Abstract
A single-step pathway for the synthesis of the compatible solute glucosylglycerate (GG) is proposed based on the activity of a recombinant glucosylglycerate synthase (Ggs) from Persephonella marina. The corresponding gene encoded a putative glycosyltransferase that was part of an operon-like structure which also contained the genes for glucosyl-3-phosphoglycerate synthase (GpgS) and glucosyl-3-phosphoglycerate phosphatase (GpgP), the enzymes that lead to the synthesis of GG through the formation of glucosyl-3-phosphoglycerate. The putative glucosyltransferase gene was expressed in Escherichia coli, and the recombinant product catalyzed the synthesis of GG in one step from ADP-glucose and d-glycerate, with Km values at 70°C of 1.5 and 2.2 mM, respectively. This glucosylglycerate synthase (Ggs) was also able to use GDP- and UDP-glucose as donors to form GG, but the efficiencies were lower. Maximal activity was observed at temperatures between 80 and 85°C, and Mg2+ or Ca2+ was required for catalysis. Ggs activity was maximal and remained nearly constant at pH values between 5.5 and pH 8.0, and the half-lives for inactivation were 74 h at 85°C and 8 min at 100°C. This is the first report of an enzyme catalyzing the synthesis of GG in one step and of the existence of two pathways for GG synthesis in the same organism.
Living cells experience fluctuations in environmental osmolarity to which they must adjust to be able to grow. To cope with increases in external osmotic pressure caused, for example, by NaCl, organisms use several strategies that involve the accumulation of inorganic solutes, including KCl, organic compatible solutes, and a mixture of potassium and negatively charged organic solutes (3, 13, 20). Many compatible solutes of thermophiles and hyperthermophiles are different from the compatible solutes of mesophiles; for example, di-myo-inositol phosphate appears to be found only in hyperthermophilic organisms with optimal growth temperatures greater than 80°C, although recently di-myo-inositol phosphate has been identified in the thermophilic and extremely radiation-resistant bacterium Rubrobacter xylanophilus (9). Mannosylglycerate (MG) has also been considered an archetypal compatible solute of thermophilic and hyperthermophilic bacteria and archaea (22), although the discovery of genes for its synthesis in the mesophilic bacterium “Dehalococcoides ethenogenes” contradicts this conclusion (8). On the other hand, glucosylglycerate (GG), a chemical analogue of MG, which has been found in the cyanobacterium Agmenellum quadruplicatum, in the gamma-proteobacterium Erwinia chrysanthemi, and in Methanohalophilus portucalensis, appeared to be a very rare compatible solute with a restricted distribution among mesophilic bacteria and archaea (5, 11, 14) until GG was unexpectedly identified in the thermophilic bacterium Persephonella marina, where it appeared to act as a compatible solute (H. Santos, personal communication). GG has also been detected in a polysaccharide from mycobacteria and in the polar head of a glycolipid in Nocardia otitidis-caviarum (15, 18).
The biosynthesis of GG in the archaeon Methanococcoides burtonii and in P. marina was recently characterized (5, 6). The pathway involved two steps, in which glucosyl-3-phosphoglycerate synthase (GpgS) catalyzes the conversion of GDP-glucose and d-3-phosphoglycerate into glucosyl-3-phosphoglycerate, which is then converted to GG by glucosyl-3-phosphoglycerate phosphatase (GpgP) (5). This pathway is very similar to one of two known pathways for the synthesis of MG in several organisms; in fact, GpgP utilizes glucosyl-3-phosphoglycerate or mannosyl-3-phosphoglycerate as a substrate (5). The gpgS and gpgP genes of P. marina are located in an operon-like structure along with genes encoding a putative glycerate kinase/dehydrogenase and a putative glycosyltransferase that are located between gpgS and gpgP. The putative glycosyltransferase gene was expressed in Escherichia coli, and the recombinant product catalyzed the synthesis of GG from ADP-glucose and d-glycerate. This pathway is mechanistically similar to the single-step pathway for the synthesis of MG involving the direct conversion of GDP-mannose and d-glycerate, which has been found only in Rhodothermus marinus (16).
To understand the role of GG at high temperatures and the regulatory mechanisms involved in the adaptation of P. marina to salt or temperature stress, elucidation of the metabolic pathways is essential. In this study we determined an alternative pathway for GG synthesis in the thermophilic bacterium P. marina and characterized the enzyme glucosylglycerate synthase.
