Abstract
The fepB gene encodes a periplasmic binding protein that is essential for the uptake of ferric enterobactin by Escherichia coli. Its transcription is regulated in response to iron levels by the Fur repressor. The fepB transcript includes a 217-nucleotide leader sequence with several features suggestive of posttranscriptional regulation. To investigate the fepB leader for its contribution to fepB expression, defined deletions and substitution mutations in the leader were characterized using fepB-phoA translational fusions. The fepB leader was found to be necessary for maximal fepB expression, primarily due to the influence of an AU-rich translational enhancer (TE) located 5′ to the Shine-Dalgarno sequence. Deletions or substitutions within the TE sequence decreased fepB-phoA expression fivefold. RNase protection and in vitro transcription-translation assays demonstrated that the TE augmented translational efficiency, as well as RNA levels. Moreover, primer extension inhibition assays showed that the TE increases ribosome binding. In contrast to the enhancing effect of the TE, the natural fepB GUG start codon decreased ribosome binding and reduced fepB expression 2.5-fold compared with the results obtained with leaders bearing an AUG initiation codon. Thus, the TE-GUG organization in fepB results in an intermediate level of expression compared to the level with AUG, with or without the TE. Furthermore, we found that the TE-GUG sequence is conserved among the eight gram-negative strains examined that have fepB genes, suggesting that this organization may provide a selective advantage.
The fepB gene encodes a periplasmic binding protein that is essential for the uptake of ferric enterobactin by Escherichia coli (7, 16, 37). Its transcription is repressed under high-iron conditions by the ferric uptake regulator, Fur (7, 16). During iron starvation stress, Fur repression is relieved, and the fepB message is produced. The fepB transcript includes a 217-nucleotide (nt) leader sequence (7, 16).
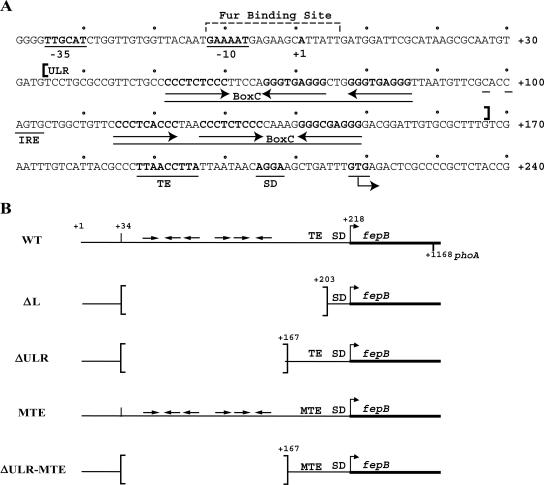
In addition to the transcriptional control of FepB synthesis, features of the fepB leader sequence are suggestive of posttranscriptional regulation. For example, the upstream leader region (ULR) of the fepB transcript contains two BoxC elements, which are present in inverted orientation (Fig. 1A). BoxC elements are extragenic sequences dispersed throughout the E. coli chromosome, and, although their locations suggest that they might influence gene expression, their role in gene regulation is not clear (1). The BoxC elements within the fepB leader might generate various secondary structures that could modulate fepB gene expression. Also within the ULR is a putative iron regulatory element (IRE), which was proposed (6) to regulate FepB expression posttranscriptionally in response to the oxidative state of the IRE-binding protein, aconitase (51).
FIG. 1.
(A) Nucleotide sequence of fepB leader region. The −10 and −35 regions and Fur binding site were defined previously (7). The transcriptional start site is designated +1, and the sequence of the nontemplate strand from position −40 to position +240 is shown. Arrows indicate the orientations of BoxC repeats within each BoxC element indicated by a line. A potential IRE is also underlined, and the ULR (positions +34 to +167) is indicated by brackets. The TE, SD sequence, and GUG start codon are underlined. (B) Schematic representation of fepB leader constructs, including WT, leader deletion (ΔL), deleted ULR (ΔULR), and MTE constructs. Bent arrows indicate fepB start codons. Horizontal arrows indicate the positions and orientations of BoxC repeats. Brackets indicate deletions.
The downstream region of the fepB leader, the translation initiation region (TIR), also has putative regulatory features. For instance, the fepB coding sequence begins with a GUG start codon. GUG, which is the initiation codon for only about 8% of E. coli genes (32), is not used as efficiently as AUG to initiate translation (13, 18, 20, 32). Thus, its presence might reduce FepB levels. The fepB TIR also has an AU-rich sequence 5′ of the Shine-Dalgarno (SD) sequence. Such AU-rich sequences have been shown to enhance translation (4, 20, 29, 35, 36, 55) by increasing the interaction with the ribosomal S1 protein (5, 28, 41).
To discern the regulatory features imparted by elements within the fepB leader sequence, we investigated the function of the 217-nt fepB leader, with an emphasis on the TIR. We found that the fepB ULR does not play a role in fepB regulation, nor is it conserved among fepB homologs. However, our results demonstrate that the fepB leader is required for maximal fepB expression and that the TIR includes a functional AU-rich translational enhancer (TE) that promotes fepB expression by increasing ribosome affinity. In addition, the fepB TIR is conserved, suggesting that the arrangement of the TE and GUG may provide an ecological advantage over a typical AUG codon.
MATERIALS AND METHODS
Strains, plasmids, and primers.
E. coli strains CC118 (31) and BN4020(fur) (2) were used as background strains. pITS346, carrying a fepB-phoA translational fusion (7), is referred to here as pfp-WT (and the gene fusion is referred to as fp-WT). The fepB leader deletions and mutations were derived from pfp-WT (7) by oligonucleotide mutagenesis (23) and two-step PCR (3). pfl-WT, carrying a fepB-lacZ translational fusion (fl-WT), was constructed by insertion of a 279-bp fragment from pfp-WT (positions −54 to +225 relative to fepB transcriptional position +1) into the EcoRI/BamHI site of the translational fusion vector pRS414 (45). Other leader constructs were cloned similarly, resulting in fepB-lacZ translational fusions with lacZ in frame at the third FepB codon. The fl-WT derivative constructs were used to generate RNA templates for primer inhibition assays. Primer sequences are available upon request.
Genome sequence alignments.
