Abstract
Salmonella enterica serovars Typhi and Paratyphi A cause systemic infections in humans which are referred to as enteric fever. Multidrug-resistant (MDR) serovar Typhi isolates emerged in the 1980s, and in recent years MDR serovar Paratyphi A infections have become established as a significant problem across Asia. MDR in serovar Typhi is almost invariably associated with IncHI1 plasmids, but the genetic basis of MDR in serovar Paratyphi A has remained predominantly undefined. The DNA sequence of an IncHI1 plasmid, pAKU_1, encoding MDR in a serovar Paratyphi A strain has been determined. Significantly, this plasmid shares a common IncHI1-associated DNA backbone with the serovar Typhi plasmid pHCM1 and an S. enterica serovar Typhimurium plasmid pR27. Plasmids pAKU_1 and pHCM1 share 14 antibiotic resistance genes encoded within similar mobile elements, which appear to form a 24-kb composite transposon that has transferred as a single unit into different positions into their IncHI1 backbones. Thus, these plasmids have acquired similar antibiotic resistance genes independently via the horizontal transfer of mobile DNA elements. Furthermore, two IncHI1 plasmids from a Vietnamese isolate of serovar Typhi were found to contain features of the backbone sequence of pAKU_1 rather than pHCM1, with the composite transposon inserted in the same location as in the pAKU_1 sequence. Our data show that these serovar Typhi and Paratyphi A IncHI1 plasmids share highly conserved core DNA and have acquired similar mobile elements encoding antibiotic resistance genes in past decades.
Salmonella enterica serovars Typhi and Paratyphi A are human-adapted bacterial pathogens that cause related systemic diseases, collectively called enteric fever or typhoid. While endemic enteric fever has been eliminated from most developed nations by improved sanitation (6), enteric fever is still a significant health threat in Southeast Asia, the Indian subcontinent, Africa, and, to a lesser extent, South America (16, 34, 38, 40). Historically, enteric fever has been a target of control programs and vaccines, but antibiotic treatment remains a central pillar of control (13). The appearance of both plasmid-mediated antibiotic resistance against conventional antityphoid drugs and chromosomal resistance to the fluoroquinolones has reduced therapeutic options to the more recently developed beta-lactams or macrolides. Resistance to both of these can be acquired through the acquisition of plasmids. This alarming situation has led to concern in countries where disease is endemic and to enhanced surveillance in countries into which enteric fever is imported.
Antibiotic resistance in serovar Typhi emerged in the 1970s, initially as chloramphenicol resistance but later as multidrug resistance (MDR) (25). MDR serovar Typhi strains have persisted in many areas and are now a huge clinical problem (22, 41). Serovar Paratyphi A is generally regarded as a less common cause of enteric fever than serovar Typhi, but this serovar causes approximately 25% of enteric fever cases in some regions (27). Unlike serovar Typhi, serovar Paratyphi A isolates have predominantly been susceptible to antibiotics (26, 35). However, in recent years there have been increasing incidents of MDR serovar Paratyphi A, particularly in Asia (2, 4, 17, 24, 32, 37, 44). A recent study in Nepal found MDR was more common among serovar Paratyphi A than serovar Typhi isolates (31). The situation is perhaps most extreme in China, where paratyphoid fever is now more common than typhoid fever in some regions and is largely drug resistant (27, 45). Additionally, many serovar Paratyphi A isolates are resistant to quinolones, and so, as with serovar Typhi-associated typhoid, the infection responds poorly to treatment with the fluoroquinolones (42).
IncHI1 plasmids have been shown to encode the MDR phenotype in the vast majority of serovar Typhi isolates analyzed. The few studies which have reported MDR in serovar Paratyphi A have pointed to a key role for plasmids in mediating resistance although few molecular studies have been undertaken (11). A large transferable plasmid of 140 MDa (∼230 kb) was found in 73% of MDR strains in Bangladesh in 1992 to 1993 (14). A similarly sized plasmid was reported in recent Chinese serovar Paratyphi A isolates (45). However, in Calcutta, India, a smaller plasmid (∼55 kb) was responsible for conferring MDR in serovar Paratyphi A isolates (23).
Here, we report the complete sequence of a 212-kb IncHI1 resistance plasmid from a serovar Paratyphi A strain isolated from a Pakistani patient in Karachi in 2002, and we provide conclusive proof that similar IncHI1 plasmids can encode the MDR phenotype in both serovar Typhi and serovar Paratyphi A. Our data show that these IncHI1 plasmids share highly conserved core DNA and have acquired similar mobile elements encoding antibiotic resistance genes on several occasions in past decades.
MATERIALS AND METHODS
The 212,711-bp plasmid pAKU_1 was sequenced as part of a whole-genome sequencing project for the parent strain (S. enterica Paratyphi A AKU_12601), the results of which will be reported elsewhere. The whole-genome shotgun consisted of 83,857 paired-end reads from libraries of 2 to 2.8 kb in pUC19, 5 to 6 kb in pMAQ1, and 6 to 9 kb in pMAQ1, giving 9.8-fold coverage. A scaffold was produced using 1,180 paired-end reads from a 20- to 30-kb library in pBACe3.6. The whole genome sequence was finished to standard criteria (28), using 9,879 directed sequencing reads. From the total of 94,916 shotgun and directed reads, 5,032 reads were used to assemble the plasmid sequence, giving around 12.9-fold final coverage of the plasmid. The sequence was annotated, and the annotation was manually curated using Artemis software (33) as previously described (28). Pairwise sequence comparisons were generated with BLASTN and visualized using ACT (3). Nucleotide differences between backbone sequences were determined using the nucmer and show-snps programs in the MUMmer package (18).