MATERIALS AND METHODS
Strains and identification, cloning, and functional expression of ggs.
P. marina strain EX-H1T (= DSM 14350T) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany. E. coli strain BL21-DE(3) was used as the host for expression vector pET30a and for protein production. Chromosomal DNA of P. marina was isolated as previously described (6). Based on the P. marina genome sequence (http://www.tigr.org/tdb/mdb/mdbinprogress.html), a gene encoding a glycosyltransferase with unknown specificity was found in the vicinity of the gpgS and gpgP genes involved in the two-step pathway for the synthesis of GG (6). The glycosyltransferase gene was amplified with a PCR SuperMix High-Fidelity kit (Invitrogen) using primers based on the 5′ and 3′ ends of the gene and designed to include NdeI and XhoI restriction sites. The cloning and expression procedures were performed as previously described (5). Cells were harvested by centrifugation (4,000 × g, 10 min, 4°C), and the pellets were suspended in 50 mM Tris-HCl (pH 7.5) containing 5 mM MgCl2, 10 μg/ml DNase I, and a protease inhibitor cocktail (Roche). The E. coli cells were disrupted by sonication, which was followed by centrifugation (15,000 × g, 20 min, 4°C) to remove cell debris. The supernatants were filtered through a 0.22-μm-pore-size filter and used for purification of the recombinant Ggs.
Protein purification.
Partial denaturation of an E. coli cell extract containing the recombinant Ggs was carried out at 70°C for 10 min. Purification of Ggs was performed by fast protein liquid chromatography; the extract was loaded onto a Q-Sepharose column (Hi-Load 16/10) equilibrated with 20 mM Tris-HCl (pH 7.5), and elution was carried out with a linear NaCl gradient (0.0 to 1.0 M). The active fractions were collected and dialyzed against the same buffer, the protein content was determined (2), and the purity was judged by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (results not shown).
Enzyme assays for purification of Ggs.
The reaction mixture (50 μl) used to detect the activity of the recombinant Ggs protein in cell extracts and during purification contained 25 μl of sample, 5 mM ADP-glucose, and 5 mM d-glycerate (hemicalcium salt) in 25 mM Tris-HCl (pH 6.5) with 10 mM MgCl2. The mixture was incubated at 70°C for 15 min and cooled on ice-ethanol. Reaction products were loaded onto thin-layer chromatography plates and visualized as previously described (7).
Enzyme assays for characterization of Ggs.
The assay used for characterization of Ggs was based on quantification of nucleoside diphosphates (NDP) released during the conversion of NDP-glucose and d-glycerate to GG. Reactions were stopped at different times by cooling on ice-ethanol, and the amount of NDP released was determined at 340 nm after incubation of the sample with 2.2 U of pyruvate kinase, 2.8 U of lactate dehydrogenase, 0.3 mM NADH, and 2 mM phosphoenolpyruvate (all obtained from Sigma) in a 1-ml (total volume) mixture for 30 min at 15°C (19).
Prior to the experiments we evaluated the purity of the ADP-glucose (Sigma) by quantifying the free ADP using the protocol described above. We determined that the level of purity of the ADP-glucose was 87%, although the supplier indicated that the lot used was 93 to 95% pure. For the substrates UDP-glucose and GDP-glucose we used levels of purity of 100 and 98.5%, respectively, as indicated by the supplier. To determine the possible degradation of ADP-glucose into ADP and glucose or the degradation of ADP to Pi during reactions, control reaction mixtures were incubated without enzyme at 70 or 90°C for up to 15 min and in the buffers described below at pH values ranging from 3.5 to 9.9 for up to 3 h, but no further degradation was observed. All experiments were performed in triplicate. The GG produced in the reaction was also quantified by high-performance liquid chromatography with an Aminex HPX-87H column (300 by 7.8 mm; Bio-Rad) equilibrated with 5 mM H2SO4. A pure GG standard was kindly provided by Helena Santos (ITQB, Oeiras, Portugal).