Genome sequences were obtained from the National Center for Biotechnology Information for eight strains of gram-negative bacteria (accession numbers are indicated in parentheses), including E. coli K-12 (NC000913), E. coli O157:H7 (AP002552), E. coli O157:H7 EDL933 (AE005239), E. coli CFT073 (AE016757), Salmonella enterica serovar Typhimurium LT2 (AE008723), S. enterica serovar Typhi (AL627267), Shigella flexneri SA101 (M63306), and S. flexneri 2a (NC004337). Alignments were constructed using the “bestfit” and “align” functions of the GCG Wisconsin Package.
Growth conditions.
Overnight cultures of E. coli K-12 strain CC118 carrying pfp-WT or pfl-WT derivatives were subcultured 1:50 into LB medium (34) containing 50 μg/ml ampicillin and grown at 37°C to the early logarithmic growth phase. Cultures were then split, and the available iron concentration in one culture was significantly decreased by addition of an iron chelator, 2,2′-dipyridyl, to a final concentration of 200 μM. The iron concentration in the other culture was supplemented with adding FeSO4 and sodium citrate to final concentrations of 20 μM and 10 mM, respectively. Cell cultures were incubated for an additional 30 min and then assayed for phosphatase activity or harvested for RNA isolation.
Phosphatase assays.
Cultures carrying fepB-phoA constructs were assayed for alkaline phosphatase activity as described previously (31), with the following modification. Samples were centrifuged for 5 min at 12,000 × g before the optical density at 600 nm (OD600) was determined. This additional step removed cell debris, allowing elimination of the absorbance measurement used for correction of light scatter (1.75 × A550) and providing more consistent results (6).
RNA isolation and RNase protection.
Bacterial cultures were harvested, and pellets were immediately resuspended in 500 μl of resuspension buffer (0.02 M Na acetate [pH 5.5], 0.5% SDS, 1 mM EDTA), which was followed by addition of an equal volume of 60°C phenol (pH 4.3). The samples were vortexed briefly and incubated at 60°C for 5 min. The aqueous phase was removed and subjected to another hot phenol extraction and one or more chloroform extractions as needed to produce clean samples. RNA were then precipitated with ammonium acetate and ethanol and resuspended in 50 μl of distilled H2O (dH2O) previously treated with diethyl pyrocarbonate (DEPC). The RNA quality and concentration were determined first visually by agarose gel electrophoresis and then spectrophotometrically by determining the OD260.
RNase protection assays (RPA) were carried out as described previously (8) with radiolabeled 300-nt fepB and bla antisense probes generated by in vitro transcription (as described below) using the linearized templates pBluSK+-fepB and pBluSK+-bla, respectively (22). The bla probe was used as a normalization control for RPA. Approximately 0.5 μg of each probe was added to 10 μg of total RNA and annealed at 42°C. The RNA hybrids were digested using RNase A and RNase T1 at concentrations of 10 μg/ml and 50 ng/ml, respectively. Samples were then cleaned by phenol extraction, heated for 2 min at 95°C, and loaded onto an 8% polyacrylamide-urea gel. After electrophoresis the gels were exposed to an Imaging Screen-K (Kodak), and each image was scanned into a Molecular Imager FX (Bio-Rad). fepB RNA levels were normalized by comparison to the bla RNA levels in each lane and were quantified using the Bio-Rad Discovery Series Quantity One software.
Coupled in vitro transcription-translation.
In vitro transcription-translation was performed using an S30 Extract for Linear Templates kit and protocol of Promega. The pBESTluc linear template was used as a positive control. The experimental template was obtained by PCR using fp-WT derivative constructs as templates and primers IGH102 and IGH114 (which anneal from positions −159 to −184 and from positions +1159 to +1136, respectively, relative to the fepB transcriptional start). This resulted in a 1,343-bp DNA product that included all but the last four fepB codons. The expected protein product was 314 amino acids long, including three [35S]methionine residues. The PCR-generated DNA templates (wild type [WT], mutation of TE [MTE], AUG, and MTE-AUG) were quantified first spectophotometrically and then by Southern analysis. Samples were loaded onto a 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel and run at 30 mA for approximately 45 min. Dried gels were exposed to film or an Imaging Screen-K (Kodak). Each image was scanned into a Molecular Imager FX (Bio-Rad) and quantified using the Bio-Rad Discovery Series Quantity One software.
In vitro transcription.
In vitro transcription was used to generate a probe labeled with [α-32P]UTP for RPA and to produce RNA templates for toeprinting experiments. Each transcription reaction mixture (total volume, 20 μl) contained approximately 1 μg of DNA template, 2 μl of 10× T7 transcription buffer, 1 μl of RNasin, 2 μl of 5 mM nucleoside triphosphates, 1 μl of T7 polymerase (10 U), and DEPC-treated dH2O. For creation of labeled antisense probes, 8 μl of 50 μM [α-32P]UTP (4.4 × 107 dpm/μl) was added separately. The reaction mixtures were incubated for 90 min at 37°C, and then 10 U of RQ1 DNase was added, which was followed by an additional 30 min of incubation. The reaction mixtures were extracted with phenol-chloroform and then with chloroform, which was followed by precipitation of the nucleic acids with ammonium acetate and ethanol. RNA was resuspended in DEPC-treated dH2O, and OD260 values were used to determine the concentration of RNA.
Isolation of ribosomal subunits.
Ribosomal subunits were isolated from E. coli strain MRE600 by the method of Spedding (48). E. coli 30S subunits were prepared using a low-salt wash so that the S1 protein would not be lost (10, 50).
Toeprinting.
