Abstract
Lipoteichoic acids (LTAs) have been shown to act as bacterial counterparts to the receptor binding proteins of LL-H, LL-H host range mutant LL-H-a21, and JCL1032. Here we have used LTAs purified by hydrophobic interaction chromatography from different phage-resistant and -sensitive strains of Lactobacillus delbrueckii subsp. lactis. Nuclear magnetic resonance analyses revealed variation in the degree of α-glucosyl and d-alanyl substitution of the 1,3-linked poly(glycerophosphate) LTAs between the phage-sensitive and phage-resistant strains. Inactivation of phages was less effective if there was a high level of d-alanine residues in the LTA backbones. Prior incubation of the LTAs with α-glucose-specific lectin inhibited the LL-H phage inactivation. The overall level of decoration or the specific spatial combination of α-glucosyl-substituted, d-alanyl-substituted, and nonsubstituted glycerol residues may also affect phage adsorption.
Multiplication of phages requires the takeover of the host cell metabolism. The phage infection starts when phages adsorb irreversibly to the specific surface structures of the host bacterium. The rate of adsorption depends on external factors like medium, pH, temperature, and cofactors (e.g., cations) (2). Phage-host interactions during host recognition and attachment between enterobacteria phages λ, T5, and P22 and outer membrane receptors have been studied thoroughly (10, 11, 25, 40, 45). Less is known about phage receptors of gram-positive bacteria (16, 37). Accessory polymers of the cell wall (such as polysaccharides and wall teichoic acids) (12, 14, 16), the peptidoglycan layer (44), and a few membrane proteins (22, 37) have been identified to serve as receptors for some Listeria, Bacillus subtilis, Staphylococcus aureus, Lactococcus lactis, and Lactobacillus phages.
Bacteriophages cause economic losses by disrupting lactic acid bacteria during milk fermentation before the desired texture and flavor have been formed in the product. Lactobacillus delbrueckii subsp. lactis and Lactobacillus delbrueckii subsp. bulgaricus are used as starters in cheese and yogurt production. The double-stranded DNA genome of the virulent phage LL-H (34.6 kb) is so far the only L. delbrueckii phage genome to be completely sequenced (29). The LL-H gene product responsible for host recognition has been identified as Gp71 by utilizing the isolated LL-H-resistant mutant (Ads-5) of L. delbrueckii subsp. lactis ATCC 15808 and the LL-H host range mutants (35). The small isometric-headed phage LL-H, with a noncontractile tail, a baseplate, and a central tail fiber (5, 21), exhibits only limited DNA similarity to another L. delbrueckii phage, JCL1032 (20, 36). The C-terminal end of ORF474 of JCL1032 shows, however, high sequence similarity to Gp71 (35). We have previously shown that lipoteichoic acids (LTAs) interact specifically with certain L. delbrueckii phages (34). LTAs most probably act as bacterial counterparts to the receptor binding proteins of LL-H, LL-H-a21, and JCL1032, thus representing a new class of phage receptors in gram-positive bacteria. Structural features of the LTAs, which determine the affinity and specificity of interactions with L. delbrueckii phages, remained to be elucidated. To resolve these structural features, a more in-depth molecular comparison between the LTAs extracted from phage-sensitive ATCC 15808 and phage-resistant strains Ads-5, 15808(J1), and LL78 was made. Structural analyses of LTAs by nuclear magnetic resonance spectroscopy (NMR) together with other experimental data indicate that the phage inactivation properties of the investigated LTAs are related to the degree of d-alanylation and α-glucosyl substitution and possibly a complex spatial combination of α-glucosyl-substituted, d-alanyl-substituted, and nonsubstituted glycerol residues. Different L. delbrueckii phages from homology groups a to d (28, 39) were tested in phage inactivation assays to see how widely the ability to use LTAs as phage receptors occurs among L. delbrueckii phages.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and bacteriophages.
Bacteria were grown at 37°C in MRS broth (Difco Laboratories, Detroit, MI), and for phage propagation MRS broth was supplemented with 10 mM CaCl2. Bacterial strains and bacteriophages used in this study are listed in Table 1. For the isolation of LTAs the strains were grown in batch cultures to an optical density at 600 nm of 0.5 to 0.6 in growth medium supplemented with 10 mM CaCl2. ATCC 15808 and Ads-5 cells extracted with butan-1-ol or n-butanol were cultivated without CaCl2.
TABLE 1.