The substrate specificity of Ggs was examined by combining several sugar nucleotides (ADP-, GDP-, TDP-, and UDP-glucose, UDP-galactose, GDP-fucose, GDP-mannose, and ADP-ribose) and the sugar phosphates mannose-6-phosphate, glucose-1-phosphate, glucose-6-phosphate, trehalose-6-phosphate, and fructose-6-phosphate with the three-carbon compounds d-3-phosphoglycerate, d-2-phosphoglycerate, d-glycerate, l-glycerate, glycerol, and glycerol-3-phosphate at 70°C in 25 mM N-(2-acetamido)-2-aminoethanesulfonic acid (ACES) (pH 6.5) containing 20 mM MgCl2. The products were visualized by thin-layer chromatography.
The cation dependence of Ggs was examined by incubating samples with the appropriate substrates at a concentration of 5 mM in 25 mM ACES buffer (pH 6.5) at 70°C along with the chloride salt of Ba2+, Ca2+, Co2+, Fe3+, Li+, Mg2+, Mn2+ Sr2+, or Zn2+ at a concentration of 0.02 to 50 mM or in the presence of 0.5 to 5 mM of EDTA. The effect of pH on the activity of Ggs was determined at 70°C by using pH values between 3.5 and 9.9 in 20 mM MgCl2, 5 mM ADP-glucose, 5 mM d-glycerate, and 25 mM acetate buffer, morpholineethanesulfonic acid (MES), bis-Tris-propane (BTP), ACES, N-tris(hydroxymethyl)methyl-3-aminopropanesulfonic acid (TAPS), and N-cyclohexyl-3-aminopropanesulfonic acid (CAPS). The pHs were determined at room temperature, and the pHs at different temperatures were calculated using pKa/°C conversion factors of −0.002 for acetate buffer, −0.011 for MES, −0.017 for BTP, −0.03 for Tris-HCl buffer, −0.020 for ACES, 0.027 for TAPS, and −0.032 for CAPS (6).
The temperature profile of Ggs was determined at temperatures between 40 and 95°C using reaction mixtures containing 5 mM ADP-glucose and 5 mM d-glycerate in 25 mM ACES buffer (pH 6.5) with 20 mM MgCl2. Enzyme stability was determined at 70, 85, and 100°C by incubating 100 μl of an enzyme solution (42 μg/ml) in 25 mM ACES. At appropriate times, samples were withdrawn and cooled on ice, and the residual activities were determined at 60°C under the conditions described above.
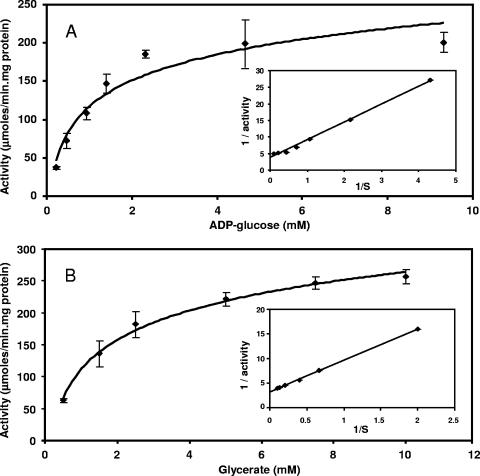
The kinetic parameters for Ggs with ADP-, GDP-, and UDP-glucose were determined at 70°C by using reaction mixtures containing 0.23 to 9.30 mM ADP-glucose, 1.48 to 9.85 mM GDP-glucose, or 2.5 to 20 mM UDP-glucose and 5 mM d-glycerate in 25 mM ACES (pH 6.5) with 20 mM MgCl2. The kinetic parameters for d-glycerate were determined by using 4.65 mM ADP-glucose and 0.25 to 10 mM d-glycerate in 25 mM ACES (pH 6.5) with 20 mM MgCl2. Km and Vmax values were obtained from Hanes plots.
GG hydrolysis by Ggs was assessed by determining the rate of glycerate formation by high-performance liquid chromatography as described above. The reaction mixtures contained 20 mM ADP, GDP, UDP, or ATP, 20 mM MgCl2, and several concentrations of GG (5 to 20 mM) in 25 mM ACES (pH 6.5) and were incubated at 70°C. The reactions were started by adding 0.5 μg of pure Ggs and were stopped by incubation on ice. A control reaction mixture contained only 20 mM GG in 25 mM ACES and 20 mM MgCl2.
Determination of the molecular mass of the recombinant enzyme.