An RNA template for toeprinting was produced by in vitro transcription (see above), using fl-WT-derived construct DNA templates generated by PCR. The downstream primer, IGH144, annealed from positions +268 to +287 relative to the fepB transcriptional start, whereas the upstream primer, IGH141, included the T7 promoter sequence followed by the fepB transcriptional sequence from positions +1 to +23. In the resulting PCR products the fepB promoter was replaced by the T7 promoter. The pET-11b template, used as a positive control for toeprinting (not shown), was also generated by PCR, using primers IGH140 and IGH143. The 3′ end of IGH143 anneals to the pET-11b vector sequence, while the 5′ end is identical to that of IGH144. This design allowed the control RNA template to be made such that toeprinting could be done using the same primer that was used with the fl-WT templates. Toeprinting was carried out essentially as described by Hartz et al. (21). [γ-32P]ATP-end-labeled primer IGH144 was mixed with the RNA template and incubated for 3 min at 60°C. Samples were placed briefly in an ice bath before addition of tRNAfmet, deoxynucleoside triphosphates, and 30S subunits if indicated. The samples were incubated for 10 min at 37°C, and then 1.5 U of avian myeloblastosis virus reverse transcriptase was added to each sample to bring the reaction volume to 10 μl. The final concentrations were 0.04 μM primer, 0.04 μM template RNA, 0.375 mM deoxynucleoside triphosphate, 2.4 μM tRNAfmet, 0.4 μM 30S subunits, and 10 mM magnesium acetate. The extension reaction was allowed to proceed for 10 min at 37°C before 15 μl formamide loading buffer (90% formamide, 0.1% xylene cyanol, 0.1% bromophenol blue, 5 mM EDTA) was added and samples were heated to 90°C for 5 min. Samples were loaded onto a 6% polyacrylamide-urea gel and subjected to electrophoresis at 1,000 V for 30 to 45 min. Samples run on 8% polyacrylamide-urea gels provided better resolution of short fragments and were used to verify the length and number of toeprint bands (not shown). Gels were exposed to an Imaging Screen-K (Kodak), and each image was scanned into a Molecular Imager FX (Bio-Rad).
RESULTS
fepB leader contains an enhancer of fepB expression.
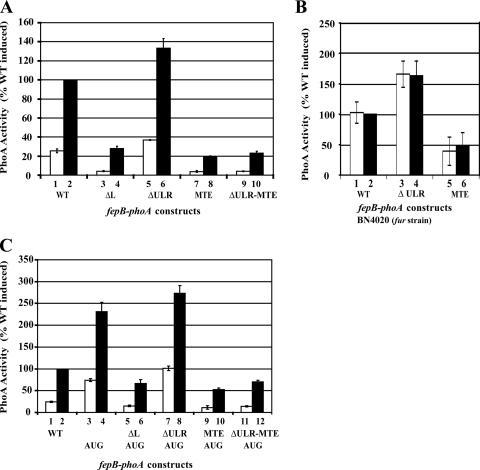
A fepB-phoA translational fusion (fp-WT), created previously to characterize regulation of transcription from the fepB-entC bidirectional promoter region (7), was first used to determine whether the 217-nt fepB leader RNA (Fig. 1A) has any influence on production of FepB. This fusion, which was previously called pITS346 (7), contains the reporter gene phoA fused in frame to the 3′ end of fepB (Fig. 1B). PhoA is active in the periplasm, making it a useful reporter for periplasmic proteins such as FepB (31). Expression of fp-WT is regulated by the transcriptional repressor Fur (ferric uptake regulator) in response to iron levels (7) (Fig. 2). In a fur null host strain, fp-WT is fully constitutive (Fig. 2B), further demonstrating that iron regulation of fepB is exerted by Fur at the transcriptional level.
FIG. 2.
Effect of leader RNA on fepB expression, measured by determining PhoA activity. The fepB leader fusion constructs were the WT, leader deletion (ΔL), deleted ULR (ΔULR), and MTE constructs. The bars indicate the averages of six experiments; the open bars indicate high-iron growth conditions, and the solid bars indicate low-iron growth conditions. The enzyme activity of each leader construct is expressed relative to the activity of the induced WT construct (WT solid bar). The error bars indicate standard deviations. (A) The fepB leader contains a TE. (B) fepB-phoA expression in the absence of the transcriptional repressor Fur. (C) Effect of AUG start codon on fepB-phoA expression.
To investigate whether the leader has a role in FepB expression, leader deletions were created in the pfp-WT fusion construct (Fig. 1B), and their effects on fepB-phoA expression were determined. The leader deletion, fp-ΔL, removed 169 nt of the leader, from nt +35 through +203 with respect to the site of transcription initiation (position +1), as determined previously (7). fp-ΔL retained 34 nt of the initial transcribed region, as well as the SD sequence and translation initiation codon (Fig. 1B), yet the mutation resulted in a threefold reduction in PhoA activity (Fig. 2A). A second leader deletion, fp-ΔULR, was created to specifically remove the BoxC repeats by deleting bases +35 through +167. In contrast to fp-ΔL, fp-ΔULR resulted in a modest increase in the level of PhoA compared to the level observed with the WT construct (Fig. 2A). However, neither of the mutants affected iron regulation. Comparison of the levels of PhoA observed with fp-ΔL and fp-ΔULR suggested that the TIR, in particular the 36-nt AU-rich region, is important for maximal fepB expression. This AU-rich region contains a sequence (5′-UUAACCUUA-3′) (Fig. 1) which is nearly identical the previously identified AU-rich TE sequence (5′-UUAACUUUA-3′) (36).
To examine whether the fepB TE sequence is responsible for improving expression, we changed this sequence to 5′-AUAGGGAAT-3′, and the resultant mutant TE fusion construct (fp-MTE) was assayed for PhoA activity. The TE mutation resulted in a fivefold reduction in the level of PhoA activity both in the context of the full-length leader, fp-MTE, and combined with the ULR deletion, fp-ΔULR-MTE (Fig. 2A). These results indicate that the fepB leader, in particular the TE sequence, is required for maximal fepB expression. In addition, none of the leader mutations altered fepB iron regulation (Fig. 2), which indicates that the leader RNA determines expression levels but does not control expression in response to iron levels.
Native start codon moderates FepB production.
The fepB coding sequence begins with a noncanonical GUG start codon, which is typically less efficient for translation than the canonical AUG start codon (13, 20, 32). To examine whether the GUG start codon negatively affects FepB expression, the GTG sequence was changed to an ATG sequence and the mutant construct, fp-AUG, was assayed for PhoA activity. As predicted, constructs with the AUG start codon had higher levels of expression than their GUG counterparts (Fig. 2C). However, the AUG substitution increased PhoA activity 2.5-fold, whereas the TE mutation resulted in a 5-fold decrease in PhoA activity. We concluded that the native fepB TIR, having both a TE and a GUG start codon, provides an intermediate level of fepB expression that is lower than the expression with fp-AUG but higher than the expression with an AUG start codon with no TE, such as fp-MTE-AUG or fp-ΔL-AUG (Fig. 2C). The start codon did not affect iron regulation.
fepB TIR, but not ULR, is conserved.