Bacterial strains and bacteriophages
| Strain or phage | Description | Source; reference(s) |
|---|---|---|
| L. delbrueckii subsp. lactis strains | ||
| ATCC 15808 | Host strain for phages LL-H, LL-K, LL-S, JCL1032, 0235, 0252, and lb539 | ATCCa |
| Ads-5 | LL-H-resistant mutant of ATCC 15808; block of LL-H, LL-K, and LL-S adsorption; host strain for LL-H-a21 | This study; 35 |
| ATCC 15808(J1) | Phage-resistant mutant of JCL1032-lysogenic ATCC 15808; block of LL-H and JCL1032 adsorption | This study |
| LL78 | Host strain for LL-Ku and c5 | Valio Ltd, Finland; 21 |
| Bacteriophages (Siphoviridae) | ||
| Group a | ||
| LL-H | Virulent phage of L. delbrueckii subsp. lactis, morphotype B1 | Valio Ltd., Finland; 5, 21 |
| LL-H-a21 | Phage LL-H host range mutant; extended host range | This study; 35 |
| LL-K | Virulent phage of L. delbrueckii subsp. lactis, morphotype B1 | Valio Ltd, Finland; 21 |
| LL-S | Virulent phage of L. delbrueckii subsp. lactis, morphotype B1 | Valio Ltd, Finland; 21 |
| lb539 | Temperate phage of L. delbrueckii subsp. bulgaricus, morphotype B1 | R. Raya, Argentina; 7, 8 |
| Group b | ||
| LL-Ku | Virulent phage of L. delbrueckii subsp. lactis, morphotype B1 | Valio Ltd, Finland; 20 |
| c5 | Virulent phage of L. delbrueckii subsp. bulgaricus, morphotype B1 | 1, 20 |
| Group c | ||
| JCL1032 | L. delbrueckii subsp. lactis phage, morphotype B3 | J. Jimeno, Switzerland; 4, 20 |
| 0235 | Temperate phage of L. delbrueckii subsp. lactis, morphotype B3 | CNRZ9024b; 39 |
| Group d | ||
| 0252 | Temperate phage of L. delbrueckii subsp. lactis, morphotype B1 | CNRZ9026; 39 |
ATCC, American Type Culture Collection.
CNRZ, Collection of the Unité de Recherches Laitières et Génétique Appliquée, INRA, Jouy-en-Josas Cedex, France.
Purification of LTAs.
LTAs were extracted from freeze-dried cells with hot 80% (wt/vol) aqueous phenol as previously described (34) or with n-butanol (31) after enzymatic treatment with a concentrate of phage LL-H lysin in 100 mM sodium citrate (pH 4.6) and 10 mM MgCl2 (43). Freeze-dried LTA samples were suspended in chromatography start buffer (15% n-propanol in 0.1 M ammonium acetate, pH 4.7), centrifuged at 26,900 × g for 60 min, and filtered (0.2 μm) before hydrophobic interaction chromatography (HIC) was performed. LTAs were subjected to HIC on octyl-Sepharose using a linear gradient of 15% to 60% propan-1-ol in 0.1 M ammonium acetate (pH 4.7). LTAs were eluted as two separate pools between propan-1-ol concentrations of 30% and 40% (pool 1) and 40% and 45% (pool 2). LTA-phosphorus was quantitated according to a protocol described by Ames (6). Estimates for LTA-phosphorus were used to compare yields between different LTA pools and to calculate weighted averages. Ratios for pool 1 and pool 2 were 3.9:1.0 for phenol-extracted ATCC 15808, 3.7:1.0 for phenol-extracted Ads-5, 1,6:1.0 for phenol-extracted ATCC 15808(J1), 2.7:1.0 for phenol-extracted LL78, and 4.3:1.0 for n-butanol-extracted ATCC 15808 LTAs. d-Alanine and α-glucosyl substitution of different LTAs (expressed as percentages) are presented as weighted averages in Results and Discussion.
Dealanylation of LTAs.
d-Alanyl ester groups were removed from HIC-purified LTAs according to the method of Fisher et al. (18), by dialyzing the LTAs against 100 mM Tris-HCl (pH 8.0) overnight at 37°C and then by dialyzing them against milli-Q water.
Phage inactivation assays.
Inactivation properties of the LTAs were compared by determining the minimum concentration of the LTA-phosphorus needed for significant inactivation (≥50%) of L. delbrueckii phages (34). In the inactivation assays phage (106 PFU) was incubated in 1 ml of 10 mM Tris-HCl (pH 7.0) supplemented with 10 mM MgCl2. LTAs were added at final concentrations of 1 pg to 100 ng of LTA-phosphorus per ml. After 20 min of incubation at 37°C samples were directly diluted in MRS broth for the standard plaque assay (38). Each experiment was repeated three times. A reduction in plaque count was considered indicative of phage inactivation.
Inhibition of phage inactivation by ConA.
Concanavalin A (ConA) from Vector Laboratories was incubated with 1 ng of LTA-phosphorus in 1 ml of buffer (see above) for 10 min at 37°C before addition of the phage (106 PFU). ConA (2 mg per ml diluted in 10 mM Tris-HCl [pH 7.0]-10 mM MgCl2) was added to obtain a final concentration of 0, 5, 20, or 50 μg per ml. After 10 min of incubation at 37°C samples were directly diluted in MRS broth for the standard plaque assay. Each experiment was repeated three times.
Structural analyses of LTAs.
