the substrates of the first two enzymes are structurally much more similar. The current analysis reveals several instances of the convergent evolution of substrate specificity. For example, BphD1RHA1 from the PCB-degrading Rhodococcus sp. strain RHA1 is evolutionarily divergent from the class I BphDs, such as BphDLB400, clustering with ThnDTFA from the tetralin catabolic pathway of Sphingomonas macrogoltabida TFA. However, they have functionally converged to transform MCPs of bicyclic compounds. Sequence alignment suggests that the key determinants for recognizing the C-6 phenyl ring are conserved in these two enzymes. Similarly, the three MCP hydrolases that act on 6-propionate HODA, MhpCW3110, HppCPWD1 from Rhodococcus globerulus PWD1, and OhpCRHA1 from Rhodococcus sp. strain RHA1, appear to be evolutionarily divergent despite possessing similar substrate specificities. We expect our understanding of the phylogeny of MCP hydrolases to improve as additional structural and functional data for these enzymes become available, particularly with respect to the enzymes that do not belong to one of the three identified classes. Functional characterization of OrfG7 would be especially interesting in light of the residues that constitute this enzyme's P subsite. In particular, the replacement of Arg180 by Ile in OrfG7 suggests that this enzyme may not be an MCP hydrolase. Arg180 is absolutely conserved among MCP hydrolases, neutralizing the negatively charged C-1 carboxylate of the substrate (7, 18).
The sequence alignment provides useful insight into the structural determinant of substrate specificity in MCP hydrolases. More particularly, the residues comprising the NP subsite to which the C-6 substituent of the substrate binds (positions 106, 131, 132, 135, 146, 149, 150, 196, 200, 203, 206, 229, and 230) appear to correlate with the specificities of the class I, II, and III MCP hydrolases. In the class II enzymes, which transform substrates with smaller alkyl C-6 substituents, the NP subsite residues tend to be bulky hydrophobic residues, such are Phe106, Phe135, Trp150, and Phe197. In contrast, in the class I and class III enzymes, which transform HOPDA, these tend to be smaller aliphatic residues, presumably creating a larger substrate binding pocket to accommodate the C-6 phenyl substituent. Of particular interest are the residues at positions 196 and 203. Residue 196 is conserved as Thr for class III enzymes, except BphD_JF8, and may interact with the ortho-hydroxyl or amine group of the substrate's phenyl ring. In class II enzymes, this residue is Met196 and is part of a conserved motif: MFPXP. Within this motif, Phe197 interacts with two of the key determinants of class II enzymes noted above: Trp150 and Phe106. Residue 203 is notable in that it is lysine or arginine in the divergent hydrolases that utilize substrates with a 6-propionate HODA (MhpC, OhpC, and HppC), suggesting that the positive charge interacts with the negatively charged carboxylate of the substrate. In class III enzymes, residue 203 is asparagine or serine and may form a hydrogen bond with the 8-hydroxyl group of the substrate produced in dibenzofuran degradation. In the other MCP hydrolases, this residue is hydrophobic.
Considering that 8-OH HOPDA is produced during the catabolism of dibenzofuran (Fig. 1) and is the likely physiological substrate of DxnB2, it is somewhat surprising that DxnB2 has a sevenfold-lower specificity for 8-Cl HOPDA than for HOPDA. Structure-based sequence alignment suggests that this may be due to Thr196 and/or Asn203 of DxnB2, which appears to be a significant determinant of substrate specificity in class III MCP hydrolases as discussed above. Thus, the hydrogen bonding that we predict to occur between these residues and the 8-OH group of 8-OH HOPDA could not occur with the chloro substituent of 8-Cl HOPDA. Alternatively, it is possible that the physiological substrate of DxnB2 is substituted 8-OH HODPA. For example, it has been reported that S. wittichii RW1 metabolizes 4-chlorodibenzofuran and 2,7-dichlorodibenzo-p-dioxin to form 9-Cl-, 10-Cl-, or 11-Cl-substituted 8-OH HOPDA (1, 17). Consistent with this possibility, DxnB2 possessed higher specificity for 9-Cl and 10-Cl HOPDAs than for unchlorinated HOPDA (Table 1).