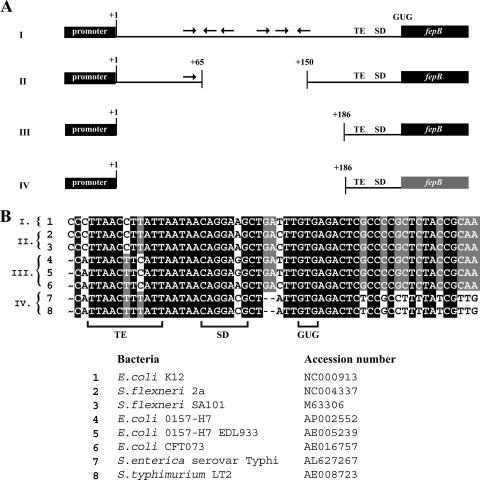
To investigate the evolutionary significance of the fepB leader, sequences from genomes that encode enterobactin systems and FepB orthologs were compared, and the level of sequence conservation was determined (Fig. 3). The fepB sequences of eight gram-negative strains (three virulent E. coli strains, two Shigella strains, two types of Salmonella, and the E. coli K-12 lab strain, which was considered the wild type in this study) were aligned and analyzed. This analysis revealed that the fepB TE and GUG start codon were conserved among these strains. All eight genomic sequences exhibited a high degree of identity in the entC-fepB intergenic region and the fepB coding sequence. However, the fepB sequences could easily be divided into four groups (Fig. 3A) based primarily on the conservation of the fepB leader sequence. E. coli K-12 (group I in Fig. 3) and Shigella sp. (group II in Fig. 3) were almost identical except for an 84-bp insertion/deletion within the BoxC element. The leader regions of the virulent E. coli strains (group III in Fig. 3) were also identical to the K-12 region except for a 187-bp insertion/deletion consisting of most of the fepB leader. The Salmonella genomes (group IV in Fig. 3A) were missing the same leader segments as the virulent E. coli strains, and the sequences that were present were somewhat less conserved in the Salmonella strains than in the other bacteria. A comparison of these sequences indicated that the ULR is not essential for fepB expression or a functional enterobactin system. Moreover, mutational analysis of the ULR, including the putative IRE, did not detect a role for this portion of the leader in fepB regulation (22). In contrast, the fepB TIR including the TE and GUG start codon is a highly conserved motif present in all of these species (Fig. 3B). Therefore, we focused on the TIR.
FIG. 3.
Genomic comparison of fepB leader regions. (A) Schematic representation of sequence data comparing the fepB leader regions of related bacteria. Genomic sequences of eight strains of gram-negative bacteria were grouped based on sequence conservation as follows: group I, E. coli K-12; group II, S. flexneri SA101 and S. flexneri 2a; group III, E. coli O157:H7, E. coli O157:H7 EDL933, and E. coli CFT073; and group IV, S. enterica serovar Typhimurium LT2 and S. enterica serovar Typhi. The black boxes and lines indicate nearly identical sequences. Increased variability in the fepB coding sequence of Salmonella sp. (group IV) is indicated by the gray fepB box. (B) Alignment of fepB TIR genome sequences. The TE sequence, SD sequence, and GUG start codon are indicated. Shading indicates the degree of conservation.
TE increases translational efficiency and RNA levels.
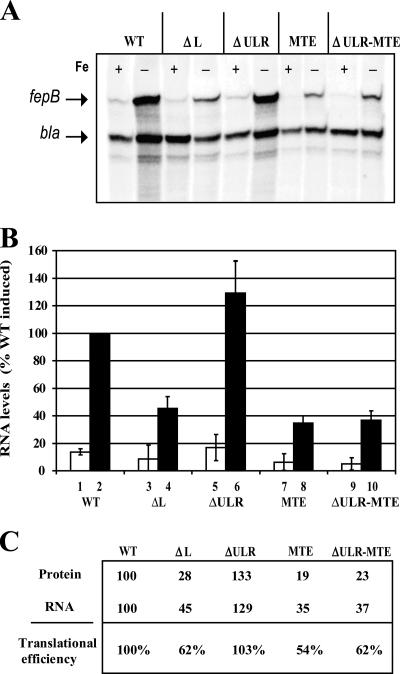
To determine whether the fepB enhancer increases fepB expression by enhancing translation initiation or by affecting the level of RNA, we compared the fusion protein levels (as assessed by PhoA activity) with the levels of steady-state fepB RNA for both WT and mutant constructs. RPA revealed that fepB mRNA levels were reduced threefold when the TE was mutated (Fig. 4A and B), whereas the PhoA assay demonstrated that the TE mutation resulted in a fivefold reduction in PhoA activity, suggesting that fusion protein levels decreased by a comparable amount. Thus, the reduction in fepB mRNA levels does not account completely for the observed decrease in fusion protein levels. When the translational efficiency was calculated by dividing the PhoA activity by the fepB mRNA level, the TE mutation accounted for a twofold decrease in fepB translational efficiency (Fig. 4C). This indicates that the TE sequence both increases translational efficiency and increases the level of fepB mRNA. This can be explained by the correlation between translation and RNA stability in bacteria (12, 26, 27, 33, 39). Transcripts that are efficiently translated are bound by ribosomes that help protect the message from degradation, thus maintaining higher steady-state mRNA levels. It is likely then that the reduced PhoA activity levels associated with the ΔL, MTE, and ΔULR-MTE mutations (Fig. 2) resulted from reduced ribosome binding, which made the mRNA more susceptible to degradation. The slight increase in protein expression in fp-ΔULR compared to the protein expression in fp-WT may also be explained by the ULR negatively affecting stability but not translation.
FIG. 4.
Effect of fepB leader RNA on translational efficiency. (A) RPA of total RNA isolated from cells containing fepB leader constructs. Bacteria were grown in medium supplemented with 2,2′-dipyridyl and in medium supplemented with FeSO4 plus sodium citrate to create low-iron (−) and high-iron (+) conditions, respectively. In addition to the fepB specific probe, a bla probe was also added to each reaction mixture as an internal control. (B) Quantitation for RPA was done with the Bio-Rad Quantity One software. Each lane was normalized using bla as a control. The open and solid bars indicate high- and low-iron growth conditions, respectively. The bars indicate the averages of three experiments, and the data are expressed relative to the WT level under inducing conditions (WT solid bar). The error bars indicate standard deviations. (C) Calculation of translational efficiency: relative protein level for each construct, as determined by PhoA activity, divided by the relative RNA level for each construct, as determined by RPA.