LTAs from ATCC 15808 (extracted with phenol and n-butanol), Ads-5, and LL78 were analyzed by 1H and 13C NMR. NMR spectra were obtained on a Bruker Avance 600 spectrometer at 300 K. Spectra were measured for solutions in D2O using sodium 3-trimethylsilyl-3,3,2,2-tetradeuteropropanoate as an internal standard for 1H NMR (δH, 0.00 ppm) and acetone for 13C NMR (δC, 30.02 ppm). Assignments were taken from two-dimensional homonuclear DQF-COSY (double-quantum-filtered correlation spectroscopy), TOCSY (total correlation spectroscopy), and ROESY (rotating-frame Overhauser enhancement spectroscopy) experiments with water suppression technique and two-dimensional heteronuclear HSQC (heteronuclear single-quantum correlation) (1H-13C) spectra. LTAs from ATCC 15808(J1) were examined for amino acids, polyols, and sugars by gas-liquid chromatography (GLC) (34). The average chain length of the poly(glycerophosphate) backbone, the degree of substitution, and the chain length of the fatty acids in the membrane anchor were determined by integration of the pertinent peak volumes in the proton spectra.
RESULTS AND DISCUSSION
Phage inactivation assays with different LTAs.
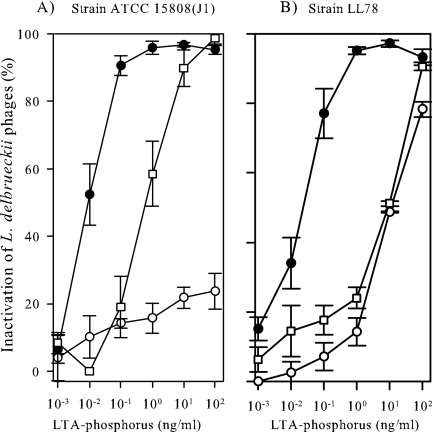
Strains ATCC 15808(J1) and LL78 already showed resistance to phage LL-H and phage JCL1032 at the adsorption stage, but they were sensitive to LL-H host range mutant LL-H-a21 (data not shown). As phage adsorption resistance could arise for a number of different reasons, for instance from steric hindrance of the surrounding molecules, HIC-purified LTAs from ATCC 15808(J1) and LL78 were tested in phage inactivation assays. The phage titer did not decrease when the LTAs from ATCC 15808(J1) were incubated with the LL-H virions (Fig. 1A). The concentration needed for the significant (≥50%) inactivation of phage LL-H was previously estimated as 100 pg of LTA-phosphorus from ATCC 15808 per ml (34). To get the equivalent response with the LTAs from strain LL78, 100-fold more LTA-phosphorus, i.e., 10 ng per ml, was needed (Fig. 1B). Phage JCL1032 was inactivated by the LTAs from ATCC 15808(J1) and LL78 (Fig. 1). Compared to the LTAs from ATCC 15808 (34), however, approximately 10 to 100 times more LTA-phosphorus was needed for the significant inactivation of phage JCL1032. LL-H host range mutant LL-H-a21 was inactivated by both the LTAs with no more than 10 pg of LTA-phosphorus per ml (Fig. 1). LTAs isolated for strains JCL15808(J1) and LL78 were confirmed to be suitable for structural analyses.
FIG. 1.
Inactivation of L. delbrueckii subsp. lactis phages LL-H (○), LL-H-a21 (•), and JCL1032 (□) with the LTAs derived from different L. delbrueckii subsp. lactis strains. (A) Strain ATCC 15808(J1). (B) Strain LL78. The data represent the means ± standard errors from three independent experiments.
Structural analysis of LTAs.
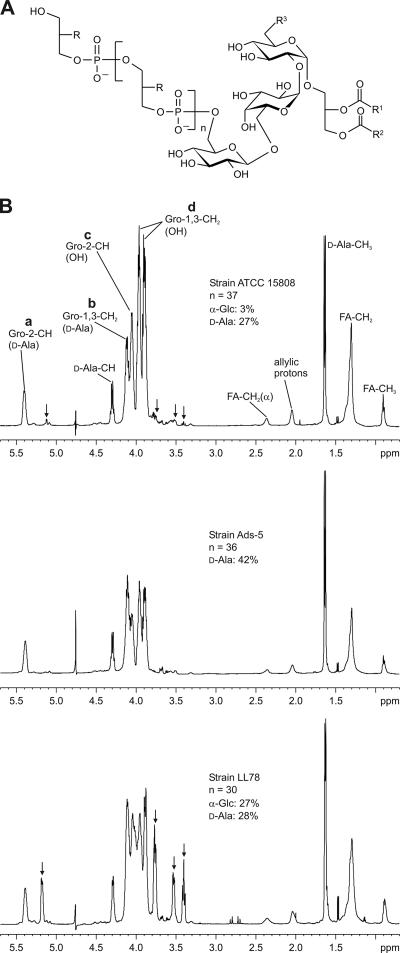
The molecular structure of the LTAs purified from the phage-sensitive strain ATCC 15808 and the phage-resistant L. delbrueckii strains Ads-5 and LL78 were investigated by NMR. For continuity with our previous work the LTAs were extracted with hot aqueous phenol before HIC purification and NMR analyses were carried out. The basic structure that was found for the L. delbrueckii strains investigated is depicted in Fig. 2A. All the LTAs investigated eluted as two separate pools during HIC. LTAs extracted from different lactobacilli, lactococci, and streptococci have been previously shown to be separated into molecular species with two and three fatty acids (19). We assume that pool 1 represents the LTAs with two fatty acids and pool 2 represents the LTAs with three fatty acids.