The molecular mass of the recombinant Ggs protein was estimated by gel filtration on a Superdex 200 column at room temperature equilibrated with BTP (pH 8.0), using the molecular mass standards albumin (67 kDa), aldolase (158 kDa), catalase (232 kDa), ferritin (440 kDa), and thyroglobulin (669 kDa); blue dextran 2000 (GE Healthcare) was used to determine the void volume.
RESULTS
Identification of the gene coding for Ggs.
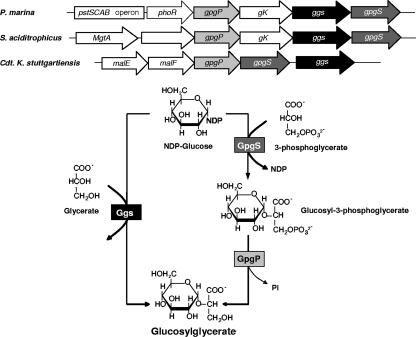
The glucosylglycerate synthase gene (ggs), annotated as a putative glucosyltransferase gene in the draft genome sequence (http://www.tigr.org/tdb/mdb/mdbinprogress.html), is located immediately upstream from the P. marina gpgS gene and contains 1,254 bp coding for a polypeptide with 417 amino acids and a calculated molecular mass of 48.4 kDa (Fig. 1). The Ggs sequence exhibited significant levels of amino acid identity with only a few sugar transferases, including those found in Synthrophus aciditrophicus (62%), in “Candidatus Kuenenia stuttgartiensis” (46%), in Thermotoga maritima and Thermotoga petrophila (42%), in Pyrococcus spp. (42 to 44%), and in Syntrophobacter fumaroxidans (38%). In P. marina the ggs gene is part of an operon-like structure containing the gpgS and gpgP genes for the two-step pathway for synthesis of GG, a putative glycerate kinase/dehydrogenase gene, and a phoR gene encoding a putative histidine kinase that is presumably involved in the regulation of the operon for a high-affinity phosphate (Pi)-specific transporter system (Fig. 1). The operon-like structure containing the ggs gene of S. aciditrophicus also includes the genes for the two-step pathway for GG synthesis and the putative glycerate kinase/dehydrogenase gene. “Candidatus Kuenenia stuttgartiensis” also contains adjacent ggs, gpgS, and gpgP genes, but the glycerate kinase/dehydrogenase gene is not present in this structure. In T. maritima, Pyrococcus spp., and S. fumaroxidans the genes for the two-step pathway for GG are not present in the genomes. The Ggs protein from P. marina and the functionally related Mgs protein from R. marinus exhibit 27% amino acid identity.
FIG. 1.
Proposed pathways for the synthesis of GG in P. marina and genomic organization and flanking regions of the GG operon in P. marina, S. aciditrophicus, and “Candidatus Kuenenia stuttgartiensis.” The arrows represent genes and their directions. pstSCAB, genes encoding a phosphate uptake system; phoR, gene encoding a putative PhoR histidine kinase; gpgP, gene encoding glucosyl-3-phosphoglycerate phosphatase; gK, gene encoding a putative glycerate kinase/dehydrogenase; ggs, gene encoding glucosylglycerate synthase; gpgS, gene encoding glucosyl-3-phosphoglycerate synthase; MgtA, gene encoding cation-transporting ATPase; malE, gene encoding a putative trehalose/maltose binding protein; malF, gene encoding a putative trehalose/maltose transport protein.
Cloning, functional expression of ggs from P. marina in E. coli, and purification of the recombinant Ggs protein.
PCR amplification of ggs yielded products that were the expected sizes. The gene was expressed in E. coli and functionally characterized as a glucosylglycerate synthase (Ggs) catalyzing the condensation of ADP-glucose and d-glycerate into GG in one step (Fig. 1). Activity assays carried out with E. coli cell extracts harboring Ggs showed that GG was synthesized, and the negative control extracts from E. coli with the empty vector did not synthesize GG. Extra bands at about 48 kDa were visualized on the SDS-PAGE gel with cell extracts of Ggs-containing E. coli clones induced with isopropyl-β-d-thiogalactopyranoside (IPTG). Heat treatment of a Ggs-containing cell extract followed by chromatography on a Q-Sepharose column resulted in purification of Ggs almost to homogeneity, as determined by SDS-PAGE (results not shown). During gel filtration chromatography the recombinant Ggs behaved like a tetramer with a calculated molecular mass of about 210 ± 3.0 kDa.