Nucleotide sequence accession number.
The sequence of the pAKU annotated plasmid has been submitted to the EMBL database under accession number AM412236.
RESULTS
Plasmid pAKU_1 has a conserved backbone highly related to other IncHI1 plasmids.
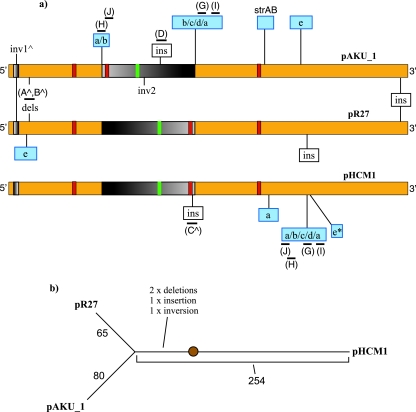
The DNA sequence of plasmid pAKU_1 encoding MDR harbored in an S. enterica serovar Paratyphi A isolate from Pakistan was determined. Comparison with sequence databases revealed high DNA sequence similarity between pAKU_1 and two IncHI1 plasmids: pR27 from S. enterica serovar Typhimurium isolated in the United Kingdom in 1961 (12, 36) (EMBL accession no. AF250878) and pHCM1 from serovar Typhi CT18 isolated in Vietnam in 1993 (29) (EMBL accession no. AL513383). Detailed comparative analysis of the three plasmid sequences revealed a 164.4-kb shared IncHI1-associated backbone, which showed 99.7% nucleotide identity across the three plasmids. This shared backbone constitutes 83% of pAKU_1 sequence and includes the IncHI1 incompatibility locus and three potential replicon elements (RepHI1A, RepHI1B, and RepFIA) characteristic of IncHI1 plasmids (9), the locations of which are indicated in Fig. 1a. The rest of the shared backbone harbors genes involved in the core plasmid functions of replication, maintenance, and conjugative transfer, as well as many hypothetical genes with no database matches to sequences outside pR27, pHCM1, and the Serratia marcescens IncHI2 plasmid pR478 (10) (EMBL accession no. BX66401; August 2006).
FIG. 1.
(a) Representative alignment of the 164-kb IncHI1 backbone sequences of pAKU_1, pR27, and pHCM1, with the sites of major insertions, deletions, and inversions indicated. Note that the plasmids are actually circular and are shown as linear here merely for ease of comparison. Red boxes show the sites of IncHI1 replicons; the green box represents the incompatibility region. Blue boxes represent resistance gene insertions, scaled to indicate relative size compared to backbone, and are labeled as in Fig. 2 (a, Tn9; b, Tn21; c, class 1 integron; d, bla/sul/str; e, Tn10; e*, truncated Tn10). Other insertions are shown in white boxes. Inversions are shown as graded black and gray boxes; the gradient indicates direction. Black bars indicate PCR target amplicons, labeled (A to J) as in Table 2 and Fig. 3. (b) Tree showing the relationship between the 164-kb IncHI1 backbone sequences of the three plasmids, based on single-nucleotide changes. Branch lengths are proportional to the number of changes, indicated next to branches. The positions of four major rearrangements (marked by a caret in a) are indicated; the position of the root is imprecise due to a lack of suitable plasmid sequences for use as outgroups.
The shared IncHI1 backbone sequences of pAKU_1, pR27, and pHCM1 were aligned, and nucleotide differences were determined using MUMmer (see Materials and Methods). This analysis found that pAKU_1 shared 99.71% nucleotide identity with pHCM1 and 99.89% with pR27. Figure 1b shows an unrooted phylogenetic tree based on the number of single-nucleotide changes found among the three IncHI1 backbones. As the tree shows, the plasmid backbone of pAKU_1 is clearly closer to that of pR27 than pHCM1. This is supported by the presence of shared variations in the backbones of pAKU_1 and pR27 relative to pHCM1. These are marked with a caret in Fig. 1a and include a small inversion near the 5′ end, two deletions downstream of this inversion, and a gene (annotated as R0107 in pR27 and SPA0320 in pAKU_1) inserted at the 3′ end of the shared backbone. A large region is inverted on pAKU_1 relative to pR27 and pHCM1; however, this occurred on pAKU_1 or a similar precursor plasmid rather than a common ancestor of pR27 and pHCM1 (see Fig. 3).
FIG. 3.
The inferred ancestral version of the composite transposon (b) and its rearrangements in three plasmids. *, genes inserted relative to the composite transposon; ∧, genes in the variable integron gene cassette. Colors and genes are the same as described in the legend of Fig. 2; pale green indicates variable sequences at equivalent loci. Upper and lower bands represent forward and reverse strands, open arrowheads represent inverted repeats, and filled arrowheads represent direct repeats. Regions of identical sequences are joined by colored boxes (tan when in direct orientation and green when inverted). Black lines indicate PCR amplicons (E to J); labels correspond to those in Table 2 and Fig. 1.