FIG. 2.
(A) The basic structure of the LTAs of L. delbrueckii subsp. lactis presented in this study. R represents −OH, d-Ala, or α-glucose; R1 and R2 represent alkyl groups, and R3 represents −OH or an acyl group. Depending on the strain investigated the LTAs were substituted with d-alanine and α-glucose (ATCC 15808 and LL78) or d-alanine only (Ads-5). (B) 1H-NMR spectra (600 MHz, 300 K) and specific features of the LTAs purified from L. delbrueckii subsp. lactis ATCC 15808, Ads-5, and LL78. LTAs extracted with hot phenol were eluted with propan-1-ol concentrations of between 30% and 40% and between 40% and 45% by HIC (pool 1 and pool 2, respectively). 1H-NMR spectra are shown only for pool 1 of each strain. The resonance signal intensities allow for the quantification of the substituents and estimation of the average poly(glycerophosphate) chain lengths. Resonance signals from α-glucose (substituent) are marked by arrows. The substitution of the glycerol moiety is indicated by giving the substituent in brackets. a, b, c, and d indicate the resonance signals from substituted Gro-2-CH (R = d-Ala) and Gro-1,3-CH2 (R = d-Ala) and unsubstituted Gro-2-CH and Gro-1,3-CH2, respectively.
Differences in d-alanyl substitution can be observed immediately if the ratios of the signal intensities of d-alanyl-substituted and nonsubstituted glycerol residues (a/c or b/d) are compared between different 1H-NMR spectra (Fig. 2B). In the proton spectra of the LTAs of ATCC 15808, weak signals could be observed (e.g., at δ 5.114, 3.765, 3.532, and 3.396 ppm), which were assigned to a glucosyl substituent (α-glucose) of the poly(glycerophosphate) backbone using 2D-NMR measurements (Fig. 2B). While these signals were absent from the 1H-NMR spectra of Ads-5, indicating that the backbone was nonglycosylated, they were present at high intensities in the spectra of LL78 (Fig. 2B). Table 2 shows the average chain lengths and the percentages of d-alanyl and α-glycosyl substitutions integrated from pertinent peak volumes in the proton spectra. Small variations in chain length and substitution between the LTA pools of each strain could be detected. On average, 26% of all the glycerophosphate residues of ATCC 15808 were d-alanyl substituted (Table 2). The corresponding figures were 27% for LL78 LTAs and 44% for Ads-5 LTAs. As a new finding to add to the previous GLC analyses of ATCC 15808 LTAs (34), a low degree of α-glucose substitution was detected. Approximately 3% of the glycerophosphate residues of ATCC 15808 and 27% of LL78 were α-glucosyl substituted, whereas LTAs from Ads-5 had no glucosyl substitution (Table 2). The results definitely show that the L. delbrueckii LTAs investigated are 1,3-linked poly(glycerophosphate)s with variable degrees of d-alanyl and α-glucosyl substitution. The structures of the LTAs from ATCC 15808(J1) are probably similar to those from Ads-5: according to GLC analysis ca. 30% (pool 1) and 50% (pool 2) of the glycerophosphate residues of ATCC 15808(J1) were substituted with d-alanyl esters (Table 2), and the glucose detected was connected to the glycolipid anchor. The trisaccharide moiety (that is, two α-glucoses and one β-glucose) of the glycolipid anchor or the overall fatty acid composition did not vary between the investigated LTAs (Fig. 2B). Thus, where phage inactivation is concerned, the relevant differences between the phage-sensitive and phage-resistant strains appear to lie in the degree of α-glucosyl and/or d-alanyl substitution and consequently in the degree of overall substitution of the backbones.
TABLE 2.
Comparison of 1,3-poly(glycerophosphate) backbones of different LTAsa
| Strain | LTA fraction | Chain length [(Gro-P)n] | Chain substitution (%)
|
||
|---|---|---|---|---|---|
| d-Ala | α-Glc | None | |||
| ATCC 15808 | 1 | 37 | 27 | 3 | 70 |
| 2 | 29 | 21 | 3 | 76 | |
| Ads-5 | 1 | 36 | 42 | ND | 58 |
| 2 | 31 | 49 | ND | 51 | |
| ATCC 15808(J1)b | 1 | 32 | ND | 68 | |
| 2 | 54 | ND | 46 | ||
| LL78 | 1 | 30 | 28 | 27 | 45 |
| 2 | 34 | 25 | 26 | 49 | |
LTAs were extracted with hot aqueous phenol and purified by HIC from different L. delbrueckii subsp. lactis strains. Unless stated otherwise, the values are based on NMR analyses. ND, not detected.