Catalytic properties of Ggs.
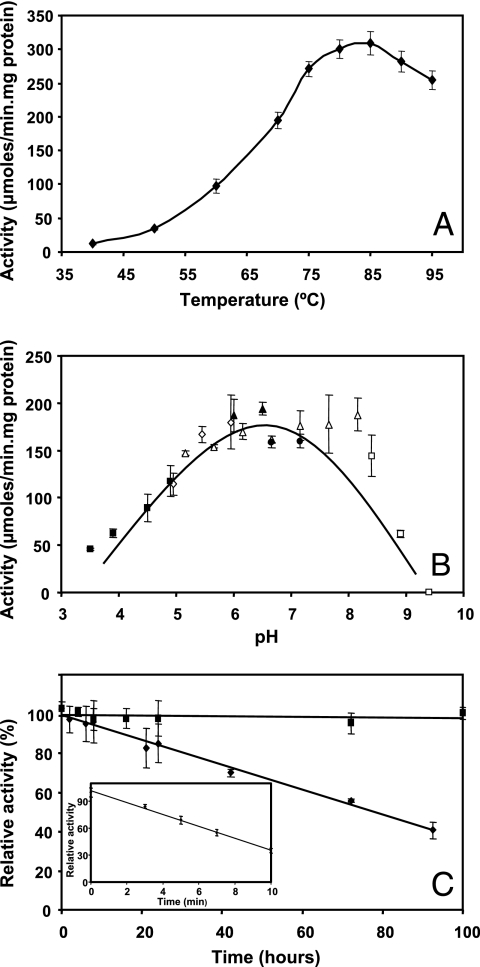
Of the sugar donors examined, ADP-glucose was the preferred substrate (284.3 ± 42.6 μmol/min·mg), but it could be partially replaced by UDP-glucose or GDP-glucose in decreasing order of efficiency (47.0 ± 3.7 and 27.4 ± 8.5 μmol/min·mg, respectively). Activity was not observed with any other sugar donor tested, and d-glycerate was the only acceptor for these glucosyl donors. Ggs exhibited Michaelis-Menten kinetics at 70°C (Fig. 2), and the Km values for the substrates were determined from double-reciprocal plots (Table 1). The Ggs was active at temperatures between 40 and 95°C, but the maximum activity was observed at temperatures between 80 and 85°C (Fig. 3A). In the pH range examined (pH 3.5 to 9.9) the activity of the enzyme at 70°C was maximal and nearly constant at values between pH 5.5 and 8.0 (Fig. 3B). The lack of activity after addition of EDTA to the reaction mixture indicated that the enzyme was strictly dependent on divalent cations. Of all the cations tested, only Mg2+ and Ca2+ (20 mM) strongly stimulated Ggs activity (results not shown). The other cations tested did not stimulate Ggs activity at any concentration; NaCl and KCl at concentrations of 0 to 100 mM and 0 to 500 mM, respectively, had no effect on Ggs activity (results not shown). The half-lives for thermal inactivation were 74 h at 85°C and 8 min at 100°C (Fig. 3C). Incubation of the enzyme for 1 week at 70°C did not affect the activity.
FIG. 2.
Dependence of the activity of recombinant Ggs on ADP-glucose (A) and d-glycerate (B). The insets show double-reciprocal plots of the rate versus the corresponding substrate concentration.
TABLE 1.
Substrate specificity of recombinant Ggs, determined at 70°C
| Fixed substrate | Varied substrate | Vmax (μmol/min·mg) | Km |
|---|---|---|---|
| ADP-glucose | d-Glycerate | 341.9 ± 51.3 | 2.2 ± 0.5 |
| d-Glycerate | ADP-glucose | 284.3 ± 42.6 | 1.5 ± 0.3 |
| d-Glycerate | GDP-glucose | 27.4 ± 8.5 | 7.1 ± 2.6 |
| d-Glycerate | UDP-glucose | 47.0 ± 3.7 | 6.8 ± 1.4 |
FIG. 3.