Prior to further analysis of the sequenced plasmids, we determined whether IncHI1 plasmids are more generally responsible for MDR in serovar Paratyphi A. A large number of MDR serovar Paratyphi A isolates from Pakistan obtained from 2002 to 2004 were analyzed for MDR and IncHI1 plasmid content. Of 81 serovar Paratyphi A isolates analyzed, 68 were MDR, and 67 of these were determined by PCR to contain the IncHI1 replicon (primers are given in Table 1). In contrast, none of the susceptible serovar Paratyphi A isolates tested positive for IncHI1. To confirm the presence of plasmids in MDR serovar Paratyphi A, plasmid preparations were analyzed for 33 MDR and 5 susceptible serovar Paratyphi A strains. All 33 MDR serovar Paratyphi A isolates harbored a single plasmid of approximately 220 kb, similar in size to pAKU_1 (212 kb); 4 of the susceptible serovar Paratyphi A isolates were plasmid free, and 1 contained two small plasmids.
TABLE 1.
PCR primers used in this studya
| PCR target ampliconb | Primer
|
Length in plasmid (bp)
|
Target featured | ||
|---|---|---|---|---|---|
| Directionc | Sequence | pAKU_1 | pHCM1 | ||
| IncHI1 | F | CGAAATCGGTCCAACCCATTG | 110 | NAe | RepHI1A |
| R | CGACAACTCATCAGAAGCGTCAAC | ||||
| A | F | GAAAGGAATCATCCACCTTCA | 419 | 990 | Deletion on pAKU_1 |
| R | AACTGTCGCTACGCCTGACT | ||||
| B | F | ATCCAGCGTGCAAAGATTTC | 407 | 2,589 | Deletion on pAKU_1 |
| R | TGGGGGAGAACACCACTTTA | ||||
| C | F | AAAGATGCAATGGGAGGAGA | 289 | 4,399 | Insertion on pHCM1 |
| R | GCGCAGCTGCTTCAATTA | ||||
| D | F | TAGGGTTTGTGCGGCTTC | 3,138 | None | Insertion on pAKU_1 |
| R | CCTTCTTGTCGCCTTTGC | ||||
| E | F | TCAAGGCAGATGGCATTCCC | 156 | None | sul1 |
| R | CGACGAGTTTGGCAGATGATTTC | ||||
| F | F | GTGTCGAGGAAAGGAATTTCAAGCTC | 191 | None | dfrA7 |
| R | TCACCTTCAACCTCAACGTGAACAG | ||||
| G | F | GATGGAGAAGAGGAGCAACG | 989 | 989 | strB, tniAΔ |
| R | TTCGTTCCTGGTCGATTTTC | ||||
| H | F | GTGCTGTGGAACACGGTCTA | 271 | 1,598 | tnpA, Tn21 IR |
| R | TCATCAACGCTTCCTGAATG | ||||
| I | F | ACGAAAGGGGAATGTTTCCT | 163 | 1,490 | merR, Tn21 IR |
| R | CGAGTGGGAATCCATGGTAG | ||||
| J | F | CAAAATGTTCTTTACGATGCC | 2,219 | None | cat, backbone (trhN) |
| R | CCAGACAGGAAAACGCTCA | ||||
Primers used to detect the IncHI1 replicon in seovar Paratyphi A plasmids from Pakistan and to detect features of the IncHI1 backbone and resistance gene insertions in pAKU_1, pHCM1, and two additional serovar Typhi plasmids from Vietnam (A to J).
The locations of amplicons A to J in pAKU_1 are shown in Fig. 1; the locations of E to J are shown in Fig. 3.
F, forward; R, reverse.
IR, inverted repeat; parentheses indicate backbone gene targeted by PCR.
NA, not applicable.
Comparison of MDR genes in serovar Paratyphi A and serovar Typhi plasmids.
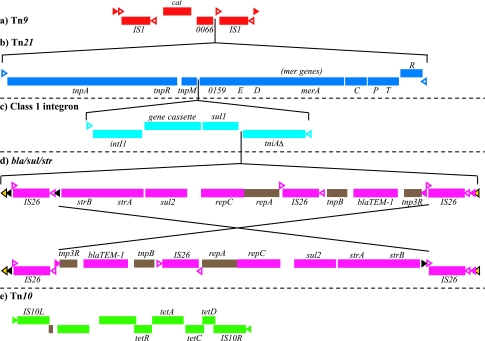
The pAKU_1 plasmid sequence contained multiple antibiotic resistance gene elements inserted into the IncHI1 backbone. Interestingly, these insertions are highly clustered relative to the conserved IncHI1 backbone and are also related to antibiotic resistance genes found on pHCM1 but not pR27, as shown in Fig. 1a. These resistance genes can be attributed to the independent insertion of previously described transposable elements (Fig. 2) into different positions in the plasmids' IncHI1 backbones. Tn10, containing the tetracycline resistance (tet) gene (Fig. 2e) (19), is present on pAKU_1, pR27, and pHCM1, although part of the transposon is missing from pHCM1. The site of the Tn10 insertion into the backbone is different in each plasmid, indicating that the transposon was independently acquired in each rather than by a common ancestor. No further resistance insertions are present on pR27. Tn9, with identical copies of the chloramphenicol resistance gene cat (1) (Fig. 2a) is present on pAKU_1 and pHCM1. The transposition of Tn9 is accompanied by target site duplications which show that the Tn9 insertion site was different in pAKU_1 and pHCM1. Tn21, harboring a class I integron and mercury resistance (mer) operon (20), can also be identified on both pAKU_1 and pHCM1, although in each case the primary transposable element has been disrupted by IS26 insertions and subsequent sequence rearrangements. Tn21 is inserted at the same site within Tn9 in pAKU_1 and pHCM1; pHCM1 also harbors a second, divergent copy of Tn21 elsewhere on the plasmid. The resistance gene cassettes associated with the class I integrons (Fig. 2c) differ in the two plasmids: sul1 and dfrA7 in pAKU_1 (encoding sulfonamide and trimethoprim resistance respectively) and dfrA14 in pHCM1.