Polyols and α-glucose were quantified by GLC, and amino acids were quantified by the use of an amino acid analyzer.
Structural features of LTAs critical to phage inactivation.
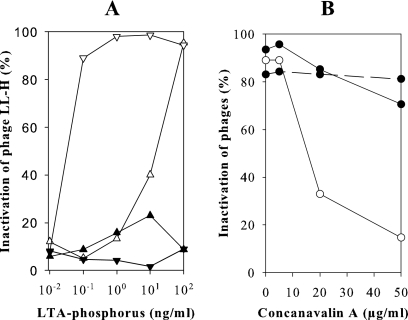
The influence of d-alanyl substitution on phage adsorption was investigated by deliberate hydrolysis of d-alanyl esters from n-butanol-extracted and HIC-purified LTAs. Compared to extraction with hot phenol, n-butanol extraction yielded LTAs with a higher level of d-alanyl esters (69%), as observed earlier by Morath et al. (31). The α-glucosyl substitution did not vary between the two extraction methods. The concentration of LTAs needed for significant LL-H inactivation was 100 times lower after the dealanylation of ATCC 15808 LTAs (Fig. 3A). The phages LL-H-a21 and JCL1032 reacted in the same way as LL-H, although their reaction was less pronounced. The concentration needed for the significant inactivation of these phages was approximately 10 times lower after the dealanylation (data not shown). The hydrolysis of d-alanyl esters from Ads-5 LTAs did not improve LL-H inactivation (Fig. 3A), which indirectly indicates the importance of α-glucosyl residues for LL-H binding. Further evidence was obtained from phage inactivation assays with ATCC 15808 LTAs preincubated with ConA (Fig. 3B). This α-glucose-specific lectin effectively inhibited phage LL-H inactivation: without preincubation with ConA 90% of the LL-H virions were inactivated, whereas after preincubation with ConA at a concentration of 20 μg per ml only 33% of the LL-H phages were inactivated. The corresponding figures for phage LL-H-a21 were 93% and 85%, respectively. Inactivation of phage LL-H-a21 by Ads-5 LTAs was not affected by ConA (Fig. 3B). This shows that inhibition of LL-H inactivation by ConA is based on ConA binding to α-glucose substituents and not to the trisaccharide moiety of the glycolipid anchor.
FIG. 3.
(A) Inactivation of the phage LL-H with the LTAs from L. delbrueckii subsp. lactis strains ATCC 15808 and Ads-5 before and after hydrolysis of d-alanyl esters. LTAs were extracted with n-butanol before HIC purification. LTAs from ATCC 15808 before (▵) and after (▿) hydrolysis and LTA from Ads-5 before (▴) and after (▾) hydrolysis. (B) Inhibition of phage LL-H (○) and phage LL-H-a21 (•) inactivation by α-glucose-specific lectin. ATCC 15808 and Ads-5 LTAs (solid and dashed lines, respectively) were treated with 0 to 50 μg ConA per ml before phage inactivation assays.
It is interesting that the degree of d-alanyl substitution affects the phage inactivation properties of the LTAs. LL-H infection requires external Ca2+ or Mg2+ at a concentration of 5 mM or more for the optimal rate of LL-H adsorption and possibly also for the transport of LL-H DNA into the host cell (3). Less-alanylated teichoic acids bind more Mg2+ and Ca2+ ions (23, 27). They may also inhibit the activity of autolytic enzymes, stabilize some secreted proteins, and play a role in bacterial coaggregation (9, 24, 32). External factors, such as pH, temperature, and sodium chloride concentration have been known to affect the d-alanine decoration of LTAs for some time now (17). For the incorporation of d-alanine into LTAs, four proteins encoded by the dlt operon are required (13), and the amount of information on the regulation mechanisms of the dlt operon is gradually increasing (15, 26, 33). According to recent findings, mRNA synthesis of the dlt operon of Staphylococcus aureus is acutely repressed by moderate concentrations (10 to 50 mM) of divalent cations Mg2+ and Ca2 (26). This is mediated by cis-acting elements upstream of the dlt operon and possibly by the two-component regulatory system ArlSR (26). How strongly the 10 mM calcium supplement in the MRS medium may have down-regulated the dlt operon of L. delbrueckii subsp. lactis remains to be elucidated.
Diversity of L. delbrueckii phages inactivated by LTAs.