Temperature (A) and pH (B) dependence of recombinant Ggs activity. The activity was determined at temperatures between 40 and 95°C, and the pH dependence was determined at pHs between 3.5 and 9.9 in the following buffers: acetate buffer for pH 3.5 to 4.9 (▪), MES for pH 5.0 to 6.0 (⋄), BTP for pH 5.2 to 8.2 (▵), ACES for pH 6.0 to 6.5 (▴), TAPS for pH 6.7 to 7.2 (•), and CAPS for pH 8.4 to 9.9 (□). The data are the means of three independent experiments. (C) Thermostability of recombinant Ggs at 70°C (▪) and 85°C (⧫). The inset shows the thermostability of the enzyme at 100°C. The half-lives for thermal inactivation of Ggs at 85 and 100°C were 74 h and 8 min, respectively.
Formation of very low levels of glycerate was observed in reaction mixtures containing GG and ADP (results not shown). A measurable rate of reaction (5.8 μmol/min·mg) required 20 mM GG, indicating that the Km for this substrate was higher and that the formation of GG was strongly favored.
DISCUSSION
GG was originally detected in the cyanobacterium A. quadruplicatum (14). Recently, GG was also found in E. chrysanthemi, where it behaved like the major compatible solute during osmotic adjustment to high salinity in low-nitrogen medium (11). Trace amounts of this organic solute were also detected in a mutant strain of the halophilic bacterium Halomonas elongata and in the methanogen Methanohalophilus portucalensis strain FDF-1, where its role remains in doubt (4, 21). However, characterization of a pathway for GG synthesis was first reported in the cold-adapted methanogenic archaeon M. burtonii, in which accumulation of GG still has not been demonstrated (5). This pathway involves the enzymes GpgS and GpgP, which catalyze the synthesis and dephosphorylation of glucosyl-3-phosphoglycerate to GG, respectively. Identification of the genes encoding these enzymes allowed us to detect homologs in a large number of genomes, all of which were genomes of mesophilic or psychrophilic organisms (5). Surprisingly, these genes were also found in the genome of the thermophilic bacterium P. marina (6), in which accumulation of GG was recently demonstrated (Santos, personal communication).
In this paper we propose an alternative route for the synthesis of GG in P. marina via direct condensation of NDP-glucose and d-glycerate instead of d-3-phosphoglycerate (Fig. 2) by glucosylglycerate synthase (Ggs). The presence of two pathways for the synthesis of a compatible solute in the same organism is not unprecedented. For example, R. marinus has two different pathways for MG synthesis, a single-step reaction that converts GDP-mannose and d-glycerate to MG via mannosylglycerate synthase (Mgs) and a two-step pathway in which GDP-mannose and d-3-phosphoglycerate are the substrates for mannosyl-3-phosphoglycerate synthase (MpgS) for production of mannosyl-3-phosphoglycerate, which is dephosphorylated by mannosyl-3-phosphoglycerate phosphatase (MpgP) to MG (7, 16). The two pathways could hypothetically be implicated in different metabolic functions, one involving the synthesis of MG and the other involving catabolism of MG; however, hydrolysis of MG was not observed with Mgs (16). Hypothetically, the catabolism of GG could also be favored in P. marina by Ggs, but we did not observe significant involvement of this enzyme in the hydrolysis of this glucose derivative.
Characterization of Mgs from R. marinus unequivocally demonstrated that the α configuration of the substrate GDP-mannose was maintained during synthesis of MG, leading to creation of glycosyltransferase family GT78 to accommodate this “retaining” enzyme (http://afmb.cnrs-mrs.fr/CAZY/fam/GT78.html) (12, 16). The α configuration of the GG identified in P. marina (Santos, personal communication) strongly suggests that there is a “retaining” mechanism for Ggs, as well as for the enzyme of the two-step pathway, GpgS. The level of sequence identity (27%) between this Ggs and the Mgs from R. marinus also indicates that this enzyme belongs to the GT78 family.
Ggs is able to use ADP-glucose, as well as GDP-glucose and UDP-glucose, with significant efficiency, and this is not common among glucosyltransferases (16). This characteristic is also shared by GpgS from P. marina, which exhibits considerable activity with UDP-glucose, ADP-glucose, GDP-glucose, and TDP-glucose (6). Broad substrate specificity may reflect an absolute requirement for GG in P. marina, as observed for the synthesis of trehalose in mycobacteria, which is used for several purposes (17). On the other hand, the GpgS from M. burtonii was strictly dependent on GDP-glucose (5). Furthermore, MG is synthesized solely from GDP-mannose, although the Mgs protein can use trace levels of other NDP-sugar donors (10, 16). The weak intrinsic thermostability of GpgS, in contrast to the extremely high thermostability of Ggs, could indicate that the former enzyme requires extrinsic stabilizing molecules or that GpgS is functional at lower temperatures in vivo, while Ggs could be active at temperatures above the optimum temperature for the growth. However, the thermostabilities in vitro of many enzymes from thermophiles are extremely variable, and no conclusions can be drawn at this stage (23).