TE enhances protein synthesis in vitro.
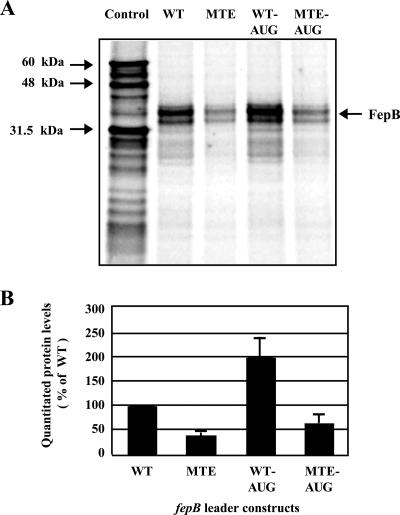
We next investigated FepB protein levels directly in vitro using transcription-translation reactions (Fig. 5). The in vitro S30 extract system included the components necessary for coupled transcription-translation, like that which occurs in the bacterial cytoplasm (17). A DNA template generated from the fp-WT, fp-MTE, fp-AUG, or fp-MTE-AUG construct was added to the reaction mixtures in the presence of [35S]methionine, yielding the expected FepB protein product, which migrated at a molecular mass of approximately 34 kDa on an SDS-PAGE gel (Fig. 5A).
FIG. 5.
FepB levels assessed by in vitro transcription-translation. (A) SDS-PAGE of [35S]Met-labeled FepB protein produced by in vitro transcription-translation reactions. The band at approximately 34 kDa is consistent with FepB from which the signal sequence has been cleaved and which migrates just below unprocessed FepB. The control experiment resulted in several products, which were used as size markers (luciferase, 60.7 kDa; internal luciferase start, 48 kDa; and β-lactamase, 31.5 kDa). (B) Quantitation of protein levels. The bars indicate the averages of six experiments, and the data are expressed relative to the value obtained for the WT, which was defined as 100%. The error bars indicate standard deviations.
Quantitation of the FepB protein generated in each reaction revealed that FepB protein production was reduced two- or threefold with templates containing the TE mutant compared with the protein production with templates containing the natural TE sequence (Fig. 5B). Also as expected, the fp-AUG and fp-MTE-AUG templates resulted in twofold-higher protein yields than the corresponding GUG templates.
The in vitro data recapitulated the results of the in vivo experiments obtained using fepB-phoA fusions to assess FepB production (Fig. 2). Interestingly, the in vitro transcription-translation experiments gave the same results when they were carried out in the presence of an RNase inhibitor (not shown). This may be because RNases are less active in the in vitro system than in vivo. Consistent with this, the in vitro protein levels were remarkably similar to the values obtained by calculating translational efficiency (Fig. 4), which indicated that the TE directly enhances fepB translation approximately twofold.
TE improves 30S binding.
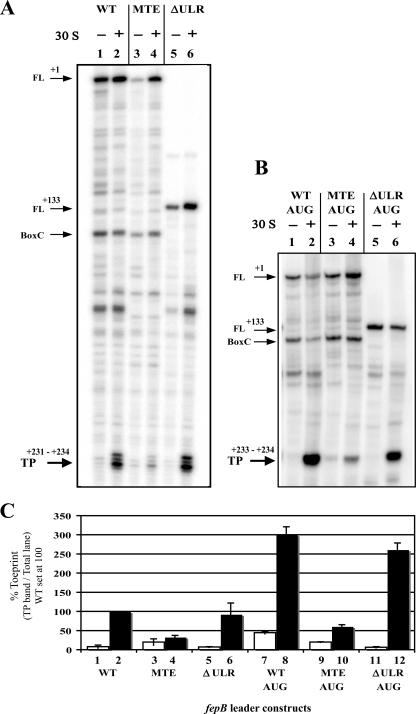
We next used primer extension inhibition assays (toeprints) to determine whether the TE sequence has a positive effect on fepB translation due to enhancement of ribosome binding. A toeprint results from a primer extension reaction that terminates when reverse transcriptase encounters a significant block, such as the 3′ boundary of a translational initiation complex. Footprinting data, as well as toeprinting data, have demonstrated that the 3′ boundary of the 30S complex extends approximately 15 nt downstream of the first nucleotide of the start codon; therefore, this is where the toeprint band is expected (21, 49). The technique allows the relative affinity of 30S ribosomal subunits for a given TIR to be estimated because 30S affinity has been shown to correlate with gene expression (21).
Using the fepB leader template, we observed the full-length product (Fig. 6A and B), as well as other 30S-independent termination products. These products were probably a result of the reverse transcriptase coming upon the 3′ end of a stable hairpin structure in the RNA and dissociating from the template. For example, a significant product was formed due to the extensive complementarities of the BoxC region (Fig. 6A and B, lanes 1 through 4). Removal of the BoxC elements in the ΔULR deletion mutant eliminated this band (Fig. 6A, lanes 5 and 6). The authentic 30S ribosomal subunit toeprint was seen upon addition of 30S subunits. With either fl-WT or fl-ΔULR templates, a strong toeprint was seen (Fig. 6A, lanes 2 and 6). In contrast, a much weaker toeprint was produced when the fl-MTE template was used (Fig. 6A, lane 4). These data demonstrate that ribosome binding is enhanced as much as fivefold when TE is present.
FIG. 6.
Primer extension inhibition assays (toeprints) of fepB leader transcripts. Each primer extension reaction was performed in the presence of tRNAfmet with (+) and without (−) 30S subunits using the fepB mRNA indicated. The following major primer extension inhibition products (described in text) are indicated by arrows: FL, full-length product; BoxC, product corresponding to the 3′ end of the BoxC repeat region; TP, 30S subunit-dependent toeprint products. (A and B) Toeprint assays of fepB leader constructs containing the native GUG start codon (A) or the mutant AUG start codon (B). The length and number of 30S subunit-dependent bands (TP) were verified with 8% polyacrylamide-urea gels (not shown), which provided better resolution of shorter fragments than the 6% polyacrylamide-urea gel shown provided. (C) Quantitation of toeprint assays was done with the Bio-Rad Quantity One software. The bars indicate the toeprint percentage for each lane obtained by dividing the toeprint product band value by the total lane value. The open and solid bars indicate reactions performed without (−) and with (+) 30S subunits, respectively. The bars indicate the averages of three experiments, and the data are expressed relative to the value obtained for the WT with 30S subunits (WT solid bar). The error bars indicate standard deviations.