FIG. 2.
Transposons identified in pAKU_1. The gene order shown here agrees with the consensus from other sequences; note that Tn9 and Tn21 have been disrupted by rearrangements in pAKU_1 and pHCM1 (Fig. 3a and d). The bla/sul/str sequence (d) appears to have been inverted in between the flanking IS26 elements in pAKU_1 and pHCM1. Upper and lower bands represent forward and reverse strands, open arrowheads represent inverted repeats, and filled arrowheads represent direct repeats (target site duplications). The insertion sites shown for panel d into panel c, panel c into panel b, and panel b into panel a are conserved in pAKU_1, pHCM1, and pRSB107.
An identical ∼9-kb sequence, incorporating blaTEM-1 (beta-lactam resistance), sul2 (sulfonamide resistance), and strAB (streptomycin resistance) genes flanked by IS26 elements (Fig. 2d), is present on both pAKU_1 and pHCM1. BLAST searching (EMBL, November 2006) revealed that this is a promiscuous sequence, referred to hereafter as bla/sul/str, that is also present in the 120-kb IncF plasmid pRSB107 (unknown host, Germany, 2005; EMBL accession no. AJ851089) (39) and the F-like plasmid pU302L of serovar Typhimurium strain G8430 (CDC, Atlanta, GA; EMBL accession no. AY333434) (5). The sequence has also previously been described in the genome of serovar Typhimurium strain DT193 (Ireland, 1998; EMBL accession no. AY524415) and (in part) in an IncI plasmid of S. enterica serovar Enteritidis (Italy, 1997; EMBL accession no. AJ628353) (7). As Chen et al. suggest (5), it is likely that this bla/sul/str sequence has moved as a single unit among enteric bacteria. The flanking IS26 elements are members of the IS6 family, for which a transposition mechanism has been demonstrated via two elements in direct orientation (21). Recombination via such elements is also a possibility. However in pAKU_1, after accounting for inversions between IS26 elements (Fig. 3), we identified 6-bp direct repeats which were likely target site duplications created upon insertion of the outermost IS26 elements (to construct the composite sequence) and upon transposition of the whole unit into Tn21. These direct repeats are shown as black, pink, and yellow filled arrowheads in Fig. 2 and 3 and appear in the annotation. These are also present (although similarly rearranged) in pHCM1 and pRSB107; thus, the insertion sites of bla/sul/str into Tn21 are identical in these three plasmids.
A composite resistance transposon.
Although the transposons of pAKU_1 and pHCM1 have been disrupted by several insertion elements and sequence rearrangements, sequence identity at the boundaries of Tn21 and the bla/sul/str insertions suggests that Tn9, Tn21, and bla/sul/str may have been acquired consecutively in one location and subsequently transferred as a single unit between plasmids. Specifically, it is hypothesized that some plasmid X first acquired Tn9, followed by the transposition of Tn21 into Tn9, 3′ of the cat gene (Fig. 2a and b). At some point bla/sul/str was inserted into the integron in Tn21, adjacent to tniAΔ (Fig. 2c and d); this may have occurred before or after the acquisition of Tn21 by plasmid X. Subsequently, the resulting 24-kb composite transposon was transferred into other plasmids. The transposition mechanism is presumably mediated by the IS1 ends of Tn9, as direct repeats are evident at opposite ends of the IS1 elements in pAKU_1. The same composite transposon is evident in plasmid pRSB107, sequenced from an unknown bacterial host from a wastewater treatment plant, albeit with additional resistance gene insertions (Fig. 3c).
Once inserted into the ancestors of pAKU_1 and pHCM1, the composite transposon sequence was disrupted by rearrangements mediated by insertion elements (Fig. 3). In pAKU_1 two IS26 insertions mediated two inversions in the 5′ end of the composite transposon (Fig. 3a); this is supported by analysis of the configuration of IS26 target site duplications, which were inverted along with the rest of the sequence between IS26 elements. One large inversion is responsible for separating the 5′ ends of Tn9 (IS1 and cat) and Tn21 (the tnpA and tnpR 3′ fragment) from the rest of the composite transposon in pAKU_1 (Fig. 3a). This presumably has deactivated the composite transposon in this plasmid, as the IS1 genes are now in opposite orientation and separated by 62 kb, thus disrupting Tn9. Tn21 is similarly disrupted, although bla/sul/str is presumably still capable of transposition. In pHCM1, recombination between an IS26 element inserted between tnpA and tnpR and the 5′ IS26 element of bla/sul/str resulted in deletion of tnpR, tnpM, intI1, and the integron gene cassette (Fig. 3d). IS4321 elements were also inserted within the Tn21 inverted flanking repeats, demonstrated to be a preferred target site for this IS element (30). In pRSB107, there are two additional resistance gene insertions within the composite transposon (Fig. 3c). The Tn4352B kanamycin/neomycin resistance transposon is inserted at the 3′ end of bla/sul/str. This transposon comprises the aph gene flanked by IS26 elements and so may have been inserted at this position via recombination with the 5′ end IS26 element of bla/sul/str. A macrolide resistance module is also inserted between the integron gene cassette and bla/sul/str.