Although DxnB2 transforms HOPDAs substituted on the dienoate moiety better than either BphDLB400 or BphDP6, the effects of such substituents are similar in the three enzymes. For example, the kcat/Km for DxnB2 for 3,9,11-triCl HOPDA was ∼300-fold lower than for 9,11-diCl HOPDA. This is consistent with the proposed catalytic mechanism of MCP hydrolases, which involves ketonization of the dienoate moiety prior to the hydrolytic step (22). Substituents on the dienoate moiety could interfere with ketonization due to steric and/or electronic effects. Nevertheless, despite the relatively low specificities of the three enzymes for HOPDAs substituted on the dienoate moiety, DxnB2 has a higher specificity for such compounds than either BphDLB400 or BphDP6. For example, DxnB2 has at least a 10-fold-higher specificity towards 3-Cl and 4-OH HOPDAs than either BphDLB400 or BphDP6. Moreover, the Km of DxnB2 for 3-Cl HOPDA is 30-fold higher than for HOPDA, implying that this enzyme is not as strongly competitively inhibited by this compound as BphDLB400. This complementarity in substrate specificity between DxnB2 and BphDLB400 could be exploited to improve the biodegradation of mixtures of PCBs. In light of the current data, it will be interesting to test whether appropriate derivatives of 4,9-DSHA inhibit HsaDH37Rv.
Acknowledgments
This research was funded by Strategic (STP0193182) and Discovery grants from the Natural Sciences and Engineering Research Council of Canada (to L.D.E.).
Footnotes
Published ahead of print on 6 April 2007.
REFERENCES
- 1.Arfmann, H. A., K. N. Timmis, and R. M. Wittich. 1997. Mineralization of 4-chlorodibenzofuran by a consortium consisting of Sphingomonas sp. strain RW1 and Burkholderia sp. strain JWS. Appl. Environ. Microbiol. 63:3458-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armengaud, J., B. Happe, and K. N. Timmis. 1998. Genetic analysis of dioxin dioxygenase of Sphingomonas sp. strain RW1: catabolic genes dispersed on the genome. J. Bacteriol. 180:3954-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armengaud, J., K. N. Timmis, and R.-M. Wittich. 1999. A functional 4-hydroxysalicylate/hydroxyquinol degradative pathway gene cluster is linked to the initial dibenzo-p-dioxin pathway genes in Sphingomonas sp. strain RW1. J. Bacteriol. 181:3452-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Bünz, P. V., R. Falchetto, and A. M. Cook. 1993. Purification of two isofunctional hydrolases (EC 3.7.1.8) in the degradative pathway for dibenzofuran in Sphingomonas sp. strain RW1. Biodegradation 4:171-178. [DOI] [PubMed] [Google Scholar]
- 5.Cornish-Bowden, A. 1995. Analysis of enzyme kinetic data. Oxford University Press, New York, NY.
- 6.Dente, L., and R. Cortese. 1987. pEMBL: a new family of single-stranded plasmids for sequencing DNA. Methods Enzymol. 155:111-119. [DOI] [PubMed] [Google Scholar]
- 7.Dunn, G., M. G. Montgomery, F. Mohammed, A. Coker, J. B. Cooper, T. Robertson, J. L. Garcia, T. D. H. Bugg, and S. P. Wood. 2005. The structure of the C—C bond hydrolase MhpC provides insights into its catalytic mechanism. J. Mol. Biol. 346:253-265. [DOI] [PubMed] [Google Scholar]
- 8.Felsenstein, J. 2006. PHYLIP (Phylogeny Inference Package), version 3.66. Department of Genome Sciences, University of Washington, Seattle.