Toeprint assays with templates containing GUG or AUG start codons.
The effect of the initiation codon was also examined by using ribosomal toeprints. The fl-AUG and fl-MTE-AUG templates produced stronger toeprint bands than their GUG counterparts (Fig. 6C). Also, the GUG start codon resulted in four toeprint bands, while the AUG start codon yielded only two very strong bands (Fig. 6A and B), suggesting that the 3′ end of the ternary complex is more stable when AUG is in the decoding site. As observed with the other assays, the combination of TE plus GUG found in WT fepB leader RNA produced an intermediate result. The fl-WT toeprint was nearly two times that of AUG without the TE sequence, fl-MTE-AUG, but was one-third that of fl-AUG, which had both the TE and AUG codon (Fig. 6C). The toeprint results argue that the differences in translational efficiency occur at the ribosome binding step.
DISCUSSION
Iron is a critical component of bacterial metabolism, and the efficiency of the enterobactin system allows E. coli to thrive in an iron-poor environment. However, by contributing to Fenton chemistry, an abundance of iron can result in the production of hydroxyl radicals, leading to DNA damage and disruption of protein function (19, 24, 38). Thus, the uncontrolled influx of iron can be toxic, and the uptake of iron must be tightly regulated. The FepB permease plays an important role in the enterobactin iron uptake system and is known to be regulated at the transcriptional level by the Fur protein. Here we investigated the 217-nt fepB leader to determine the role that it plays in control of fepB expression.
We found that the leader does not affect regulation in response to iron levels but does determine the efficiency of translation. We demonstrated that the fepB TIR, but not the ULR, is required for maximal fepB expression. Consistent with this finding, our genomic comparisons revealed that the ULR is not conserved and in fact is absent or only partially represented in several other bacterial genomes (Fig. 3). However, it is reasonable that addition of 190 nt, capable of extensive secondary structure, affects protein levels if only by altering the RNA stability. Indeed, our data indicate that the ULR negatively influences the basal level of FepB production in E. coli K-12, as fp-ΔULR increased expression relative to the expression with fp-WT by increasing the level of steady-state RNA (Fig. 4C).
We also demonstrated that the fepB TIR contains an AU-rich TE. The fepB TE improves expression due to increased translation efficiency by increasing ribosome binding and, as a consequence, the level of RNA. In contrast to the enhancement provided by the TE sequence, the fepB GUG start codon is less efficient than the typical AUG codon. The fepB TIR was highly conserved in all genomes analyzed, which implies that the combination of the TE and the GUG start codon is advantageous. The TE may act as another translational signal such that a given combination of TE, SD sequence, and start codon sequence can produce a wide array of expression levels.
TE are strikingly similar to the extensively studied transcriptional UP elements. UP elements are AT-rich sequences located upstream of promoter regions that improve transcription initiation via interaction with the C-terminal α-domains of RNA polymerase, which are attached to the RNA polymerase by flexible linkers (42). The C-terminal α-domains also interact nonspecifically with upstream DNA if an UP element sequence is not present (43). TE are AU-rich sequences located 5′ of the SD sequence and start codon that improve translation initiation via interaction with the S1 protein (5, 52), which is loosely attached to the ribosome. Cryo-electron microscope studies have provided evidence that S1 interacts with 11 nt of mRNA 5′ of the SD sequence (44). When this interaction is nonspecific, it stabilizes ribosome binding, but when a TE sequence is present, the interaction can improve ribosome binding. Our findings demonstrate that the fepB AU-rich sequence enhances translation by improving ribosome binding, consistent with improved interaction with the S1 protein. AU-rich sequences located at various distances from the initiation codon have been identified as translational enhancers; e.g., the poly(U) tract within rnd is at positions −35 to −22 relative to the initiation codon (55), while the AU-rich sequence from T7 gene 10 is at positions −24 to −14 (36). The fepB TE is located at positions −29 to −20 5′ of the GUG codon and is therefore reasonably positioned to interact with the S1 protein.
Like the availability of a transcriptional UP element, the availability of a TE may also provide a regulatory opportunity. This could be due to cis or trans regulation. Translation initiation depends on both the affinity of ribosomes for the TIR (determined by the primary sequence) and the accessibility of the TIR (determined by the secondary structures). Changes that improve ribosome binding or weaken structure formation improve expression levels (14). The TE may improve both of these steps, by increasing ribosome binding and also by providing a “standby site,” which increases the local concentration of 30S (15). This work suggested that when ribosomes bind at a “standby site” near the start codon, the frequency of initiation from RNA that has a secondary structure within the TIR is greatly improved. Whether the TE within the fepB TIR improves translation in part by functioning as a “standby site” has yet to be demonstrated. However, secondary structure has been predicted within the fepB leader (6, 16), and initial toeprint experiments using template RNA generated from fepB-phoA constructs resulted in 30S independent bands within the TIR (22). This in vitro result was consistent with the presence of a secondary structure in the fepB TIR that prevented 30S binding and raises the possibility that in vivo a secondary structure may influence ribosome binding and make fepB translation more reliant upon the TE.
An AU-rich TE could also be a target of a trans-acting factor which influences protein production in response to cellular conditions. RNase E (9, 27, 47), HfQ (40, 53, 54), and the T4 phage protein RegA (11) have each been shown to act as a translational regulator by recognizing an AU-rich sequence within mRNA. Factors such as these may compete with S1 protein for binding and thereby regulate expression.
In this way, the TE-S1 protein interaction could itself provide a form of regulation. Under stressful conditions, including the stationary phase, ribosomes are limiting (25, 30), and a TIR which contains a TE sequence may be more competitive than a TIR with only an SD sequence (5, 46). Therefore, fepB and other transcripts with S1 recognition elements would be expected to adjust their levels of expression to the growth conditions, while protein production from genes lacking TE-like sequences may be less responsive. This could create a hierarchy of gene expression, based on recognition by the S1 protein (5). Thus, the presence of an AU-rich TE may boost protein synthesis, while simultaneously providing a way to quickly adjust transcript levels via RNA decay in response to environmental conditions.