Comparison of pAKU_1 to serovar Typhi IncHI1 resistance plasmids.
The serovar Paratyphi A plasmid pAKU_1 was compared to two IncHI1 plasmids isolated from serovar Typhi strains during outbreaks in Vietnam in 1996. PCR primers (Table 1) were designed based on the pAKU_1 sequence to detect (i) unique features of the pAKU_1 IncHI1 backbone compared to the serovar Typhi plasmid pHCM1 (Fig. 1a, A to D), (ii) transposon boundaries within the composite transposon (Fig. 1a and 3, G to I), and (iii) the insertion site of the composite transposon into the IncHI1 backbone (Fig. 1a and 3, J). Results from PCR assays designed to detect genes in the intI1 variable gene cassette were previously reported by Wain et al. (43) (Fig. 3, E and F). The regions amplified by PCR in pAKU_1 and pHCM1 are indicated in Fig. 1 and 3; results of the PCR assays are given in Table 2.
TABLE 2.
PCR assays in pAKU_1, pHCM1, pR27, and two serovar Typhi plasmids from Vietnam
| PCR region and amplicona | PCR product in the indicated plasmidb
|
||||
|---|---|---|---|---|---|
| pR27 | pHCM1 | pSTY6 | pSTY7 | pAKU_1 | |
| Backbone | |||||
| A | X | 0 | X | X | X |
| B | X | 0 | X | X | X |
| C | X | 0 | X | X | X |
| D | − | − | X | X | X |
| Integron cassette | |||||
| E | − | − | ND | X | X |
| F | − | − | X | X | X |
| Composite transposon | |||||
| G | − | X | X | X | X |
| H | − | 0 | X | X | X |
| I | − | 0 | X | X | X |
| J | − | − | − | X | X |
PCR amplicons A and B target deletions in the pR27 and pAKU_1 backbone compared to pHCM1, C targets an insertion in the pHCM1 backbone, and D targets an insertion in the pAKU_1 backbone. Their positions are shown in Fig. 1. PCR amplicons E and F are PCR results for the detection of dfrA7 and sul1, reported in Wain et al. (43), which are present in the variable integron gene cassette (Fig. 2c) in pAKU_1 but not in pHCM1; G targets one end of the insertion of bla/sul/str into Tn21; H and I target the boundaries of the insertion of Tn21 into Tn9, and J targets the insertion site of Tn9 into the IncHI1 backbone.
X, amplification of product the same size as for pAKU_1; 0, amplified product of different size; −, no amplification; ND, not determined.
DISCUSSION
pAKU_1 is the first MDR plasmid from serovar Paratyphi A to be sequenced and analyzed in detail. Like IncHI1 plasmids isolated from MDR serovar Typhi, the DNA sequence is composed of an IncHI1 backbone with numerous insertions of mobile elements encoding resistance to chloramphenicol, streptomycin, beta-lactams, trimethoprim, sulfonamides, and tetracycline. Analysis of 81 serovar Paratyphi A isolates from Pakistan confirmed that IncHI1 plasmids of similar size to pAKU_1 are responsible for MDR in the majority of clinical isolates analyzed from this area. Plasmids of similar size have also been associated with MDR serovar Typhi in other regions (14, 45). The pAKU_1 plasmid shares its IncHI1 backbone with two plasmids that have been sequenced previously: pR27 from serovar Typhimurium and pHCM1 from serovar Typhi. This shared backbone was inherited vertically from a common ancestral plasmid and was therefore analyzed separately from the mobile elements contained in the plasmid sequences, which can theoretically be readily transferred horizontally into distinct DNA backbones. Comparative analysis of the three IncHI1 backbone sequences revealed that pAKU_1 is more closely related to pR27 than to pHCM1, at the level of single-nucleotide changes as well as larger insertions, deletions, and rearrangements (Fig. 1b).
Plasmids pAKU_1 and pHCM1 share very similar resistance gene complements, while pR27 has only one resistance gene element (Tn10). It was hypothesized that the accumulation of resistance since the 1960s when pR27 was first isolated is the result of independent acquisition of resistance genes by two distinct IncHI1 plasmid lineages. Plasmids pAKU_1 and pR27 share a related backbone, but pHCM1 and pAKU_1 have acquired most of the same mobile elements. All three plasmids encode the tetracycline resistance transposon Tn10; however, the insertion site is at different positions in the IncHI1 backbone (Fig. 1a), suggesting that it, too, has been independently acquired by each of the plasmids since their divergence.
The high degree of similarity in the resistance gene complement of pAKU_1 and pHCM1 is due to the independent acquisition of a single composite transposon by both plasmids (Fig. 2 and 3), which has since been subject to different rearrangements in each (Fig. 3). The proposed composite transposon includes Tn9, Tn21, and a stretch of sequence including the blaTEM-1, sul2, and strAB resistance genes that may itself be mobile (Fig. 2d). The insertion sites of the composite transposon are different in pAKU_1 and pHCM1, supporting the hypothesis that the plasmids acquired their similar resistance genes independently by horizontal transfer rather than by vertical inheritance from a common ancestral plasmid. A BLAST search of the proposed composite transposon sequence (Fig. 3b) against the EMBL database revealed its presence, without rearrangements, in a plasmid from an unknown source, pRSB107 (Fig. 3c). This plasmid has a distinct IncF backbone; thus, the composite transposon appears capable of insertion into a variety of genetic contexts. The strongest evidence for the transfer of the composite transposon as a single unit is the 100% sequence identity in pAKU_1, pHCM1, and pRSB107 across the boundaries of insertion of (i) Tn21 into Tn9 and (ii) bla/sul/str into Tn21. If Tn9, Tn21, and bla/sul/str were acquired independently in each plasmid, it is highly unlikely that the insertion sites of Tn21 and bla/sul/str and the resulting target site duplication for bla/sul/str would be identical at the nucleotide level as they are in these three sequences. Thus, the most likely explanation is that the insertions occurred once to form a composite transposon, which was then able to move between distinct plasmid backbones as a single unit using the IS1 ends of Tn9.