- 9.Fleming, S. M., T. A. Robertson, G. J. Langley, and T. D. H. Bugg. 2000. Catalytic mechanism of a C—C hydrolase enzyme: evidence for a gem-diol intermediate, not an acyl enzyme. Biochemistry 39:1522-1531. [DOI] [PubMed] [Google Scholar]
- 10.Focht, D. D. 1995. Strategies for the improvement of aerobic metabolism of polychlorinated-biphenyls. Curr. Opin. Biotechnol. 6:341-346. [Google Scholar]
- 11.Furukawa, K., J. Hirose, A. Suyama, T. Zaiki, and S. Hayashida. 1993. Gene components responsible for discrete substrate specificity in the metabolism of biphenyl (bph operon) and toluene (tod operon). J. Bacteriol. 175:5224-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa, K., N. Tomizuka, and A. Kamibayashi. 1979. Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Appl. Environ. Microbiol. 38:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fushinobu, S., T. Saku, M. Hidaka, S. Y. Jun, H. Nojiri, H. Yamane, H. Shoun, T. Omori, and T. Wakagi. 2002. Crystal structures of a meta-cleavage product hydrolase from Pseudomonas fluorescens IP01 (CumD) complexed with cleavage products. Protein Sci. 11:2184-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habe, H., K. Morii, S. Fushinobu, J. W. Nam, Y. Ayabe, T. Yoshida, T. Wakagi, H. Yamane, H. Nojiri, and T. Omori. 2003. Crystal structure of a histidine-tagged serine hydrolase involved in the carbazole degradation (CarC enzyme). Biochem. Biophys. Res. Commun. 303:631-639. [DOI] [PubMed] [Google Scholar]
- 15.Hernáez, M. J., E. Andujar, J. L. Rios, S. R. Kaschabek, W. Reineke, and E. Santero. 2000. Identification of a serine hydrolase which cleaves the alicyclic ring of tetralin. J. Bacteriol. 182:5448-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickey, W. J., G. Sabat, A. S. Yuroff, A. R. Arment, and J. Perez-Lesher. 2001. Cloning, nucleotide sequencing, and functional analysis of a novel, mobile cluster of biodegradation genes from Pseudomonas aeruginosa strain JB2. Appl. Environ. Microbiol. 67:4603-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong, H. B., Y. S. Chang, I. H. Nam, P. Fortnagel, and S. Schmidt. 2002. Biotransformation of 2,7-dichloro- and 1,2,3,4-tetrachlorodibenzo-p-dioxin by Sphingomonas wittichii RW1. Appl. Environ. Microbiol. 68:2584-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horsman, G. P., J. Ke, S. Dai, S. Y. K. Seah, J. T. Bolin, and L. D. Eltis. 2006. Kinetic and structural insight into the mechanism of BphD, a C—C bond hydrolase from the biphenyl degradation pathway. Biochemistry 45:11071-11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, T. A., J. Y. Zou, S. W. Cowan, and M. Kjeldgaard. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47:110-119. [DOI] [PubMed] [Google Scholar]
- 20.Kleywegt, G. J., and T. A. Jones. 1997. Detecting folding motifs and similarities in protein structures. Methods Enzymol. 277:525-545. [DOI] [PubMed] [Google Scholar]
- 21.Kohler, H. P. E., A. Schmid, and M. Vandermaarel. 1993. Metabolism of 2,2′-dihydroxybiphenyl by Pseudomonas sp. strain Hbp1: production and consumption of 2,2′,3-trihydroxybiphenyl. J. Bacteriol. 175:1621-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam, W. W. Y., and T. D. H. Bugg. 1997. Purification, characterization, and stereochemical analysis of a C—C hydrolase: 2-hydroxy-6-keto-nona-2,4-diene-1,9-dioic acid 5,6-hydrolase. Biochemistry 36:12242-12251. [DOI] [PubMed] [Google Scholar]