Acknowledgments
We thank the following individuals for helpful discussions: Michael J. Calcutt and John Cannon of the University of Missouri and Travis J. Barnard, Deborah M. Hinton, and Richard Bonocora of the National Institutes of Health. We also thank Marc Dreyfus of Laboratoire de Génétique Moléculaire, Ecole Normale Supérieure, for supplying bacterial strain MRE600.
I.G.H.-B. was supported by NIH training grant 5 T32 AI07276. This research was supported in part by grant GM54243 from the NIH and by grant URB-08-055 from the University of Missouri Research Board.
Footnotes
Published ahead of print on 30 March 2007.
REFERENCES
- 1.Bachellier, S., W. Saurin, D. Perrin, M. Hofnung, and E. Gilson. 1994. Structural and functional diversity among bacterial interspersed mosaic elements (BIMEs). Mol. Microbiol. 12:61-70. [DOI] [PubMed] [Google Scholar]
- 2.Bagg, A., and J. B. Neilands. 1987. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol. Rev. 51:509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnard, T. J., M. E. Watson, Jr., and M. A. McIntosh. 2001. Mutations in the Escherichia coli receptor FepA reveal residues involved in ligand binding and transport. Mol. Microbiol. 41:527-536. [DOI] [PubMed] [Google Scholar]
- 4.Boni, I. V., V. S. Artamonova, N. V. Tzareva, and M. Dreyfus. 2001. Non-canonical mechanism for translational control in bacteria: synthesis of ribosomal protein S1. EMBO J. 20:4222-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boni, I. V., D. M. Isaeva, M. L. Musychenko, and N. V. Tzareva. 1991. Ribosome-messenger recognition: mRNA target sites for ribosomal protein S1. Nucleic Acids Res. 19:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brickman, T. J. 1991. Regulation of enterobactin gene expression from the bidirectional fepB-entC promoter-operator regions in Escherichia coli. Ph.D. thesis. University of Missouri—Columbia, Columbia.
- 7.Brickman, T. J., B. A. Ozenberger, and M. A. McIntosh. 1990. Regulation of divergent transcription from the iron-responsive fepB-entC promoter-operator regions in Escherichia coli. J. Mol. Biol. 212:669-682. [DOI] [PubMed] [Google Scholar]
- 8.Clemens, K. E., and D. J. Pintel. 1988. The two transcription units of the autonomous parvovirus minute virus of mice are transcribed in a temporal order. J. Virol. 62:1448-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, S. N., and K. J. McDowall. 1997. RNase E: still a wonderfully mysterious enzyme. Mol. Microbiol. 23:1099-1106. [DOI] [PubMed] [Google Scholar]
- 10.Dahlberg, A. E. 1974. Two forms of the 30 S ribosomal subunit of Escherichia coli. J. Biol. Chem. 249:7673-7678. [PubMed] [Google Scholar]
- 11.Dean, T. R., S. V. Allen, and E. S. Miller. 2005. In vitro selection of phage RB69 RegA RNA binding sites yields UAA triplets. Virology 336:26-36. [DOI] [PubMed] [Google Scholar]
- 12.Deana, A., and J. G. Belasco. 2005. Lost in translation: the influence of ribosomes on bacterial mRNA decay. Genes Dev. 19:2526-2533. [DOI] [PubMed] [Google Scholar]
- 13.de Boer, H. A., and A. S. Hui. 1990. Sequences within ribosome binding site affecting messenger RNA translatability and method to direct ribosomes to single messenger RNA species. Methods Enzymol. 185:103-114. [DOI] [PubMed] [Google Scholar]
- 14.de Smit, M. H., and J. van Duin. 1994. Control of translation by mRNA secondary structure in Escherichia coli. A quantitative analysis of literature data. J. Mol. Biol. 244:144-150. [DOI] [PubMed] [Google Scholar]
- 15.de Smit, M. H., and J. van Duin. 2003. Translational standby sites: how ribosomes may deal with the rapid folding kinetics of mRNA. J. Mol. Biol. 331:737-743. [DOI] [PubMed] [Google Scholar]
- 16.Elkins, M. F., and C. F. Earhart. 1989. Nucleotide sequence and regulation of the Escherichia coli gene for ferrienterobactin transport protein FepB. J. Bacteriol. 171:5443-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etchegaray, J. P., and M. Inouye. 1999. Translational enhancement by an element downstream of the initiation codon in Escherichia coli. J. Biol. Chem. 274:10079-10085. [DOI] [PubMed] [Google Scholar]
- 18.Farwell, M. A., M. W. Roberts, and J. C. Rabinowitz. 1992. The effect of ribosomal protein S1 from Escherichia coli and Micrococcus luteus on protein synthesis in vitro by E. coli and Bacillus subtilis. Mol. Microbiol. 6:3375-3383. [DOI] [PubMed] [Google Scholar]
- 19.Fridovich, I. 1978. The biology of oxygen radicals. Science 201:875-880. [DOI] [PubMed] [Google Scholar]
- 20.Hartz, D., D. S. McPheeters, and L. Gold. 1991. Influence of mRNA determinants on translation initiation in Escherichia coli. J. Mol. Biol. 218:83-97. [DOI] [PubMed] [Google Scholar]
- 21.Hartz, D., D. S. McPheeters, R. Traut, and L. Gold. 1988. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 164:419-425. [DOI] [PubMed] [Google Scholar]
- 22.Hook-Barnard, I. G. 2003. Molecular analysis of regulatory elements within the Escherichia coli fepB leader mRNA. Ph.D. thesis. University of Missouri, Columbia.