There are currently no other resistance plasmid sequences from serovar Typhi or serovar Paratyphi A available for comparative analysis. However, two IncHI1 serovar Typhi plasmids, isolated in 1996 from the same location in Vietnam as pHCM1, were available for genetic analysis. PCR assays found that both of these plasmids, pSTY6 and pSTY7 isolated in 1996, matched the backbone of pAKU_1 rather than pHCM1, contained resistance genes present in pAKU_1 and not pHCM1, and contained the composite transposon inserted at the same site as in pAKU_1 (Table 2). It is highly unlikely that the composite transposon has been inserted independently in exactly the same position in both pAKU_1 and the serovar Typhi plasmids. The more probable explanation is that the serovar Typhi plasmids pSTY6 and pSTY7 and the serovar Paratyphi A plasmid pAKU_1 share a recent common ancestor from which they have each inherited the backbone and composite transposon insertion in a vertical fashion. This is supported by the matching results for PCR targeting the IncHI1 backbone (Table 2, A to D).
The observation that very closely related plasmids that share a backbone carrying identical resistance insertions are present in serovar Typhi and serovar Paratyphi A strongly suggests that transfer of a plasmid between these two serovars has occurred. However, it remains to be determined when this plasmid transfer may have occurred and whether the direction of transfer was from serovar Typhi to serovar Paratyphi A, or vice versa, or via another bacterial host. Direct transfer between serovar Typhi and serovar Paratyphi A may be possible, as incidents of coinfection have been reported (15), as has chromosomal recombination between serovar Typhi and serovar Paratyphi A (8). Whatever the direction of transfer, current data suggest that plasmids similar to pAKU_1 have recently replaced the pHCM1-type plasmids in some serovar Typhi populations.
This analysis clearly shows that plasmids found in serovar Typhi and serovar Paratyphi A have independently acquired a single composite transposon encoding MDR. This provides a mechanism for the acquisition of a large number of drug resistance genes in a single transfer and should serve as a warning that MDR can be acquired rapidly by human pathogens in a single step. It also suggests that selection for resistance to one antibiotic may lead to the proliferation of resistance to many. Moreover, if MDR can be transferred rapidly from one serovar to the other, whether by horizontal gene acquisition or plasmid transfer, then the impact of selection for resistance in one serovar can affect resistance in the other. This is an important consideration in a clinical environment as it suggests that treatment choices for serovar Typhi can impact treatment options for serovar Paratyphi A infection and vice versa.
ADDENDUM IN PROOF
Since acceptance of the manuscript, it has been suggested to us that the following should be clarified. The transposon Tn2670 (reviewed in reference 20) contains Tn21 inserted at the same site in Tn9, but no bla/sul/str element, and is therefore a possible progenitor for the composite transposon inferred here.
Acknowledgments
This project was funded by the Wellcome Trust through its support of the Sanger Institute. We acknowledge the support of the WTSI core sequencing and informatics groups. K.E.H. is supported by a WTSI Ph.D. studentship.
Footnotes
Published ahead of print on 23 March 2007.
REFERENCES
- 1.Alton, N. K., and D. Vapnek. 1979. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature 282:864-869. [DOI] [PubMed] [Google Scholar]
- 2.Butt, T., R. N. Ahmad, M. Salman, and S. Y. Kazmi. 2005. Changing trends in drug resistance among typhoid salmonellae in Rawalpindi, Pakistan. East Mediterr. Health J. 11:1038-1044. [PubMed] [Google Scholar]
- 3.Carver, T. J., K. M. Rutherford, M. Berriman, M. A. Rajandream, B. G. Barrell, and J. Parkhill. 2005. ACT: the Artemis comparison tool. Bioinformatics 21:3422-3423. [DOI] [PubMed] [Google Scholar]
- 4.Chandel, D. S., R. Chaudhry, B. Dhawan, A. Pandey, and A. B. Dey. 2000. Drug-resistant Salmonella enterica serotype Paratyphi A in India. Emerg. Infect. Dis. 6:420-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, C. Y., G. W. Nace, B. Solow, and P. Fratamico. 2007. Complete nucleotide sequences of 84.5- and 3.2-kb plasmids in the multi-antibiotic resistant Salmonella enterica serovar Typhimurium U302 strain G8430. Plasmid 57:29-43. [DOI] [PubMed] [Google Scholar]
- 6.Crump, J. A., S. P. Luby, and E. D. Mintz. 2004. The global burden of typhoid fever. Bull W. H. O. 82:346-353. [PMC free article] [PubMed] [Google Scholar]