- 23.Li, C., M. G. Montgomery, F. Mohammed, J. J. Li, S. P. Wood, and T. D. H. Bugg. 2005. Catalytic mechanism of C—C hydrolase MhpC from Escherichia coli: kinetic analysis of His263 and Ser110 site-directed mutants. J. Mol. Biol. 346:241-251. [DOI] [PubMed] [Google Scholar]
- 24.Li, J. J., C. Li, C. A. Blindauer, and T. D. H. Bugg. 2006. Evidence for a gem-diol reaction intermediate in bacterial C—C hydrolase enzymes BphD and MhpC from C-13 NMR spectroscopy. Biochemistry 45:12461-12469. [DOI] [PubMed] [Google Scholar]
- 25.Mukerjee-Dhar, G., M. Shimura, D. Miyazawa, K. Kimbara, and T. Hatta. 2005. bph genes of the thermophilic PCB degrader, Bacillus sp. JF8: characterization of the divergent ring-hydroxylating dioxygenase and hydrolase genes upstream of the Mn-dependent BphC. Microbiology 151:4139-4151. [DOI] [PubMed] [Google Scholar]
- 26.Nandhagopal, N., A. Yamada, T. Hatta, E. Masai, M. Fukuda, Y. Mitsui, and T. Senda. 2001. Crystal structure of 2-hydroxyl-6-oxo-6-phenylhexa-2,4-dienoic acid (HPDA) hydrolase (BphD enzyme) from the Rhodococcus sp. strain RHA1 of the PCB degradation pathway. J. Mol. Biol. 309:1139-1151. [DOI] [PubMed] [Google Scholar]
- 27.Nerdinger, S., C. Kendall, R. Marchhart, P. Riebel, M. R. Johnson, C. F. Yin, L. D. Eltis, and V. Snieckus. 1999. Directed ortho metalation and Suzuki-Miyaura cross-coupling connections: regiospecific synthesis of all isomeric chlorodihydroxybiphenyls for microbial degradation studies of PCBs. Chem. Commun. 22:2259-2260. [Google Scholar]
- 28.Ollis, D. L., E. Cheah, M. Cygler, B. Dijkstra, F. Frolow, S. M. Franken, M. Harel, S. J. Remington, I. Silman, J. Schrag, J. L. Sussman, K. H. G. Verscheuren, and A. Goldman. 1992. The alpha/beta hydrolase fold. Protein Eng. 5:197-211. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Seah, S. Y. K., G. Labbé, S. R. Kaschabek, F. Reifenrath, W. Reineke, and L. D. Eltis. 2001. Comparative specificities of two evolutionarily divergent hydrolases involved in microbial degradation of polychlorinated biphenyls. J. Bacteriol. 183:1511-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seah, S. Y. K., G. Labbé, S. Nerdinger, M. R. Johnson, V. Snieckus, and L. D. Eltis. 2000. Identification of a serine hydrolase as a key determinant in the microbial degradation of polychlorinated biphenyls. J. Biol. Chem. 275:15701-15708. [DOI] [PubMed] [Google Scholar]
- 32.Seah, S. Y. K., G. Terracina, J. T. Bolin, P. Riebel, V. Snieckus, and L. D. Eltis. 1998. Purification and preliminary characterization of a serine hydrolase involved in the microbial degradation of polychlorinated biphenyls. J. Biol. Chem. 273:22943-22949. [DOI] [PubMed] [Google Scholar]
- 33.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Geize, R., K. Yam, T. Heuser, M. H. Wilbrink, H. Hara, M. C. Anderton, E. Sim, L. Dijkhuizen, J. E. Davies, W. W. Mohn, and L. D. Eltis. 2007. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc. Natl. Acad. Sci. USA 104:1947-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkes, H., R. M. Wittich, K. N. Timmis, P. Fortnagel, and W. Francke. 1996. Degradation of chlorinated dibenzofurans and dibenzo-p-dioxins by Sphingomonas sp. strain RW1. Appl. Environ. Microbiol. 62:367-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wittich, R. M., H. Wilkes, V. Sinnwell, W. Francke, and P. Fortnagel. 1992. Metabolism of dibenzo-p-dioxin by Sphingomonas sp. strain RW1. Appl. Environ. Microbiol. 58:1005-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]