Seven L. delbrueckii phages selected from homology groups a to d were tested with the LTAs from ATCC 15808 or LL78, depending on their adsorption specificity for the respective bacteria. LTAs from ATCC 15808 were able to inactivate phages LL-S, LL-K, and 0235 (Table 3). LL-Ku and c5, the only phages capable of infecting strain LL78 (20), did not react with the LTAs (data not shown). Table 3 summarizes the results from different phage inactivation assays. Phage 0235 was inactivated by the LTAs from ATCC 15808(J1), Ads-5, and LL78 with minimum concentrations comparable to that for phage JCL1032 (Table 3). LL-K and LL-S were inactivated by the LTAs from LL78, but as in the case of phage LL-H, higher LTA concentrations were needed for phage inactivation (Table 3). Table 3 strongly supports our previous results showing that LTAs act as phage receptors for certain L. delbrueckii phages (34). The ability to use LTAs as receptor molecules occurs among L. delbrueckii subsp. lactis phages from homology groups a and c. The data imply that L. delbrueckii phages most probably utilize more than one type of cell surface structure in host recognition. General relatedness between phages, as indicated by L. delbrueckii phages lb539 (this study) and mv4 (34), does not guarantee that the phages have the same or even similar specificities for bacterial surface structures. On the other hand, the modular organization of phage genomes and the genetic exchange enable even more distantly related phages to use the same receptors (30, 41).
TABLE 3.
Comparison of phage inactivation properties of different LTAs purified from Lactobacillus delbrueckii subsp. lactis strainsa
| Strain | Inactivation ratio for indicated phage
|
|||||
|---|---|---|---|---|---|---|
| LL-H | LL-H-a21 | LL-K | LL-S | JCL1032 | 0235 | |
| ATCC 15808 | 1b | 1b | 1 | 1 | 1b | 1 |
| Ads-5 | <10−3b | 0.2b | <10−3 | <10−2 | 0.2b | 0.3 |
| ATCC 15808(J1) | <10−3 | 1 | <10−3 | <10−2 | 0.1 | 0.1 |
| LL78 | 10−2 | 1 | 0.1 | 10−2 | 10−2 | <10−2 |
First, the amount of LTA-phosphorus needed for 50% of phage inactivation was estimated (see Materials and Methods). Each L. delbrueckii phage was considered separately. A value of 1 was given to the LTAs that could inactivate the phage with the smallest amount of LTA-phosphorus. All other LTA samples were compared to this figure. Concentrations of LTA-phosphorus of ATCC 15808 needed for 50% phage inactivation were as follows: for LL-H-a21, 10 pg per ml; for LL-H, LL-K, JCL1032, and 0235, 100 pg per ml; for LL-S, 1 ng per ml.
Previous results (34).
Summary.
This is the first time a detailed structure of the LTAs purified from industrially important L. delbrueckii subsp. lactis is described. NMR analyses revealed differences in the degree of α-glucosyl and d-alanyl substitution of the LTA backbones between phage-sensitive and phage-resistant strains. The capacity of the LTAs to inactivate L. delbrueckii phages was increased by dealanylation, indicating that a high level of ester-linked d-alanine residues inhibits phage adsorption. A glucose-specific lectin, ConA, inhibited inactivation by LTAs, especially of phage LL-H, which shows that α-glucose plays a significant role in phage LL-H adsorption. Also, the overall level of decoration or possibly the specific spatial combination of α-glucosyl-substituted, d-alanyl-substituted, and nonsubstituted glycerol residues may affect phage adsorption. Characterization of the genes responsible for d-alanylation of (lipo)teichoic acids together with the recently published genomic sequence from L. delbrueckii subsp. bulgaricus (42) may help us in the future to pinpoint the genetic changes in the LL-H and JCL1032 adsorption-resistant mutants of ATCC 15808.
Acknowledgments
We thank Franz Fiedler (Munich, Germany) for the opportunity to analyze the LTAs using GLC in his laboratory and Mika Mahosenaho (Sotkamo, Finland) for skillful technical assistance.
This study was supported by grants from the Academy of Finland (SA 46921), the EC Biotech II program (BIO4-CT98-0406), and the National Technology Agency in Finland (Tekes 70062/01) and by personal grants from the Oulu University Scholarship Foundation, the University of Oulu, Betty Väänänen Foundation, and Societas Amicorum Naturae Ouluensis to L.R.
Footnotes
Published ahead of print on 6 April 2007.
REFERENCES
- 1.Accolas, J.-P., and H. Spillmann. 1979. Morphology of bacteriophages of Lactobacillus bulgaricus, L. lactis and L. helveticus. J. Appl. Bacteriol. 47:309-319. [Google Scholar]
- 2.Ackermann, H.-W., and M. S. DuBow. 1987. Viruses of prokaryotes 1 & 2. CRC Press, Boca Raton, FL.
- 3.Alatossava, T. 1987. Molecular biology of Lactococcus lactis bacteriophage LL-H. Ph.D. thesis. University of Oulu, Oulu, Finland.