- 7.Daly, M., L. Villa, C. Pezzella, S. Fanning, and A. Carattoli. 2005. Comparison of multidrug resistance gene regions between two geographically unrelated Salmonella serotypes. J. Antimicrob. Chemother. 55:558-561. [DOI] [PubMed] [Google Scholar]
- 8.Didelot, X., M. Achtman, J. Parkhill, N. R. Thomson, and D. Falush. 2007. A bimodal pattern of relatedness between the Salmonella Paratyphi A and Typhi genomes: convergence or divergence by homologous recombination? Genome Res. 17:61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabant, P., P. Newnham, D. Taylor, and M. Couturier. 1993. Isolation and location on the R27 map of two replicons and an incompatibility determinant specific for IncHI1 plasmids. J. Bacteriol. 175:7697-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilmour, M. W., N. R. Thomson, M. Sanders, J. Parkhill, and D. E. Taylor. 2004. The complete nucleotide sequence of the resistance plasmid R478: defining the backbone components of incompatibility group H conjugative plasmids through comparative genomics. Plasmid 52:182-202. [DOI] [PubMed] [Google Scholar]
- 11.Goh, Y. L., S. D. Puthucheary, R. Chaudhry, Z. A. Bhutta, M. Lesmana, B. A. Oyofo, N. H. Punjabi, A. Ahmed, and K. L. Thong. 2002. Genetic diversity of Salmonella enterica serovar Paratyphi A from different geographical regions in Asia. J. Appl. Microbiol. 92:1167-1171. [DOI] [PubMed] [Google Scholar]
- 12.Grindley, N. D. F., G. O. Humphreys, and E. S. Anderson. 1973. Molecular studies of R factor compatibility groups. J. Bacteriol. 115:387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman, C. A., S. Borsutzky, M. Griot-Wenk, I. C. Metcalfe, J. Pearman, A. Collioud, D. Favre, and G. Dietrich. 2006. Vaccines against typhoid fever. Vaccine 24:3804-3811. [DOI] [PubMed] [Google Scholar]
- 14.Hasan, Z., K. M. Rahman, M. N. Alam, A. Afroza, Z. H. Asna, P. K. Ghosh, and N. Alam. 1995. Role of a large plasmid in mediation of multiple drug resistance in Salmonella Typhi and Paratyphi A in Bangladesh. Bangladesh Med. Res. Counc. Bull. 21:50-54. [PubMed] [Google Scholar]
- 15.Joshi, S., C. Wattal, A. Sharma, and K. J. Prasad. 2002. Mixed salmonella infection: a case report. Indian J. Med. Microbiol. 20:113-114. [PubMed] [Google Scholar]
- 16.Kariuki, S., G. Revathi, J. Muyodi, J. Mwituria, A. Munyalo, S. Mirza, and C. A. Hart. 2004. Characterization of multidrug-resistant typhoid outbreaks in Kenya. J. Clin. Microbiol. 42:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanum, S., Noor-us-Sabab, M. Qayyum, B. Islam, and A. A. Qazilbash. 2006. Emergence of multi-drug resistant strains of Salmonella Typhi and Paratyphi A in the Rawalpindi/Islamabad. J. Med. Sci. 6:68. [Google Scholar]
- 18.Kurtz, S., A. Phillippy, A. L. Delcher, M. Smoot, M. Shumway, C. Antonescu, and S. L. Salzberg. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawley, T. D., V. Burland, and D. E. Taylor. 2000. Analysis of the complete nucleotide sequence of the tetracycline-resistance transposon Tn10. Plasmid 43:235-239. [DOI] [PubMed] [Google Scholar]
- 20.Liebert, C. A., R. M. Hall, and A. O. Summers. 1999. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63:507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malla, S., P. Kansakar, O. Serichantalergs, M. Rahman, and S. Basnet. 2005. Epidemiology of typhoid and paratyphoid fever in Kathmandu: two years study and trends of antimicrobial resistance. J. Nepal Med. Assoc. 44:18-22. [PubMed] [Google Scholar]
- 23.Mandal, S., M. D. Mandal, and N. K. Pal. 2006. Antibiotic resistance of Salmonella enterica serovar Paratyphi A in India: emerging and reemerging problem. J. Postgrad. Med. 52:163-166. [PubMed] [Google Scholar]
- 24.Maskey, A. P., J. N. Day, Q. T. Phung, G. E. Thwaites, J. I. Campbell, M. Zimmerman, J. J. Farrar, and B. Basnyat. 2006. Salmonella enterica serovar Paratyphi A and S. enterica serovar Typhi cause indistinguishable clinical syndromes in Kathmandu, Nepal. Clin. Infect. Dis. 42:1247-1253. [DOI] [PubMed] [Google Scholar]
- 25.Mered, B., F. Poittevin, and B. Louli. 1977. Surveillance of drug resistance in pathogenic enterobacteria in Algeria. I. Study of the resistance of major and minor Salmonella species to antibiotics in 1973-1974. Arch. Inst. Pasteur Alger. 52:17-35. [PubMed] [Google Scholar]
- 26.Mirza, S. H., N. J. Beeching, and C. A. Hart. 1995. The prevalence and clinical features of multi-drug resistant Salmonella typhi infections in Baluchistan, Pakistan. Ann. Trop. Med. Parasitol. 89:515-519. [DOI] [PubMed] [Google Scholar]