The presence of two pathways for the synthesis of GG in P. marina could mean that there is greater flexibility for the regulation of GG synthesis in response to stress. In R. marinus, for example, differential expression of the pathways for the synthesis of MG occurs when the organism is challenged with either salt or thermal stress (1). Moreover, the presence in P. marina of an operon encoding a phosphate uptake system (pstSCAB) upstream of an operon-like structure containing phoR, gpgP, gK, ggS, and gpgS, where phoR may be involved in the regulation of the former operon, leads to the hypothesis that Ggs is involved in the synthesis of GG when the levels of phosphate are low, since there is no need to synthesize a phosphorylated intermediate (6). The operon-like organization of the genes for both pathways indicates that they are under the control of the same promoter, suggesting that regulation is likely to occur at the posttranscriptional level (6). Genome sequencing projects have revealed that there is broad distribution of the genes of the two-step pathway for GG synthesis, but operon-like structures containing the genes for both pathways were found only in S. aciditrophicus and “Candidatus Kuenenia stuttgartiensis.” P. marina is the only one of these organisms in which the GG gene cluster seems to be associated with phosphate uptake genes (6).
The hyperthermophilic archaea Pyrococcus furiosus and Pyrococcus horikoshii, which respond to increases in the salinity of the medium by accumulating primarily MG (9), also have the gene coding for Ggs. Preliminary experiments have shown that recombinant Ggs from P. horikoshii synthesizes GG from ADP-glucose and d-glycerate in vitro. Since free GG has never been detected in these organisms, it is possible that it may be synthesized and incorporated into an unknown macromolecule.
The elucidation of an additional pathway for the synthesis of GG in P. marina and the characterization of the genetic structure and the biochemical properties of the key enzyme represent an additional step toward understanding GG synthesis and regulation in the adaptation of thermophilic bacteria to stress.
Acknowledgments
This work was supported by Fundaçcão para a Ciência e a Tecnologia (FCT), Portugal, and by FEDER (project A005/2005, action V.5.1). C. Fernandes and N. Empadinhas acknowledge scholarships from FCT (SFRH/BD/19599/2004 and SFRH/BPD/14828/2003).
We thank Helena Santos (ITQB, Oeiras, Portugal) for providing the information concerning the accumulation of compatible solutes in P. marina and for supplying pure GG.
Footnotes
Published ahead of print on 16 March 2007.
REFERENCES
- 1.Borges, N., J. D. Marugg, N. Empadinhas, M. S. da Costa, and H. Santos. 2004. Specialized roles of the two pathways for the synthesis of mannosylglycerate in osmoadaptation and thermoadaptation of Rhodothermus marinus. J. Biol. Chem. 279:9892-9898. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Brown, A. D. 1976. Microbial water stress. Bacteriol. Rev. 40:803-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cánovas, D., N. Borges, C. Vargas, A. Ventosa, J. J. Nieto, and H. Santos. 1999. Role of N-γ-acetyldiaminobutyrate as an enzyme stabilizer and an intermediate in the biosynthesis of hydroxyectoine. Appl. Environ. Microbiol. 65:3774-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa, J., N. Empadinhas, L. Gonçcalves, P. Lamosa, H. Santos, and M. S. da Costa. 2006. Characterization of the biosynthetic pathway of glucosylglycerate in the archaeon Methanococcoides burtonii. J. Bacteriol. 188:1022-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa, J., N. Empadinhas, and M. S. da Costa. 2007. Glucosylglycerate biosynthesis in the deepest lineage of bacteria. Characterization of the thermophilic GpgS and GpgP from Persephonella marina. J. Bacteriol. 189:1648-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Empadinhas, N., J. D. Marugg, N. Borges, H. Santos, and M. S. da Costa. 2001. Pathway for the synthesis of mannosylglycerate in the hyperthermophilic archaeon Pyrococcus horikoshii. Biochemical and genetic characterization of key enzymes. J. Biol. Chem. 276:43580-43588. [DOI] [PubMed] [Google Scholar]