- 4.Alatossava, T., P. Forsman, M. Mikkonen, L. Räisänen, and A. Vasala. 1998. Molecular genetics and evolution of Lactobacillus phage LL-H and its related phage. Recent Res. Dev. Agric. Biol. Chem. 2:345-360. [Google Scholar]
- 5.Alatossava, T., and M. J. Pyhtilä. 1980. Characterization of a new Lactobacillus lactis bacteriophage. IRCS Med. Sci. 8:297-298. [Google Scholar]
- 6.Ames, B. N. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 8:115-118. [Google Scholar]
- 7.Auad, L., L. Räisänen, R. R. Raya, and T. Alatossava. 1999. Physical mapping and partial genetic characterization of the Lactobacillus delbrueckii subsp. bulgaricus bacteriophage lb539. Arch. Virol. 144:1503-1512. [DOI] [PubMed] [Google Scholar]
- 8.Auad, L., A. P. P. de Ruiz Holdago, P. Forsman, T. Alatossava, and R. R. Raya. 1997. Isolation and characterization of a new Lactobacillus delbrueckii ssp. bulgaricus temperate phage. J. Dairy Sci. 80:2706-2712. [Google Scholar]
- 9.Baddiley, J. 2000. Teichoic acids in bacterial coaggregation. Microbiology 146:1257-1258. [DOI] [PubMed] [Google Scholar]
- 10.Bonhivers, M., L. Plancon, A. Ghazi, P. Boulanger, M. le Maire, O. Lambert, J. L. Rigaud, and L. Letellier. 1998. FhuA, an Escherichia coli outer membrane protein with a dual function of transporter and channel which mediates the transport of phage DNA. Biochimie 80:363-369. [DOI] [PubMed] [Google Scholar]
- 11.Charbit, A., C. Werts, V. Michel, P. E. Klebba, P. Quillardet, and M. Hofnung. 1994. A role for residue 151 of LamB in bacteriophage lambda adsorption: possible steric effect of amino acid substitutions. J. Bacteriol. 176:3204-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyette, J., and J.-M. Ghuysen. 1968. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. IX. Teichoic acid and phage adsorption. Biochemistry 7:2385-2389. [DOI] [PubMed] [Google Scholar]
- 13.Debabov, D. V., M. Y. Kiriukhin, and F. C. Neuhaus. 2000. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in d-alanylation. J. Bacteriol. 182:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas, L., and J. M. Wolin. 1971. Cell wall polymers and phage lysis of Lactobacillus plantarum. Biochemistry 10:1551-1555. [DOI] [PubMed] [Google Scholar]
- 15.Dunman, P. M., E. Murphy, S. Haney, D. Palacios, G. Tucker-Kellogg, S. Wu, E. L. Brown, R. J. Zagursky, D. Shlaes, and S. J. Projan. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 183:7341-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont, K., T. Janzen, F. K. Vogensen, J. Josephsen, and B. Stuer-Lauridsen. 2004. Identification of Lactococcus lactis genes required for bacteriophage adsorption. Appl. Environ. Microbiol. 70:5825-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer, W. 1988. Physiology of lipoteichoic acids in bacteria. Adv. Microb. Physiol. 29:233-302. [DOI] [PubMed] [Google Scholar]
- 18.Fischer, W., H. U. Koch, P. Rösel, and F. Fiedler. 1980. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier: isolation, structural and functional characterization. J. Biol. Chem. 255:4557-4562. [PubMed] [Google Scholar]
- 19.Fischer, W., T. Mannsfeld, and G. Hagen. 1990. On the basic structure of poly(glycerophosphate) lipoteichoic acids. Biochem. Cell Biol. 68:33-43. [DOI] [PubMed] [Google Scholar]
- 20.Forsman, P. 1993. Characterization of a prolated-headed bacteriophage of Lactobacillus delbrueckii subsp. lactis, and its DNA homology with isometric-headed phages. Arch. Virol. 132:321-330. [DOI] [PubMed] [Google Scholar]
- 21.Forsman, P., and T. Alatossava. 1991. Genetic variation of Lactobacillus delbrueckii subsp. lactis bacteriophages isolated from cheese processing plants in Finland. Appl. Environ. Microbiol. 57:1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geller, B. L., R. G. Ivey, J. E. Trempy, and B. Hettinger-Smith. 1993. Cloning of a chromosomal gene required for phage infection of Lactococcus lactis subsp. lactis. J. Bacteriol. 175:5510-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Höltje, J.-V., and T. Tomasz. 1975. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc. Natl. Acad. Sci. USA 72:1690-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyyryläinen, H.-L., M. Vitikainen, J. Thwaite, H. Wu, M. Sarvas, C. R. Harwood, V. P. Kontinen, and K. Stephenson. 2000. D-Alanine substitution of teichoic acids as modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J. Biol. Chem. 275:26696-26703. [DOI] [PubMed] [Google Scholar]