- 27.Ochiai, R. L., X. Wang, L. von Seidlein, J. Yang, Z. A. Bhutta, S. K. Bhattacharya, M. Agtini, J. L. Deen, J. Wain, D. R. Kim, M. Ali, C. J. Acosta, L. Jodar, and J. D. Clemens. 2005. Salmonella Paratyphi A rates, Asia. Emerg. Infect. Dis. 11:1764-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M. A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 29.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 30.Partridge, S. R., and R. M. Hall. 2003. The IS1111 family members IS4321 and IS5075 have subterminal inverted repeats and target the terminal inverted repeats of Tn21 family transposons. J. Bacteriol. 185:6371-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pokharel, B. M., J. Koirala, R. K. Dahal, S. K. Mishra, P. K. Khadga, and N. R. Tuladhar. 2006. Multidrug-resistant and extended-spectrum beta-lactamase (ESBL)-producing Salmonella enterica (serotypes Typhi and Paratyphi A) from blood isolates in Nepal: surveillance of resistance and a search for newer alternatives. Int. J. Infect. Dis. 10:434-438. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi, A. H., N. Mushahid, A. Ijaz, A. Ahmad, M. Ateque, M. H. Marri, M. A. Qamar, and N. Ali. 2001. Changing drug susceptibility pattern of Salmonella Paratyphi A. J. Coll. Physicians Surg. Pak. 13:31. [Google Scholar]
- 33.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M. A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 34.Salve, A., M. Pichel, M. Wiesner, M. Hidalgo, R. Terragno, A. Alvarez, C. I. Agudelo, E. Castaneda, and N. Binsztein. 2006. Molecular subtyping of Salmonella enterica serovar Typhi isolates from Colombia and Argentina. Foodborne Pathog. Dis. 3:142-152. [DOI] [PubMed] [Google Scholar]
- 35.Saxena, S. N., and R. Sen. 1966. Salmonella Paratyphi A infection in India: incidence and phage types. Trans. R. Soc. Trop. Med. Hyg. 60:409-411. [DOI] [PubMed] [Google Scholar]
- 36.Sherburne, C. K., T. D. Lawley, M. W. Gilmour, F. R. Blattner, V. Burland, E. Grotbeck, D. J. Rose, and D. E. Taylor. 2000. The complete DNA sequence and analysis of R27, a large IncHI plasmid from Salmonella typhi that is temperature sensitive for transfer. Nucleic Acids Res. 28:2177-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sood, S., A. Kapil, N. Dash, B. K. Das, V. Goel, and P. Seth. 1999. Paratyphoid fever in India: an emerging problem. Emerg. Infect. Dis. 5:483-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sur, D., L. von Seidlein, B. Manna, S. Dutta, A. K. Deb, B. L. Sarkar, S. Kanungo, J. L. Deen, M. Ali, D. R. Kim, V. K. Gupta, R. L. Ochiai, A. Tsuzuki, C. J. Acosta, J. D. Clemens, and S. K. Bhattacharya. 2006. The malaria and typhoid fever burden in the slums of Kolkata, India: data from a prospective community-based study. Trans. R. Soc. Trop. Med. Hyg. 100:725-733. [DOI] [PubMed] [Google Scholar]
- 39.Szczepanowski, R., S. Braun, V. Riedel, S. Schneiker, I. Krahn, A. Puhler, and A. Schluter. 2005. The 120 592 bp IncF plasmid pRSB107 isolated from a sewage-treatment plant encodes nine different antibiotic-resistance determinants, two iron-acquisition systems and other putative virulence-associated functions. Microbiology 151:1095-1111. [DOI] [PubMed] [Google Scholar]
- 40.Tran, H. H., G. Bjune, B. M. Nguyen, J. A. Rottingen, R. F. Grais, and P. J. Guerin. 2005. Risk factors associated with typhoid fever in Son La province, northern Vietnam. Trans. R. Soc. Trop. Med. Hyg. 99:819-826. [DOI] [PubMed] [Google Scholar]
- 41.Wain, J., and C. Kidgell. 2004. The emergence of multidrug resistance to antimicrobial agents for the treatment of typhoid fever. Trans. R. Soc. Trop. Med. Hyg. 98:423-430. [DOI] [PubMed] [Google Scholar]
- 42.Wain, J., N. T. T. Hoa, N. T. Chinh, H. Vinh, M. J. Everett, T. S. Diep, N. P. J. Day, T. Solomon, N. J. White, L. J. V. Piddock, and C. M. Parry. 1997. Quinolone-resistant Salmonella typhi in Viet Nam: molecular basis of resistance and clinical response to treatment. Clin. Infect. Dis. 25:1404-1410. [DOI] [PubMed] [Google Scholar]
- 43.Wain, J., L. T. Diem Nga, C. Kidgell, K. James, S. Fortune, T. Song Diep, T. Ali, P. O Gaora, C. Parry, J. Parkhill, J. Farrar, N. J. White, and G. Dougan. 2003. Molecular analysis of IncHI1 antimicrobial resistance plasmids from Salmonella serovar Typhi strains associated with typhoid fever. Antimicrob. Agents Chemother. 47:2732-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woods, C. W., D. R. Murdoch, M. D. Zimmerman, W. A. Glover, B. Basnyat, L. Wolf, R. H. Belbase, and L. B. Reller. 2006. Emergence of Salmonella enterica serotype Paratyphi A as a major cause of enteric fever in Kathmandu, Nepal. Trans. R. Soc. Trop. Med. Hyg. 100:1063-1067. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, Z.-K., Y.-N. Huang, and B.-C. Guo. 2004. Surveillance of the antibiotic resistance and plasmid of Salmonella paratyphoid. Chin. J. Antibiot. 29:610-613. [Google Scholar]