- 8.Empadinhas, N., L. Albuquerque, J. Costa, S. H. Zinder, M. A. Santos, H. Santos, and M. S. da Costa. 2004. A gene from the mesophilic bacterium Dehalococcoides ethenogenes encodes a novel mannosylglycerate synthase. J. Bacteriol. 186:4075-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Empadinhas, N., and M. S. da Costa. 2006. Diversity and biosynthesis of compatible solutes in hyper/thermophiles. Int. Microbiol. 9:199-206. [PubMed] [Google Scholar]
- 10.Flint, J., E. Taylor, M. Yang, D. N. Bolam, L. E. Tailford, C. Martinez-Fleites, E. J. Dodson, B. G. Davis, H. J. Gilbert, and G. J. Davies. 2005. Structural dissection and high-throughput screening of mannosylglycerate synthase. Nat. Struct. Mol. Biol. 12:608-614. [DOI] [PubMed] [Google Scholar]
- 11.Goude, R., S. Renaud, S. Bonnassie, T. Bernard, and C. Blanco. 2004. Glutamine, glutamate, and alpha-glucosylglycerate are the major osmotic solutes accumulated by Erwinia chrysanthemi strain 3937. Appl. Environ. Microbiol. 70:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrissat, B., and P. M. Coutinho. 2001. Classification of glycoside hydrolases and glycosyltransferases from hyperthermophiles. Methods Enzymol. 330:183-201. [DOI] [PubMed] [Google Scholar]
- 13.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170:319-330. [DOI] [PubMed] [Google Scholar]
- 14.Kollman, V. H., J. L. Hanners, R. E. London, E. G. Adame, and T. E. Walker. 1979. Photosynthetic preparation and characterization of ′3C-labeled carbohydrates in Agmenellum quadruplicatum. Carbohydr. Res. 73:193-202. [Google Scholar]
- 15.Lee, Y. C., and C. E. Ballou. 1964. 6-O-methyl-d-glucose from mycobacteria. J. Biol. Chem. 239:3602-3603. [PubMed] [Google Scholar]
- 16.Martins, L. O., N. Empadinhas, J. D. Marugg, C. Miguel, C. Ferreira, M. S. da Costa, and H. Santos. 1999. Biosynthesis of mannosylglycerate in the thermophilic bacterium Rhodothermus marinus. Biochemical and genetic characterization of a mannosylglycerate synthase. J. Biol. Chem. 274:35407-35414. [DOI] [PubMed] [Google Scholar]
- 17.Pan, Y. T., J. D. Carroll, and A. D. Elbein. 2002. Trehalose-phosphate synthase of Mycobacterium tuberculosis: cloning, expression and purification of the recombinant enzyme. Eur. J. Biochem. 269:6091-6100. [DOI] [PubMed] [Google Scholar]
- 18.Pommier, M. T., and G. Michel. 1981. Structure of 2′,3′-di-O-acyl-α-d-glucopyranosyl-(1→2)-d-glyceric acid, a new glycolipid from Nocardia caviae. Eur. J. Biochem. 118:329-333. [DOI] [PubMed] [Google Scholar]
- 19.Qu, Q., S. J. Lee, and W. Boos. 2004. TreT, a novel trehalose glycosyltransferring synthase of the hyperthermophilic archaeon Thermococcus litoralis. J. Biol. Chem. 279:47890-47897. [DOI] [PubMed] [Google Scholar]
- 20.Roberts, M. F. 2005. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson, D. E., M. C. Lai, R. P. Gunsalus, and M. F. Roberts. 1992. Composition, variation, and dynamics of major osmotic solutes in Methanohalophilus strain FDF1. Appl. Environ. Microbiol. 58:2438-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos, H., and M. S. da Costa. 2002. Compatible solutes of organisms that live in hot saline environments. Environ. Microbiol. 4:501-509. [DOI] [PubMed] [Google Scholar]
- 23.Sterner, R., and W. Liebl. 2001. Thermophilic adaptation of proteins. Crit. Rev. Biochem. Mol. Biol. 36:39-106. [DOI] [PubMed] [Google Scholar]