- 25.Killmann, H., G. Videnov, G. Jung, H. Schwarz, and V. Braun. 1995. Identification of receptor binding sites by competitive peptide mapping: phages T1, T5, and φ80 and colicin M bind to the gating loop of FhuA. J. Bacteriol. 177:694-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koprivnjak, T., V. Mlakar, L. Swanson, B. Fournier, A. Peschel, and J. P. Weiss. 2006. Cation-induced transcriptional regulation of the dlt operon of Staphylococcus aureus. J. Bacteriol. 188:3622-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert, P. A., I. C. Hancock, and J. Baddiley. 1975. Influence of alanyl ester residues on the binding of magnesium ions to teichoic acids. Biochem. J. 151:671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mata, M., A. Trautwetter, G. Luthaud, and P. Ritzenthaler. 1986. Thirteen virulent and temperate bacteriophages of Lactobacillus bulgaricus and Lactobacillus lactis belong to a single DNA homology group. Appl. Environ. Microbiol. 52:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikkonen, M., L. Räisänen, and T. Alatossava. 1996. The early gene region completes the nucleotide sequence of Lactobacillus delbrueckii subsp. lactic phage LL-H. Gene 175:49-57. [DOI] [PubMed] [Google Scholar]
- 30.Montag, D., H. Schwartz, and U. Henning. 1989. A component of the side tail fiber of Escherichia coli bacteriophage λ can functionally replace the receptor-recognizing part of a long tail fiber protein of the unrelated bacteriophage T4. J. Bacteriol. 171:4378-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poyart, C., M. C. Lamy, C. Boumaila, F. Fiedler, and P. Trieu-Cuot. 2001. Regulation of d-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 183:6324-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Räisänen, L., K. Schubert, T. Jaakonsaari, and T. Alatossava. 2004. Characterization of lipoteichoic acids as Lactobacillus delbrueckii phage receptor components. J. Bacteriol. 186:5529-5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravin, V., L. Räisänen, and T. Alatossava. 2002. A conserved C-terminal region in Gp71 of the small isometric-head phage LL-H and ORF474 of the prolate-head phage JCL1032 is implicated in specificity of adsorption to its host, Lactobacillus delbrueckii. J. Bacteriol. 184:2455-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riipinen, K.-A., and T. Alatossava. 2004. Two self-splicing group I introns interrupt two late transcribed genes from prolate-headed Lactobacillus delbrueckii phage JCL1032. Arch. Virol. 149:2013-2024. [DOI] [PubMed] [Google Scholar]
- 37.São-José, C., C. Baptista, and M. A. Santos. 2004. Bacillus subtilis operon encoding a membrane receptor for bacteriophage SPP1. J. Bacteriol. 186:8337-8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarimo, S. S., M. Hartiala, and L. Aaltonen. 1976. Preparation and partial characterization of a Lactobacillus lactis bacteriophage. Arch. Microbiol. 107:193-197. [DOI] [PubMed] [Google Scholar]
- 39.Sechaud, L., P.-J. Cluzel, M. Rousseau, A. Baumgartner, and J.-P. Accolas. 1988. Bacteriophages of lactobacilli. Biochimie 70:401-410. [DOI] [PubMed] [Google Scholar]
- 40.Steinbacher, S., R. Seckler, S. Miller, B. Steipe, R. Huber, and P. Reinemer. 1994. Crystal structure of P22 tailspike protein: interdigitated subunits in a thermostable trimer. Science 265:283-386. [DOI] [PubMed] [Google Scholar]
- 41.Tétart, F., C. Desplats, and H. M. Krisch. 1998. Genome plasticity in the distal tail fiber locus of the T-even bacteriophage: recombination between conserved motifs swaps adhesin specificity. J. Mol. Biol. 282:543-556. [DOI] [PubMed] [Google Scholar]
- 42.van de Guchte, M., S. Penaud, C. Grimaldi, V. Barbe, K. Bryson, P. Nicolas, C. Robert, S. Oztas, S. Mangenot, A. Couloux, V. Loux, R. Dervyn, R. Bossy, A. Bolotin, J.-M. Batto, T. Walunas, J.-F. Gibrat, P. Bessières, J. Weissenbach, S. D. Ehrlich, and E. Maguin. 2006. The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proc. Natl. Acad. USA 103:9274-9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasala, A., M. Välkkilä, J. Caldentey, and T. Alatossava. 1995. Genetic and biochemical characterization of the Lactobacillus delbrueckii subsp. lactis bacteriophage LL-H lysin. Appl. Environ. Microbiol. 61:4004-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wendlinger, G. M., J. Loessner, and S. Scherer. 1996. Bacteriophage receptor on Listeria monocytogenes cells are the N-acetylglucosamine and rhamnose substituents of teichoic acids or the peptidoglycan itself. Microbiology 142:985-992. [DOI] [PubMed] [Google Scholar]
- 45.Werts, C., V. Michel, M. Hofnung, and A. Charbit. 1994. Adsorption of bacteriophage lambda on the LamB protein of Escherichia coli K-12: point mutations in gene J of lambda responsible for extended host range. J. Bacteriol. 176:941-947. [DOI] [PMC free article] [PubMed] [Google Scholar]