- 23.Hunt, M. D., G. S. Pettis, and M. A. McIntosh. 1994. Promoter and operator determinants for fur-mediated iron regulation in the bidirectional fepA-fes control region of the Escherichia coli enterobactin gene system. J. Bacteriol. 176:3944-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imlay, J. A. 2006. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 59:1073-1082. [DOI] [PubMed] [Google Scholar]
- 25.Kajitani, M., and A. Ishihama. 1984. Promoter selectivity of Escherichia coli RNA polymerase. Differential stringent control of the multiple promoters from ribosomal RNA and protein operons. J. Biol. Chem. 259:1951-1957. [PubMed] [Google Scholar]
- 26.Klug, G., C. W. Adams, J. Belasco, B. Doerge, and S. N. Cohen. 1987. Biological consequences of segmental alterations in mRNA stability: effects of deletion of the intercistronic hairpin loop region of the Rhodobacter capsulatus puf operon. EMBO J. 6:3515-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komarova, A. V., L. S. Tchufistova, M. Dreyfus, and I. V. Boni. 2005. AU-rich sequences within 5′ untranslated leaders enhance translation and stabilize mRNA in Escherichia coli. J. Bacteriol. 187:1344-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komarova, A. V., L. S. Tchufistova, E. V. Supina, and I. V. Boni. 2002. Protein S1 counteracts the inhibitory effect of the extended Shine-Dalgarno sequence on translation. RNA 8:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loechel, S., J. M. Inamine, and P. C. Hu. 1991. A novel translation initiation region from Mycoplasma genitalium that functions in Escherichia coli. Nucleic Acids Res. 19:6905-6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnusson, L. U., T. Nystrom, and A. Farewell. 2003. Underproduction of sigma 70 mimics a stringent response. A proteome approach. J. Biol. Chem. 278:968-973. [DOI] [PubMed] [Google Scholar]
- 31.Manoil, C., and J. Beckwith. 1985. TnphoA: a transposon probe for protein export signals. Proc. Natl. Acad. Sci. USA 82:8129-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy, J. E., and C. Gualerzi. 1990. Translational control of prokaryotic gene expression. Trends Genet. 6:78-85. [DOI] [PubMed] [Google Scholar]
- 33.McCormick, J. R., J. M. Zengel, and L. Lindahl. 1994. Correlation of translation efficiency with the decay of lacZ mRNA in Escherichia coli. J. Mol. Biol. 239:608-622. [DOI] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 35.O'Connor, M., and A. E. Dahlberg. 2001. Enhancement of translation by the epsilon element is independent of the sequence of the 460 region of 16S rRNA. Nucleic Acids Res. 29:1420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olins, P. O., and S. H. Rangwala. 1989. A novel sequence element derived from bacteriophage T7 mRNA acts as an enhancer of translation of the lacZ gene in Escherichia coli. J. Biol. Chem. 264:16973-16976. [PubMed] [Google Scholar]
- 37.Ozenberger, B. A., M. S. Nahlik, and M. A. McIntosh. 1987. Genetic organization of multiple fep genes encoding ferric enterobactin transport functions in Escherichia coli. J. Bacteriol. 169:3638-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park, S., X. You, and J. A. Imlay. 2005. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx− mutants of Escherichia coli. Proc. Natl. Acad. Sci. USA 102:9317-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen, C. (ed.). 1993. Translation and mRNA stability in bacteria: a complex relationship. Academic Press, Inc., San Diego, CA.
- 40.Rasmussen, A. A., M. Eriksen, K. Gilany, C. Udesen, T. Franch, C. Petersen, and P. Valentin-Hansen. 2005. Regulation of ompA mRNA stability: the role of a small regulatory RNA in growth phase-dependent control. Mol. Microbiol. 58:1421-1429. [DOI] [PubMed] [Google Scholar]
- 41.Ringquist, S., T. Jones, E. E. Snyder, T. Gibson, I. Boni, and L. Gold. 1995. High-affinity RNA ligands to Escherichia coli ribosomes and ribosomal protein S1: comparison of natural and unnatural binding sites. Biochemistry 34:3640-3648. [DOI] [PubMed] [Google Scholar]
- 42.Ross, W., K. K. Gosink, J. Salomon, K. Igarashi, C. Zou, A. Ishihama, K. Severinov, and R. L. Gourse. 1993. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science 262:1407-1413. [DOI] [PubMed] [Google Scholar]
- 43.Ross, W., and R. L. Gourse. 2005. Sequence-independent upstream DNA-alphaCTD interactions strongly stimulate Escherichia coli RNA polymerase-lacUV5 promoter association. Proc. Natl. Acad. Sci. USA 102:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sengupta, J., R. K. Agrawal, and J. Frank. 2001. Visualization of protein S1 within the 30S ribosomal subunit and its interaction with messenger RNA. Proc. Natl. Acad. Sci. USA 98:11991-11996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 46.Sorensen, M. A., J. Fricke, and S. Pedersen. 1998. Ribosomal protein S1 is required for translation of most, if not all, natural mRNAs in Escherichia coli in vivo. J. Mol. Biol. 280:561-569. [DOI] [PubMed] [Google Scholar]
- 47.Sousa, S., I. Marchand, and M. Dreyfus. 2001. Autoregulation allows Escherichia coli RNase E to adjust continuously its synthesis to that of its substrates. Mol. Microbiol. 42:867-878. [DOI] [PubMed] [Google Scholar]
- 48.Spedding, G. 1990. Isolation and analysis of ribosomes from prokaryotes, eukaryotes, and organelles. Oxford University Press, Oxford, United Kingdom.
- 49.Steitz, J. A., and K. Jakes. 1975. How ribosomes select initiator regions in mRNA: base pair formation between the 3′ terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 72:4734-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramanian, A. R. 1983. Structure and functions of ribosomal protein S1. Prog. Nucleic Acid Res. Mol. Biol. 28:101-142. [DOI] [PubMed] [Google Scholar]
- 51.Tang, Y., and J. R. Guest. 1999. Direct evidence for mRNA binding and post-transcriptional regulation by Escherichia coli aconitases. Microbiology 145:3069-3079. [DOI] [PubMed] [Google Scholar]
- 52.van Duin, J., and P. H. van Knippenberg. 1974. Functional heterogeneity of the 30 S ribosomal subunit of Escherichia coli. 3. Requirement of protein S1 for translation. J. Mol. Biol. 84:185-195. [DOI] [PubMed] [Google Scholar]
- 53.Vecerek, B., I. Moll, and U. Blasi. 2005. Translational autocontrol of the Escherichia coli hfq RNA chaperone gene. RNA 11:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vytvytska, O., I. Moll, V. R. Kaberdin, A. von Gabain, and U. Blasi. 2000. Hfq (HF1) stimulates ompA mRNA decay by interfering with ribosome binding. Genes Dev. 14:1109-1118. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, J., and M. P. Deutscher. 1992. A uridine-rich sequence required for translation of prokaryotic mRNA. Proc. Natl. Acad. Sci. USA 89:2605-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]